Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation
Abstract
1. Introduction
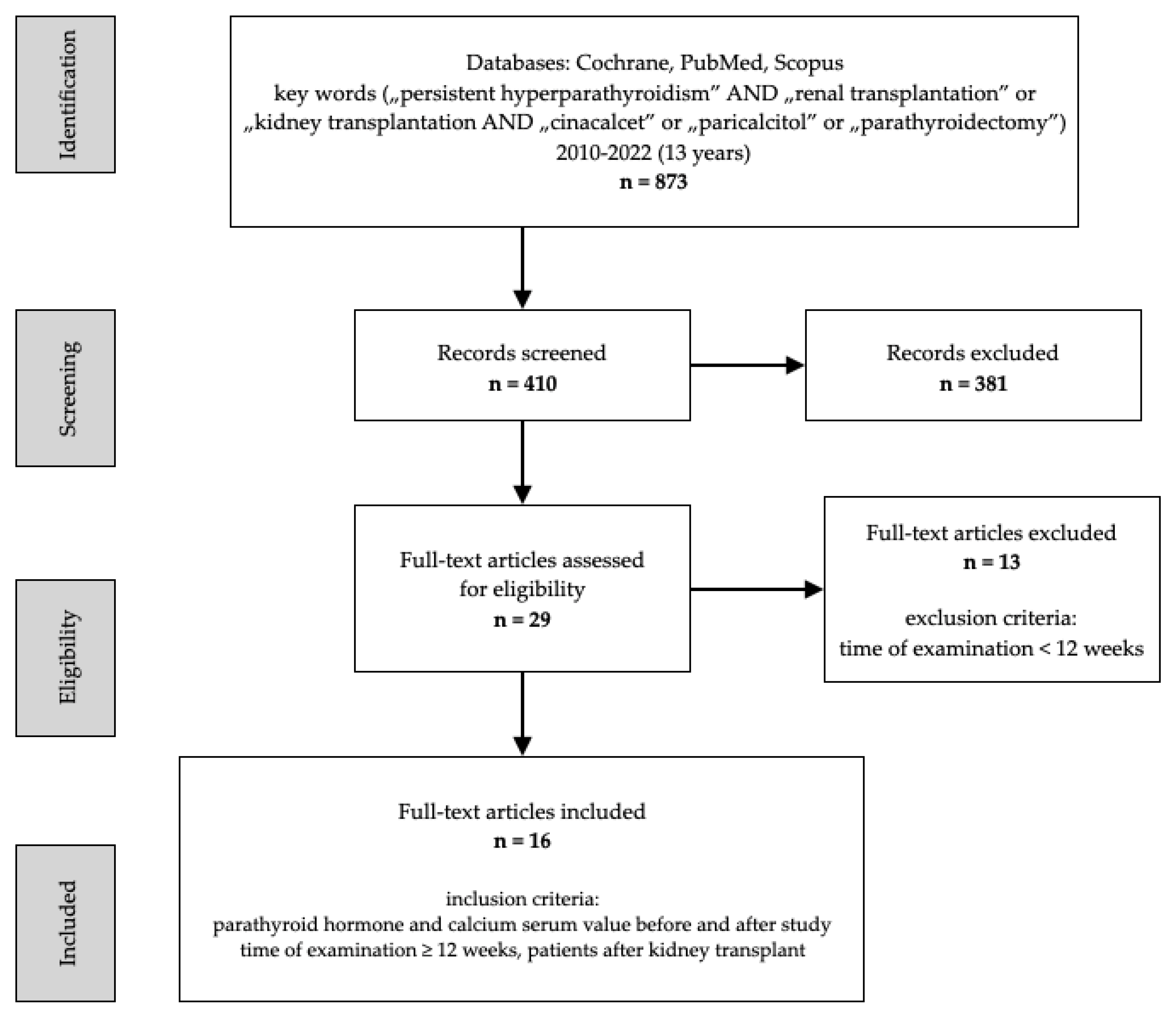
2. Materials and Methods
3. Results
3.1. Calcitriol
3.2. Paricalcitiol
3.3. Cinacalcet
3.4. Parathyreidectomy
3.5. Etelcalcetide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silver, J.; Levi, R. Regulation of PTH synthesis and secretion relevant to the management of secondary hyperparathyroidism in chronic kidney disease. Kidney Int. Suppl. 2005, 95, S8–S12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Plas, W.Y.; Noltes, M.E.; van Ginhoven, T.M.; Kruijff, S. Secondary and Tertiary Hyperparathyroidism: A Narrative Review. Scand. J. Surg. 2020, 109, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yuen, N.K.; Ananthakrishnan, S.; Campbell, M.J. Hyperparathyroidism of Renal Disease. Perm J. 2016, 20, 15–127. [Google Scholar] [CrossRef] [PubMed]
- Miedziaszczyk, M.; Idasiak-Piechocka, I.; Wiśniewski, O.W.; Lacka, K. A systematic review of the pharmacotherapy of secondary hyperparathyroidism (SHPT) in grades 3-5 Chronic Kidney Disease (CKD). Eur Rev. Med. Pharmacol Sci. 2022, 26, 232–239. [Google Scholar]
- Delos Santos, R.; Rossi, A.; Coyne, D.; Maw, T.T. Management of Post-transplant Hyperparathyroidism and Bone Disease. Drugs 2019, 79, 501–513. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Kuypers, D.; Maes, B.; Bammens, B.; Vanrenterghem, Y. Natural history of parathyroid function and calcium metabolism after kidney transplantation: A single-centre study. Nephrol Dial. Transplant. 2004, 19, 1281–1287. [Google Scholar] [CrossRef]
- Messa, P.; Sindici, C.; Cannella, G.; Miotti, V.; Risaliti, A.; Gropuzzo, M.; Di Loreto, P.L.; Bresadola, F.; Mioni, G. Persistent secondary hyperparathyroidism after renal transplantation. Kidney Int. 1998, 54, 1704–1713. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, Y.J.; Kwon, H.Y.; Koo, T.Y.; Baek, S.H.; Kim, H.J.; Huh, W.S.; Huh, K.H.; Kim, M.S.; Kim, Y.S.; et al. Impact of parathyroidectomy on allograft outcomes in kidney transplantation. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2012, 25, 1248–1256. [Google Scholar] [CrossRef]
- Nakamura, M.; Tanaka, K.; Marui, Y.; Tomikawa, S. Clinicopathological analysis of persistent hypercalcemia and hyperparathyroidism after kidney transplantation in long-term dialysis patients. Ther Apher Dial. 2013, 17, 551–556. [Google Scholar] [CrossRef]
- Taniguchi, M.; Tokumoto, M.; Matsuo, D.; Motoyama, K.; Sugitani, A.; Kuroki, S.; Yotsueda, H.; Tsuruya, K.; Hirakata, H.; Iida, M. Persistent hyperparathyroidism in renal allograft recipients: Vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int. 2006, 70, 363–370. [Google Scholar] [CrossRef]
- Garcia-Montemayor, V.; Sánchez-Agesta, M.; Agüera, M.L.; Calle, Ó.; Navarro, M.D.; Rodríguez, A.; Aljama, P. Influence of Pre-renal Transplant Secondary Hyperparathyroidism on Later Evolution After Transplantation. Transplant. Proc. 2019, 51, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lou, I.; Foley, D.; Odorico, S.K.; Leverson, G.; Schneider, D.F.; Sippel, R.; Chen, H. How Well Does Renal Transplantation Cure Hyperparathyroidism? Ann. Surg. 2015, 262, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Tominaga, Y.; Okada, M.; Hiramitsu, T.; Tsujita, M.; Goto, N.; Narumi, S.; Watarai, Y. Characteristics of Persistent Hyperparathyroidism After Renal Transplantation. World J. Surg. 2016, 40, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kirnap, N.G.; Kirnap, M.; Sayin, B.; Akdur, A.; Bascil Tutuncu, N.; Haberal, M. Risk Factors and Treatment Options for Persistent Hyperparathyroidism After Kidney Transplantation. Transplant. Proc. 2020, 52, 157–161. [Google Scholar] [CrossRef]
- Triponez, F.; Clark, O.H.; Vanrenthergem, Y.; Evenepoel, P. Surgical treatment of persistent hyperparathyroidism after renal transplantation. Ann. Surg. 2008, 248, 18–30. [Google Scholar] [CrossRef]
- Evenepoel, P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol. 2013, 33, 191–203. [Google Scholar] [CrossRef]
- Gwinner, W.; Suppa, S.; Mengel, M.; Hoy, L.; Kreipe, H.H.; Haller, H.; Schwarz, A. Early calcification of renal allografts detected by protocol biopsies: Causes and clinical implications. Am. J. Transplant. 2005, 5, 1934–1941. [Google Scholar] [CrossRef]
- Mazzaferro, S.; Pasquali, M.; Taggi, F.; Baldinelli, M.; Conte, C.; Muci, M.L.; Pirozzi, N.; Carbone, I.; Francone, M.; Pugliese, F. Progression of coronary artery calcification in renal transplantation and the role of secondary hyperparathyroidism and inflammation. Clin. J. Am. Soc. Nephrol. 2009, 4, 685–690. [Google Scholar] [CrossRef]
- Rojas, E.; Carlini, R.G.; Clesca, P.; Arminio, A.; Suniaga, O.; De Elguezabal, K.; Weisinger, J.R.; Hruska, K.A.; Bellorin-Font, E. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003, 63, 1915–1923. [Google Scholar] [CrossRef]
- Ivarsson, K.M.; Clyne, N.; Almquist, M.; Akaberi, S. Hyperparathyroidism and new onset diabetes after renal transplantation. Transplant. Proc. 2014, 46, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Paschoalin, R.P.; Torregrosa, J.V.; Barros, X.; Durán, C.E.; Soler, M.; Campistol, J.M. Withdrawal of cinacalcet at the time of renal transplantation is not a risk factor for allograft calcifications in the early posttransplantation period. Transplant. Proc. 2012, 44, 2379–2380. [Google Scholar] [CrossRef]
- Julian, B.A.; Laskow, D.A.; Dubovsky, J.; Dubovsky, E.V.; Curtis, J.J.; Quarles, L.D. Rapid loss of vertebral mineral density after renal transplantation. N. Eng. J. Med. 1991, 325, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Rufino, M.; Bartolomei, S.; González-Rinne, A.; Lorenzo, V.; Cobo, M.; Torres, A. Clinical impact of preexisting vascular calcifications on mortality after renal transplantation. Kidney Int. 2005, 67, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrøm, H.K.; Gatti, F.; Hammarström, C.; Eide, I.A.; Kasprzycka, M.; Wang, J.; Haraldsen, G.; Svensson, M.H.S.; Midtvedt, K.; Mjøen, G.; et al. Early introduction of oral paricalcitol in renal transplant recipients. An open-label randomized study. Transpl Int. 2017, 30, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Borrego Utiel, F.J.; Bravo Soto, J.A.; Merino Pérez, M.J.; González Carmelo, I.; López Jiménez, V.; García Álvarez, T.; Acosta Martínez, Y.; Mazuecos Blanca, M.A. Effect of paricalcitol on mineral bone metabolism in kidney transplant recipients with secondary hyperparathyroidism. Nefrologia 2015, 35, 363–373, (In English & Spanish). [Google Scholar] [CrossRef] [PubMed]
- Trillini, M.; Cortinovis, M.; Ruggenenti, P.; Reyes Loaeza, J.; Courville, K.; Ferrer-Siles, C.; Prandini, S.; Gaspari, F.; Cannata, A.; Villa, A.; et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J. Am. Soc. Nephrol. 2015, 26, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Rojas-Rivera, J.; Polanco, N.; Morales, E.; Morales, J.M.; Egido, J.; Amado, A.; Praga, M. Effects of oral paricalcitol on secondary hyperparathyroidism and proteinuria of kidney transplant patients. Transplantation 2013, 95, e49–e52. [Google Scholar] [CrossRef]
- Pinho, L.R.; Ribeiro Santos, M.J.; Pestana Vasconcelos, M. Cinacalcet in the treatment of persistent hyperparathyroidism after kidney transplantation. Clin. Nephrol. 2011, 75, 263–268. [Google Scholar] [CrossRef]
- Torregrosa, J.V.; Morales, E.; Díaz, J.M.; Crespo, J.; Bravo, J.; Gómez, G.; Gentil, M.A.; Rodríguez-Benot, A.; Rodríguez-García, M.; López-Jiménez, V.; et al. Cinacalcet en el manejo del hiperparatiroidismo secundario normocalcémico tras el trasplante renal: Estudio multicéntrico de un año de seguimiento [Cinacalcet in the management of normocalcaemic secondary hyperparathyroidism after kidney transplantation: One-year follow-up multicentre study]. Nefrologia 2014, 34, 62–68. (In Spanish) [Google Scholar]
- Torregrosa, J.V.; Morales, E.; Díaz, J.M.; Crespo, J.; Bravo, J.; Gómez, G.; Gentil, M.Á.; Rodríguez Benot, A.; García, M.R.; Jiménez, V.L.; et al. Cinacalcet for hypercalcaemic secondary hyperparathyroidism after renal transplantation: A multicentre, retrospective, 3-year study. Nephrology 2014, 19, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zavvos, V.; Fyssa, L.; Papasotiriou, M.; Papachristou, E.; Ntrinias, T.; Savvidaki, E.; Goumenos, D.S. Long-Term Use of Cinacalcet in Kidney Transplant Recipients With Hypercalcemic Secondary Hyperparathyroidism: A Single-Center Prospective Study. Exp. Clin. Transplant. 2018, 16, 287–293. [Google Scholar] [PubMed]
- Paschoalin, R.P.; Torregrosa, J.V.; Sánchez-Escuredo, A.; Barros, X.; Durán, C.E.; Campistol, J.M. Cinacalcet treatment for stable kidney transplantation patients with hypercalcemia due to persistent secondary hyperparathyroidism: A long-term follow-up. Transplant. Proc. 2012, 44, 2588–2589. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Cooper, K.; Holdaas, H.; Messa, P.; Mourad, G.; Olgaard, K.; Rutkowski, B.; Schaefer, H.; Deng, H.; Torregrosa, J.V.; et al. A randomized study evaluating cinacalcet to treat hypercalcemia in renal transplant recipients with persistent hyperparathyroidism. Am. J. Transplant. 2014, 14, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Henríquez-Palop, F.; Martín-Núñez, E.; Hernández-Carballo, C.; Ferri, C.; Pérez-Delgado, N.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Anti-inflammatory profile of paricalcitol in kidney transplant recipients. Nefrologia 2017, 37, 622–629, (In English & Spanish). [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; Sánchez-Escuredo, A.; Lauzurica, R.; Bayés, B.; Navarro-Muñoz, M.; Pastor, M.C.; Cañas, L.; Bonet, J.; Romero, R. Magnetic bead-based proteomic technology to study paricalcitol effect in kidney transplant recipients. Eur. J. Pharmacol. 2013, 709, 72–79. [Google Scholar] [CrossRef]
- Moreno, P.; Coloma, A.; Torregrosa, J.V.; Montero, N.; Francos, J.; Codina, S.; Manonelles, A.; Bestard, O.; García-Barrasa, A.; Melilli, E.; et al. Long-term results of a randomized study comparing parathyroidectomy with cinacalcet for treating tertiary hyperparathyroidism. Clin. Transplant. 2020, 34, e13988. [Google Scholar] [CrossRef]
- Cruzado, J.M.; Moreno, P.; Torregrosa, J.V.; Taco, O.; Mast, R.; Gómez-Vaquero, C.; Polo, C.; Revuelta, I.; Francos, J.; Torras, J.; et al. A Randomized Study Comparing Parathyroidectomy with Cinacalcet for Treating Hypercalcemia in Kidney Allograft Recipients with Hyperparathyroidism. J. Am. Soc. Nephrol. 2016, 27, 2487–2494. [Google Scholar] [CrossRef]
- Cianciolo, G.; Galassi, A.; Capelli, I.; Angelini, M.L.; La Manna, G.; Cozzolino, M. Vitamin D in Kidney Transplant Recipients: Mechanisms and Therapy. Am. J. Nephrol. 2016, 43, 397–407. [Google Scholar] [CrossRef]
- Rodriguez, M.; Munoz-Castaneda, J.R.; Almaden, Y. Therapeutic use of calcitriol. Curr Vasc Pharmacol. 2014, 12, 294–299. [Google Scholar] [CrossRef]
- Torregrosa, J.V.; Fuster, D.; Duran, C.E.; Oppenheimer, F.; Muxí, Á.; Rubello, D.; Pons, F.; Campistol, J.M. Set point of calcium in severe secondary hyperparathyroidism is altered and does not change after successful kidney transplantation. Endocrine 2015, 48, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Torregrosa, J.V.; Barros, X. Management of hypercalcemia after renal transplantation. Nefrologia 2013, 33, 751–757, (In English & Spanish). [Google Scholar] [PubMed]
- Tillmann, F.P.; Wächtler, C.; Hansen, A.; Rump, L.C.; Quack, I. Vitamin D and cinacalcet administration pre-transplantation predict hypercalcaemic hyperparathyroidism post-transplantation: A case-control study of 355 deceased-donor renal transplant recipients over 3 years. Transplant. Res. 2014, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D.; Leifheit-Nestler, M. CKD-MBD post kidney transplantation. Pediatr Nephrol. 2021, 36, 41–50. [Google Scholar] [CrossRef]
- Wada, Y.; Iyoda, M.; Iseri, K.; Arai-Nunota, N.; Saito, T.; Hamada, T.; Tachibana, S.; Ikeda, M.; Shibata, T. Combination Therapy of Denosumab and Calcitriol for a Renal Transplant Recipient with Severe Bone Loss due to Therapy-Resistant Hyperparathyroidism. Tohoku J. Exp. Med. 2016, 238, 205–212. [Google Scholar] [CrossRef]
- Robinson, D.M.; Scott, L.J. Paricalcitol: A review of its use in the management of secondary hyperparathyroidism. Drugs 2005, 65, 559–576. [Google Scholar] [CrossRef]
- Žilinská, Z.; Dedinská, I.; Breza, J.; Laca, L. Effect of Paricalcitol on Bone Density After Kidney Transplantation: Analysis of 2 Transplant Centers. Iran. J. Kidney Dis. 2017, 11, 461–466. [Google Scholar]
- Donate-Correa, J.; Henríquez-Palop, F.; Martín-Núñez, E.; Pérez-Delgado, N.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Effect of Paricalcitol on FGF-23 and Klotho in Kidney Transplant Recipients. Transplantation 2016, 100, 2432–2438. [Google Scholar] [CrossRef]
- Pérez, V.; Sánchez, A.; Bayés, B.; Navarro-Muñoz, M.; Lauzurica, R.; Pastor, M.C.; Romero, R. Effect of paricalcitol on the urinary peptidome of kidney transplant patients. Transplant. Proc. 2010, 42, 2924–2927. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar]
- Amer, H.; Griffin, M.D.; Stegall, M.D.; Cosio, F.G.; Park, W.D.; Kremers, W.K.; Heilman, R.L.; Mazur, M.J.; Hamawi, K.; Larson, T.S.; et al. Oral paricalcitol reduces the prevalence of posttransplant hyperparathyroidism: Results of an open label randomized trial. Am. J. Transplant. 2013, 13, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, W.; Zhang, X.; Li, X.; Chen, J. Efficacy and safety of paricalcitol therapy for chronic kidney disease: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2012, 7, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C.A. Safety and tolerability of paricalcitol in patients with chronic kidney disease. Expert Opin. Drug Saf. 2013, 12, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.R.; Maamoun, H.A.; Soliman, M.A.; Darwish, H.; Elbanna, E. Cinacalcet versus Parathyroidectomy in the Treatment of Secondary Hyperparathyroidism Post Renal Transplantation. Rom. J. Intern. Med. 2016, 54, 184–189. [Google Scholar] [CrossRef]
- Mogl, M.T.; Skachko, T.; Dobrindt, E.M.; Reinke, P.; Bures, C.; Pratschke, J.; Rayes, N. Surgery for Renal Hyperparathyroidism in the Era of Cinacalcet: A Single-Center Experience. Scand. J. Surg. 2021, 110, 66–72. [Google Scholar] [CrossRef]
- Guerra, R.; Auyanet, I.; Fernández, E.J.; Pérez, M.Á.; Bosch, E.; Ramírez, A.; Suria, S.; Checa, M.D. Hypercalcemia secondary to persistent hyperparathyroidism in kidney transplant patients: Analysis after a year with cinacalcet. J. Nephrol. 2011, 24, 78–82. [Google Scholar] [CrossRef]
- Boulanger, H.; Haymann, J.P.; Fouqueray, B.; Mansouri, R.; Metivier, F.; Mercadal, L.; Attaf, D.; Flamant, M.; Glotz, D. Effet du cinacalcet sur l′homéostasie calcique et le remodelage osseux chez 13 transplantés rénaux présentant une hyperparathyroïdie avec hypercalcémie [Cinacalcet impact on calcium homeostasis and bone remodeling in 13 renal transplanted patients with hyperparathyroidism and hypercalcaemia]. Nephrol Ther. 2012, 8, 47–53. (In French) [Google Scholar]
- Vaquero, E.; Esteban de la Rosa, R.J.; Oliva, N.; Fernández Castillo, R.; Fernández Gallegos, R.; Bravo Soto, J. Efecto de cinacalcet sobre la hormona paratiroidea en el hiperparatiroidismo hipercalcémico del trasplantado renal [Effect of cinacalcet on parathyroid hormone level in hypercalcemic hyperparathyroidism of patients with renal transplantation]. Med. Clin. 2011, 138, 323–326. [Google Scholar] [CrossRef]
- Paschoalin, R.P.; Torregrosa, J.V.; Barros, X.; Durán, C.E.; Campistol, J.M. Cinacalcet de novo in persistent hypercalcemia after kidney transplantation secondary to hyperparathyroidism: Long-term follow-up and effect of withdrawal. Transplant. Proc. 2012, 44, 2376–2378. [Google Scholar] [CrossRef]
- Mawad, H.; Bouchard, H.; Tran, D.; Ouimet, D.; Lafrance, J.P.; Bell, R.Z.; Bezzaoucha, S.; Boucher, A.; Collette, S.; Pichette, V.; et al. Retrospective Study Looking at Cinacalcet in the Management of Hyperparathyroidism after Kidney Transplantation. J. Transplant. 2017, 2017, 8720283. [Google Scholar] [CrossRef]
- Niel, O.; Maisin, A.; Macher, M.A.; Peuchmaur, M.; Deschênes, G. Cinacalcet in hyperparathyroidism management after pediatric renal transplantation. CEN Case Rep. 2016, 5, 141–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seager, C.M.; Srinivas, T.R.; Flechner, S.M. Development of nephrolithiasis in a renal transplant patient during treatment with Cinacalcet. Ann. Transplant. 2013, 18, 31–35. [Google Scholar] [CrossRef]
- Seikrit, C.; Mühlfeld, A.; Groene, H.J.; Floege, J. Renal allograft failure in a hyperparathyroid patient following initiation of a calcimimetic. Nat. Rev. Nephrol. 2011, 7, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.Á.; Fedor, R.; Asztalos, L.; Andrási, M.; Szabó, R.P.; Kanyári, Z.; Barna, S.; Nemes, B.; Győry, F. Surgical Treatment of Hyperparathyroidism After Kidney Transplant. Transplant. Proc. 2019, 51, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, K.M.; Akaberi, S.; Isaksson, E.; Reihnér, E.; Rylance, R.; Prütz, K.G.; Clyne, N.; Almquist, M. The effect of parathyroidectomy on patient survival in secondary hyperparathyroidism. Nephrol Dial. Transplant. 2015, 30, 2027–2033. [Google Scholar] [CrossRef]
- Li, X.; An, C.; Yu, M.; Peng, L. US-guided microwave ablation for secondary hyperparathyroidism in patients after renal transplantation: A pilot study. Int. J. Hyperthermia. 2019, 36, 322–327. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Montenegro, F.L.; Machado, D.J.; Ianhez, L.E.; Nahas, W.C.; David-Neto, E. Parathyroidectomy after kidney transplantation: Short-and long-term impact on renal function. Clinics 2011, 66, 431–435. [Google Scholar] [CrossRef]
- Chudzinski, W.; Wyrzykowska, M.; Nazarewski, S.; Durlik, M.; Galazka, Z. Does the Parathyroidectomy Endanger the Transplanted Kidney? Transplant. Proc. 2016, 48, 1633–1636. [Google Scholar] [CrossRef]
- Bures, C.; Seika, P.; Skachko, T.; Dobrindt, E.M.; Rayes, N.; Pratschke, J.; Goretzki, P.E.; Mogl, M.T. Influence of Parathyroidectomy on Kidney Graft Function in Secondary and Tertiary Hyperparathyroidism. Transplant. Proc. 2020, 52, 3134–3143. [Google Scholar] [CrossRef]
- Littbarski, S.A.; Kaltenborn, A.; Gwiasda, J.; Beneke, J.; Arelin, V.; Schwager, Y.; Stupak, J.V.; Marcheel, I.L.; Emmanouilidis, N.; Jäger, M.D.; et al. Timing of parathyroidectomy in kidney transplant candidates with secondary hyperparathryroidism: Effect of pretransplant versus early or late post-transplant parathyroidectomy. Surgery 2018, 163, 373–380. [Google Scholar] [CrossRef]
- El-Husseini, A.; Wang, K.; Edon, A.; Saxon, D.; Lima, F.; Sloan, D.; Sawaya, B.P. Value of Intraoperative Parathyroid Hormone Assay during Parathyroidectomy in Dialysis and Renal Transplant Patients with Secondary and Tertiary Hyperparathyroidism. Nephron 2018, 138, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Bautista, J.; Occhiogrosso, R.; Scott-Sheldon, L.A.; Gohh, R. Acute hypocalcemia following kidney transplantation may depend on the type of remote parathyroidectomy: A retrospective cohort study. Clin. Nephrol. 2017, 87, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; Kewalramani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 2017, 317, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Karaboyas, A.; Muenz, D.; Fuller, D.S.; Desai, P.; Lin, T.C.; Robinson, B.M.; Rossetti, S.; Pisoni, R.L. Etelcalcetide Utilization, Dosing Titration, and Chronic Kidney Disease-Mineral and Bone Disease (CKD-MBD) Marker Responses in US Hemodialysis Patients. Am. J. Kidney Dis. 2022, 79, 362–373. [Google Scholar] [CrossRef]
| The Active Substance | Dosage | Time Study [Weeks] | Study Group (n) * Completed | Control Group (n) * Completed | Placebo or Comparator |
|---|---|---|---|---|---|
| Paricalcitol [27] | 1 μg/day and next 2 μg/day | 24 | 22 * 20 | 21 * 19 | other therapy |
| Paricalcitol [25] | 2 μg/day | 44 | 37 * 35 | 40 | standard therapy |
| Paricalcitol [26] | 2–3 μg/day | 96 | 69 | - | - |
| Paricalcitol [28] | 1 μg/day | 72 | 58 | - | - |
| Paricalcitol [35] | 1 μg/day | 12 | 31 | - | - |
| Paricalcitol [36] | 1 μg/day | 48 | 31 | 31 | standard therapy |
| Cinacalcet [30] | 30 mg/day | 48 | 32 * 29 | - | - |
| Cinacalcet [29] | 30 mg/day | 48 | 18 | - | - |
| Cinacalcet [31] | 30 mg/day | 24 | 193 * 175 | - | - |
| Cinacalcet [32] | 30 mg/day | 24 | 47 * no data | - | - |
| Cinacalcet [33] | 30 mg/day | 240 | 6 | - | - |
| Cinacalcet [34] | 30 mg/day | 52 | 57 * 52 | 57 * 52 | placebo |
| Cinacalcet, Parathyreidectomy [37] | 30 mg every other day 30 mg/day 60 mg/day | 240 | 15 * 13 | 15 * 11 | parathyreidectomy |
| The Active Substance | PTH before the Study [pg/mL] | PTH after the Study [pg/mL] | Phosphorus Serum before [mmol/L] | Phosphorus Serum after [mmol/L] | Calcium Serum before [mmol/L] | Calcium Serum after [mmol/L] | Albumin (Serum Calcium Concentrations Were Corrected for Albumin Level) |
|---|---|---|---|---|---|---|---|
| Paricalcitol [27] | 115.60 | 63.25 | 1.06 | 1.10 | 2.38 | 2.40 | no data |
| Paricalcitol [25] | 105.62 | 93.36 | 0.93 | 0.95 | 2.37 | 2.39 | no data |
| Paricalcitol [26] | 288 | 193 | 1.10 | 1.16 | 2.42 | 2.40 | no data |
| Paricalcitol [28] | 333 | 181 | 1.10 | 1.13 | 2.32 | 2.40 | no data |
| Paricalcitol [35] | 216 | 167 | 1.05 | 1.04 | 2.41 | 2.43 | no data |
| Paricalcitol [36] | 100.00 | 59.70 | 1.00 | 1.01 | 2.33 | 2.36 | serum calcium concentrations were corrected for the albumin level |
| Cinacalcet [30] | 364 | 145 | 1.03 | 1.07 | 2.37 | 2.30 | no data |
| Cinacalcet [29] | 242.04 | 145.62 | 0.65 | 0.94 | 2.78 | 2.48 | no data |
| Cinacalcet [31] | 235 | 181 | 0.87 | 0.97 | 2.77 | 2.52 | no data |
| Cinacalcet [32] | 310.72 | 238.39 | 0.93 | 0.96 | 2.69 | 2.48 | serum calcium concentrations were corrected for the albumin level |
| Cinacalcet [33] | 260 | 237 | 0.90 | 1.01 | 2.74 | 2.56 | no data |
| Cinacalcet [34] | 327.7 | 169.0 | 0.86 | 1.06 | 2.81 | 2.39 | serum calcium concentrations were corrected for the albumin level |
| Cinacalcet [37] | 235.75 | 198.03 | 0.92 | 0.96 | 2.72 | 2.43 | serum calcium concentrations were corrected for the albumin level |
| Parathyreidectomy [37] | 348.91 | 47.15 | 0.93 | 1.30 | 2.78 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedziaszczyk, M.; Lacka, K.; Tomczak, O.; Bajon, A.; Primke, M.; Idasiak-Piechocka, I. Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation. Biomedicines 2023, 11, 25. https://doi.org/10.3390/biomedicines11010025
Miedziaszczyk M, Lacka K, Tomczak O, Bajon A, Primke M, Idasiak-Piechocka I. Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation. Biomedicines. 2023; 11(1):25. https://doi.org/10.3390/biomedicines11010025
Chicago/Turabian StyleMiedziaszczyk, Miłosz, Katarzyna Lacka, Oskar Tomczak, Aleksander Bajon, Marta Primke, and Ilona Idasiak-Piechocka. 2023. "Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation" Biomedicines 11, no. 1: 25. https://doi.org/10.3390/biomedicines11010025
APA StyleMiedziaszczyk, M., Lacka, K., Tomczak, O., Bajon, A., Primke, M., & Idasiak-Piechocka, I. (2023). Systematic Review of the Treatment of Persistent Hyperparathyroidism Following Kidney Transplantation. Biomedicines, 11(1), 25. https://doi.org/10.3390/biomedicines11010025








