High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions
Abstract
1. Introduction
2. The Kinetics of Vitamin C
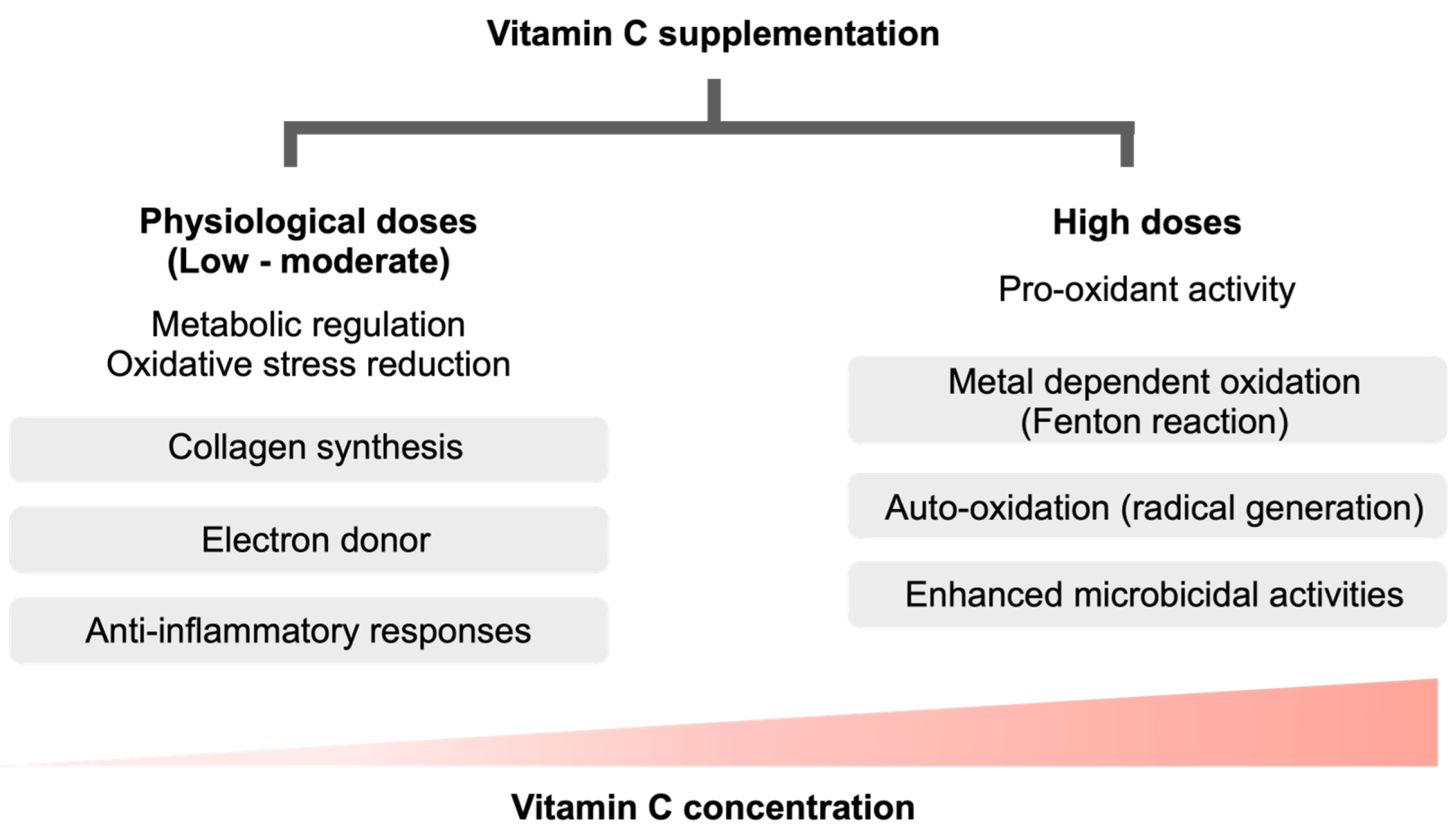
3. Pro-Oxidant Vitamin C Versus Warburg Effect of the Immune Cells in Sepsis
4. Vitamin C as a Pro-Oxidant against Microbial Agents
5. Neutrophils in Sepsis
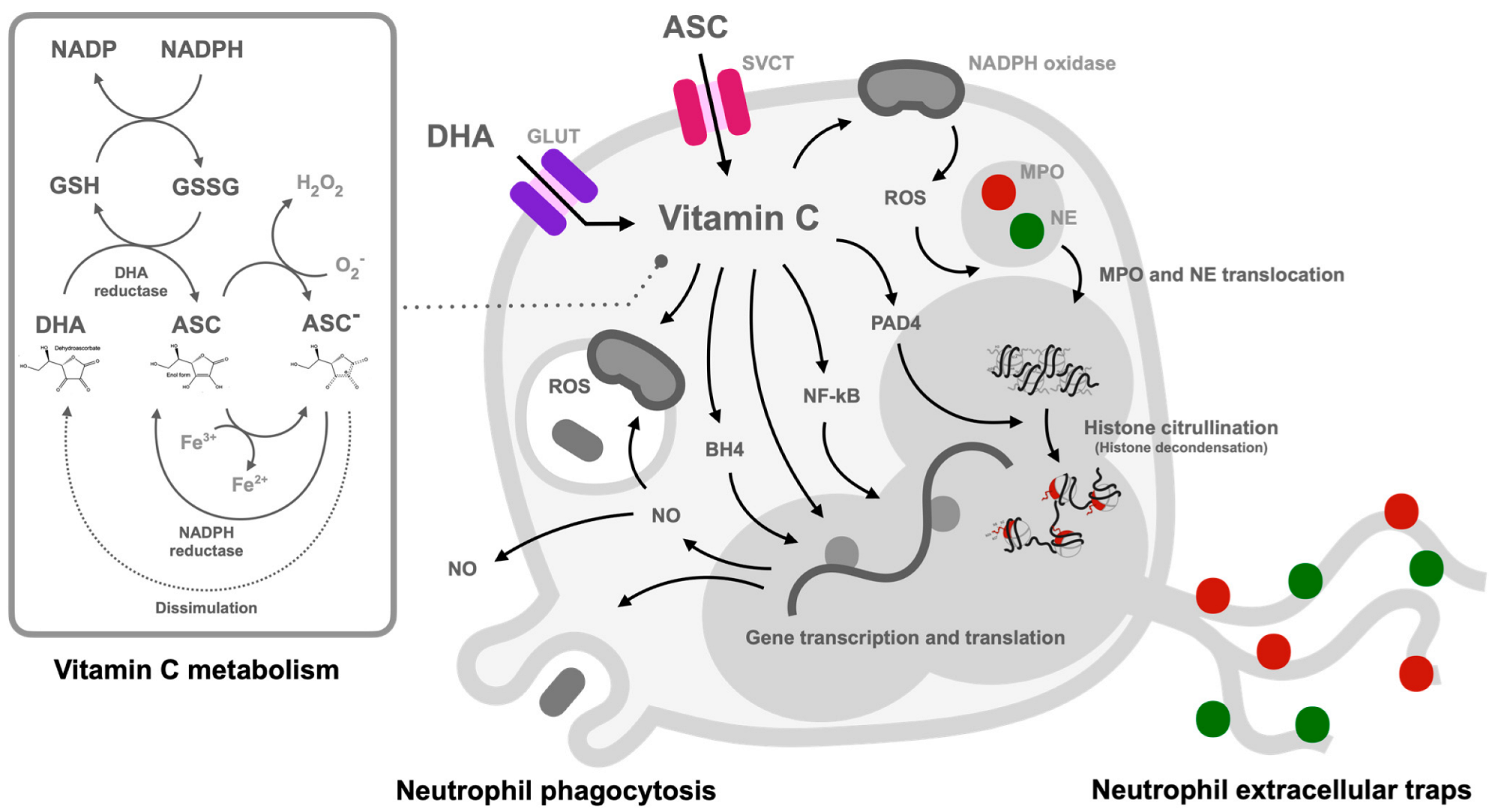
6. Vitamin C and Neutrophil Functions
7. Clinical Trials of Vitamin C Supplementation in Sepsis
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Sicignano, A.; Palazzo, M.; Moreno, R.; Boulme, R.; et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Standiford, T.J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012, 25, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C. The Role of Physiological Vitamin C Concentrations on Key Functions of Neutrophils Isolated from Healthy Individuals. Nutrients 2019, 11, 1363. [Google Scholar] [CrossRef]
- Bozonet, S.M.; Carr, A.C.; Pullar, J.M.; Vissers, M.C.M. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-rich SunGold kiwifruit. Nutrients 2015, 7, 2574–2588. [Google Scholar] [CrossRef]
- Michels, A.J.; Hagen, T.M.; Frei, B. Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function. Annu. Rev. Nutr. 2013, 33, 45–70. [Google Scholar] [CrossRef]
- Corpe, C.P.; Lee, J.H.; Kwon, O.; Eck, P.; Narayanan, J.; Kirk, K.L.; Levine, M. 6-Bromo-6-deoxy-L-ascorbic acid: An ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J. Biol. Chem. 2005, 280, 5211–5220. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.-R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Evolution and the need for ascorbic acid. Proc. Natl. Acad. Sci. USA 1970, 67, 1643–1648. [Google Scholar] [CrossRef]
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X. Regulation of vitamin C transport. Annu Rev. Nutr 2005, 25, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Hornung, T.C.; Biesalski, H.-K. Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis. Evol. Med. Public Health 2019, 2019, 221–231. [Google Scholar] [CrossRef]
- May, J.M. Is ascorbic acid an antioxidant for the plasma membrane? FASEB J. 1999, 13, 995–1006. [Google Scholar] [CrossRef]
- Chatterjee, I.B. Evolution and the biosynthesis of ascorbic acid. Science 1973, 182, 1271–1272. [Google Scholar] [CrossRef]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Washko, P.W.; Wang, Y.; Levine, M. Ascorbic acid recycling in human neutrophils. J. Biol. Chem. 1993, 268, 15531–15535. [Google Scholar] [CrossRef]
- Tu, H.; Li, H.; Wang, Y.; Niyyati, M.; Wang, Y.; Leshin, J.; Levine, M. Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid. eBioMedicine 2015, 2, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Schorah, C.J.; Downing, C.; Piripitsi, A.; Gallivan, L.; Al-Hazaa, A.H.; Sanderson, M.J.; Bodenham, A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996, 63, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Long, C.L.; Maull, K.I.; Krishnan, R.S.; Laws, H.L.; Geiger, J.W.; Borghesi, L.; Franks, W.; Lawson, T.C.; Sauberlich, H.E. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Anderson, R.; Oosthuizen, R.; Maritz, R.; Theron, A.; van Rensburg, A.J. The effects of increasing weekly doses of ascorbate on certain cellular and humoral immune functions in normal volunteers. Am. J. Clin. Nutr. 1980, 33, 71–76. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Cimmino, L.; Neel, B.G.; Aifantis, I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018, 28, 698–708. [Google Scholar] [CrossRef]
- Aguilera, O.; Muñoz-Sagastibelza, M.; Torrejón, B.; Borrero-Palacios, A.; del Puerto-Nevado, L.; Martínez-Useros, J.; Rodriguez-Remirez, M.; Zazo, S.; García, E.; Fraga, M.; et al. Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget 2016, 7, 47954. [Google Scholar] [CrossRef]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Hunyady, J. The Result of Vitamin C Treatment of Patients with Cancer: Conditions Influencing the Effectiveness. Int. J. Mol. Sci. 2022, 23, 4380. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Carrick, M.; Tanner, A., 2nd; Lieser, M.J.; Rael, L.T.; Brody, E. Overcoming the Warburg Effect: Is it the key to survival in sepsis? J. Crit. Care 2018, 43, 197–201. [Google Scholar] [CrossRef]
- Srivastava, A.; Mannam, P. Warburg revisited: Lessons for innate immunity and sepsis. Front. Physiol. 2015, 6, 70. [Google Scholar] [CrossRef]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef]
- Miliaraki, M.; Briassoulis, P.; Ilia, S.; Michalakakou, K.; Karakonstantakis, T.; Polonifi, A.; Bastaki, K.; Briassouli, E.; Vardas, K.; Pistiki, A.; et al. Oxidant/Antioxidant Status Is Impaired in Sepsis and Is Related to Anti-Apoptotic, Inflammatory, and Innate Immunity Alterations. Antioxidants 2022, 11, 231. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Somparn, P.; Bootprapan, T.; Tu, H.; Tangtanatakul, P.; Nuengjumnong, R.; Worasilchai, N.; Tiranathanagul, K.; Eiam-ong, S.; Levine, M.; et al. High-dose ascorbate with low-dose amphotericin B attenuates severity of disease in a model of the reappearance of candidemia during sepsis in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R223–R234. [Google Scholar] [CrossRef] [PubMed]
- Sripramong, C.; Visitsunthorn, K.; Srisuwatchari, W.; Pacharn, P.; Jirapongsananuruk, O.; Visitsunthorn, N. Food sensitization and food allergy in allergic Thai patients from a tertiary care center in Thailand. Asian Pac. J. Allergy Immunol. 2022, 40, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.K.; Dhingra, S.; Mittal, A.; Naresh, M.; Tyagi, J.S. Mycobacterium tuberculosis Transcriptional Adaptation, Growth Arrest and Dormancy Phenotype Development Is Triggered by Vitamin C. PLoS ONE 2010, 5, e10860. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Wu, K.; Li, Z.; Li, C.; Zeng, L.; Li, F.; Pei, N.; Liu, H.; Zhang, S.-l.; Song, Y.-z.; et al. Pharmacologic ascorbate as a pro-drug for hydrogen peroxide release to kill mycobacteria. Biomed. Pharmacother. 2019, 109, 2119–2127. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Oh, H.J.; Park, H.Y.; Kim, K.H.; Park, C.K.; Shin, H.J.; Lim, J.H.; Kwon, Y.S.; Oh, I.J.; Kim, Y.I.; Lim, S.C.; et al. Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer. J. Thorac. Dis. 2016, 8, 2530–2537. [Google Scholar] [CrossRef][Green Version]
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Zabet, M.H.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose Ascorbic acid on vasopressor’s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94–100. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; Hendrickx, I.; de Waele, E.; Spapen, H.D. Adjuvant vitamin C treatment in sepsis-how many oranges a day keep (vasopressor-dependent) septic shock away? J. Thorac. Dis. 2016, 8, E993–E995. [Google Scholar] [CrossRef]
- Wilson, J.X. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid. Redox Signal. 2013, 19, 2129–2140. [Google Scholar] [CrossRef]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit. Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Kim, J.-H.; Jeong, Y.-J.; Maeng, H.G.; Lee, Y.-T.; Kang, J.S.; Lee, W.J.; Hwang, Y.-I. Vitamin C acts indirectly to modulate isotype switching in mouse B cells. Anat. Cell Biol. 2010, 43, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Shriker, O.; Porath, A.; Riesenberg, K.; Schlaeffer, F. Vitamin C for the treatment of recurrent furunculosis in patients with imparied neutrophil functions. J. Infect. Dis. 1996, 173, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Hong, S.W.; Kim, J.H.; Jin, D.H.; Kang, J.S.; Lee, W.J.; Hwang, Y.I. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. 2011, 266, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Parkos, C.A. Neutrophil-Epithelial Interactions: A Double-Edged Sword. Am. J. Pathol. 2016, 186, 1404–1416. [Google Scholar] [CrossRef]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Kazzaz, N.M.; Sule, G.; Knight, J.S. Intercellular Interactions as Regulators of NETosis. Front. Immunol. 2016, 7, 453. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- McDonald, B.; Urrutia, R.; Yipp, B.G.; Jenne, C.N.; Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Paunel-Görgülü, A.; Flohé, S.; Hoffmann, A.; Witte, I.; MacKenzie, C.; Baldus, S.E.; Windolf, J.; Lögters, T.T. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit. Care 2012, 16, R137. [Google Scholar] [CrossRef] [PubMed]
- Czaikoski, P.G.; Mota, J.M.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142. [Google Scholar] [CrossRef]
- Delabranche, X.; Stiel, L.; Severac, F.; Galoisy, A.C.; Mauvieux, L.; Zobairi, F.; Lavigne, T.; Toti, F.; Anglès-Cano, E.; Meziani, F.; et al. Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock 2017, 47, 313–317. [Google Scholar] [CrossRef]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- Kambas, K.; Mitroulis, I.; Apostolidou, E.; Girod, A.; Chrysanthopoulou, A.; Pneumatikos, I.; Skendros, P.; Kourtzelis, I.; Koffa, M.; Kotsianidis, I.; et al. Autophagy Mediates the Delivery of Thrombogenic Tissue Factor to Neutrophil Extracellular Traps in Human Sepsis. PLoS ONE 2012, 7, e45427. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.J.; May, M.A.; Martin, K.J. The effect of vitamin E and vitamin C supplementation on LDL oxidizability and neutrophil respiratory burst in young smokers. J. Am. Coll. Nutr. 2000, 19, 361–369. [Google Scholar] [CrossRef]
- Davison, G.; Gleeson, M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 465–479. [Google Scholar] [CrossRef]
- Ferrón-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.M.; Bueno, P.; Ferrón, J.A. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J. Surg. Res. 2009, 153, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular Redox Homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef]
- Wang, Y.; Russo, T.A.; Kwon, O.; Chanock, S.; Rumsey, S.C.; Levine, M. Ascorbate recycling in human neutrophils: Induction by bacteria. Proc. Natl. Acad. Sci. USA 1997, 94, 13816–13819. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-F.; Cao, K.; Jiang, J.-P.; Guan, W.-X.; Du, J.-F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef]
- Zhu, C.-l.; Wang, Y.; Liu, Q.; Li, H.-r.; Yu, C.-m.; Li, P.; Deng, X.-m.; Wang, J.-f. Dysregulation of neutrophil death in sepsis. Front. Immunol. 2022, 13, 963955. [Google Scholar] [CrossRef]
- Chatterjee, M.; Saluja, R.; Kumar, V.; Jyoti, A.; Kumar Jain, G.; Kumar Barthwal, M.; Dikshit, M. Ascorbate sustains neutrophil NOS expression, catalysis, and oxidative burst. Free Radic. Biol. Med. 2008, 45, 1084–1093. [Google Scholar] [CrossRef]
- Sae-Khow, K.; Tachaboon, S.; Wright, H.L.; Edwards, S.W.; Srisawat, N.; Leelahavanichkul, A.; Chiewchengchol, D. Defective Neutrophil Function in Patients with Sepsis Is Mostly Restored by ex vivo Ascorbate Incubation. J. Inflamm. Res. 2020, 13, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Smit, M.J.; Joone, G.K.; van Staden, A.M. Vitamin C and cellular immune functions. Protection against hypochlorous acid-mediated inactivation of glyceraldehyde-3-phosphate dehydrogenase and ATP generation in human leukocytes as a possible mechanism of ascorbate-mediated immunostimulation. Ann. N. Y. Acad. Sci. 1990, 587, 34–48. [Google Scholar] [CrossRef]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Farkas, D.; Brophy, D.F.; Fowler, A.A., 3rd; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Raghavan, S.A.; Saini, R.; Dikshit, M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: Modulatory effect of nitric oxide. J. Leukoc. Biol. 2004, 75, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.B.; Tohme, S.; Yazdani, H.O.; Miller, D.; Huang, H.; Tsung, A. Superoxide induces Neutrophil Extracellular Trap Formation in a TLR-4 and NOX-dependent mechanism. Mol. Med. 2016, 22, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Inducible nitric oxide synthase: An asset to neutrophils. J. Leukoc. Biol. 2019, 105, 49–61. [Google Scholar] [CrossRef]
- Rojas, C.; Cadenas, S.; Herrero, A.; Méndez, J.; Barja, G. Endotoxin depletes ascorbate in the guinea pig heart. Protective effects of vitamins C and E against oxidative stress. Life Sci. 1996, 59, 649–657. [Google Scholar] [CrossRef]
- Cullen, J.J. Ascorbate induces autophagy in pancreatic cancer. Autophagy 2010, 6, 421–422. [Google Scholar] [CrossRef]
- Hong, S.W.; Jin, D.H.; Hahm, E.S.; Yim, S.H.; Lim, J.S.; Kim, K.I.; Yang, Y.; Lee, S.S.; Kang, J.S.; Lee, W.J.; et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol. Rep. 2007, 18, 811–815. [Google Scholar] [CrossRef]
- Sant, D.W.; Mustafi, S.; Gustafson, C.B.; Chen, J.; Slingerland, J.M.; Wang, G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci. Rep. 2018, 8, 5306. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis: Mechanisms of bacterial injury to the patient. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Davies, M.J.; Webster, N.R. Ascorbyl radical formation in patients with sepsis: Effect of ascorbate loading. Free Radic. Biol. Med. 1996, 20, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bharara, A.; Grossman, C.; Grinnan, D.; Syed, A.; Fisher, B.; DeWilde, C.; Natarajan, R.; Fowler, A.A. Intravenous Vitamin C Administered as Adjunctive Therapy for Recurrent Acute Respiratory Distress Syndrome. Case Rep. Crit. Care 2016, 2016, 8560871. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Iglesias, J.; Vassallo, A.V.; Patel, V.V.; Sullivan, J.B.; Cavanaugh, J.; Elbaga, Y. Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis: The ORANGES Trial. Chest 2020, 158, 164–173. [Google Scholar] [CrossRef]
- Chang, P.; Liao, Y.; Guan, J.; Guo, Y.; Zhao, M.; Hu, J.; Zhou, J.; Wang, H.; Cen, Z.; Tang, Y.; et al. Combined Treatment with Hydrocortisone, Vitamin C, and Thiamine for Sepsis and Septic Shock: A Randomized Controlled Trial. Chest 2020, 158, 174–182. [Google Scholar] [CrossRef]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients with Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Sevransky, J.E.; Rothman, R.E.; Hager, D.N.; Bernard, G.R.; Brown, S.M.; Buchman, T.G.; Busse, L.W.; Coopersmith, C.M.; DeWilde, C.; Ely, E.W.; et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients with Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021, 325, 742–750. [Google Scholar] [CrossRef]
- Lamontagne, F.; Masse, M.H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Cazzato, S.; Giovanella, S.; Ballestri, M.; Leonelli, M.; Mori, G.; Alfano, G.; Ligabue, G.; Magistroni, R.; Cenacchi, G.; et al. Oxalate Nephropathy Caused by Excessive Vitamin C Administration in 2 Patients with COVID-19. Kidney Int. Rep. 2020, 5, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Zeier, M.; Eckert, C. Acute Kidney Injury Following High-Dose Vitamin C Treatment. Dtsch. Arztebl. Int. 2019, 116, 756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sae-khow, K.; Charoensappakit, A.; Chiewchengchol, D.; Leelahavanichkul, A. High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions. Biomedicines 2023, 11, 51. https://doi.org/10.3390/biomedicines11010051
Sae-khow K, Charoensappakit A, Chiewchengchol D, Leelahavanichkul A. High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions. Biomedicines. 2023; 11(1):51. https://doi.org/10.3390/biomedicines11010051
Chicago/Turabian StyleSae-khow, Kritsanawan, Awirut Charoensappakit, Direkrit Chiewchengchol, and Asada Leelahavanichkul. 2023. "High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions" Biomedicines 11, no. 1: 51. https://doi.org/10.3390/biomedicines11010051
APA StyleSae-khow, K., Charoensappakit, A., Chiewchengchol, D., & Leelahavanichkul, A. (2023). High-Dose Intravenous Ascorbate in Sepsis, a Pro-Oxidant Enhanced Microbicidal Activity and the Effect on Neutrophil Functions. Biomedicines, 11(1), 51. https://doi.org/10.3390/biomedicines11010051





