The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedures
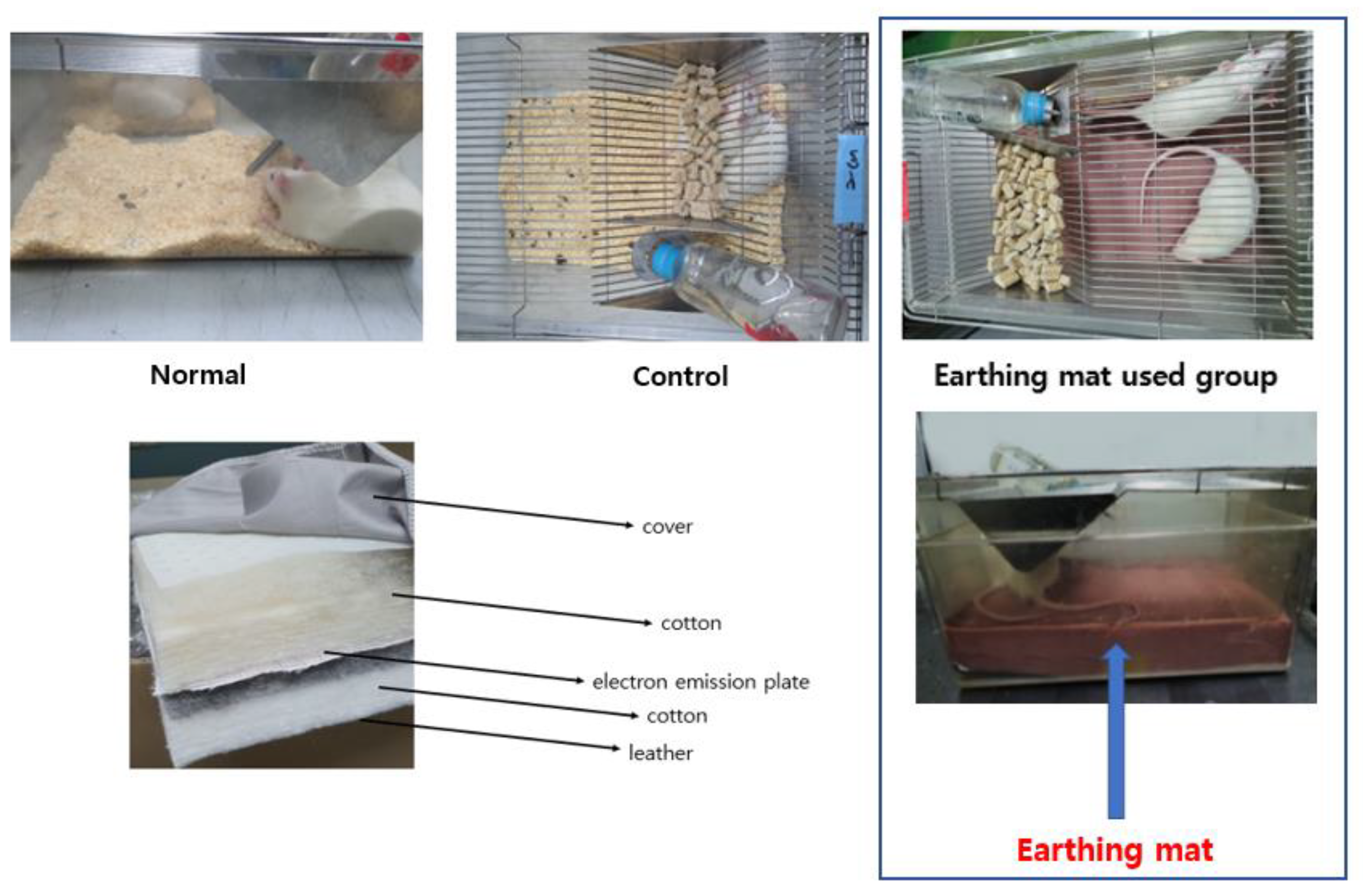
2.2. Earthing Mat
2.3. Tail Suspension Test (TST)
2.4. Forced Swimming Test (FST)
2.5. Elevated Plus Maze (EPM)
2.6. Immunohistochemistry of c-Fos and Corticotrophin Releasing Factor (CRF)
2.7. Statistical Analysis
3. Results
3.1. Forced Swimming Test (FST) and Tail Suspension Test (TST)
3.2. Elevated Plus Maze
3.3. Immunohistochemistry
3.3.1. Corticotrophin-Releasing Factor (CRF) Immunohistochemistry
3.3.2. c-Fos Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Ader, R.; Cohen, N. Psychoneuroimmunology: Conditioning and stress. Annu. Rev. Psychol. 1993, 44, 53–85. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P. Stressors, stress, and neuroendocrinology integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann. N. Y. Acad. Sci. 1998, 851, 311–335. [Google Scholar] [CrossRef] [PubMed]
- File, S.E. The use of social interaction as a method for detecting anxiolytic activity of chlorodiazepoxide-like drugs. J. Neurosci. Methods 1980, 2, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.; Villegas, M.; Martinez, C.; McEwen, B.S. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994, 639, 167–170. [Google Scholar] [CrossRef]
- Pare, W.P. The effects of chronic environmental stress and stomach ulceration, adrenal function and consummatory behavior in the rat. J. Psychol. 1964, 57, 143–151. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Park, S.Y.; Shim, I.S. Antidepressant effect and neural mechanism of Acer tegmentosum in repeated stress–induced ovariectomized female rats. Anim. Cells Syst. 2020, 24, 205–2013. [Google Scholar] [CrossRef]
- Chevalier, G.; Sinatra, S.T.; Oschman, J.L.; Sokal, K.; Sokal, P. Earthing: Health implications of reconnecting the human body to the Earth’s surface electrons. J. Environ. Public Health 2012, 2012, 291541. [Google Scholar] [CrossRef] [Green Version]
- Oschman, J.L. Perspective: Assume a spherical cow: The role of free or mobile electrons in bodywork, energetic and movement therapies. J. Bodyw. Mov. Ther. 2008, 12, 40–57. [Google Scholar] [CrossRef]
- Chevalier, G.; Patel, S.; Weiss, L.; Chopra, D.; Mills, P.J. The Effects of Grounding (Earthing) on Bodyworkers’ Pain and Overall Quality of Life: A Randomized Controlled Trial. Explore 2019, 15, 181–190. [Google Scholar] [CrossRef]
- Chevalier, G.; Sinatra, S.T.; Oschman, J.L.; Delany, R.M. Earthing (grounding) the human body reduces blood viscosity-a major factor in cardiovascular disease. J. Altern. Complement Med. 2013, 19, 102–110. [Google Scholar] [CrossRef]
- Bulmus, O.; Ercan, Z.; Kacar, E.; Serhatlioglu, I.; Yasar, A.; Kelestimur, H. Treadmill exercise training improves the high-fat diet-induced behavioral changes in the male rats. Biol. Futur. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, L.; Lu, S.; Li, C.; Bai, M.; Xu, E.; Shen, J.; Li, Y. Baicalin ameliorates CUMS-induced depression-like behaviors through activating AMPK/PGC-1α pathway and enhancing NIX-mediated mitophagy in mice. Eur. J. Pharmacol. 2022, 938, 175435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Chen, Z.Y. Electroacupuncture alleviates depression-like behaviors via a neural mechanism involving activation of Nucleus Accumbens Shell. World J. Biol. Psychiatry 2022, 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, M.; Teplitz, D. The biologic effects of grounding the human body during sleep as measured by cortisol levels and subjective reporting of sleep, pain, and stress. J. Altern. Complement Med. 2004, 10, 767–776. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Kim, J.Y.; Kim, J.Y.; Park, S.K.; Shim, I. Ginseng Purified Dry Extract, BST204, Improved Cancer Chemotherapy-Related Fatigue and Toxicity in Mice. eCAM 2015, 2015, 197459. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Shim, H.S.; Shim, I. The Differential Role of Cytokines on Stress Responses in a Menopause Rat Model. Front. Psychiatry 2020, 11, 577561. [Google Scholar] [CrossRef]
- Bailly, J.; Allain, F.; Schwartz, E.; Tirel, C.; Dupuy, C.; Petit, F.; Diana, M.A.; Darcq, E.; Kieffer, B.L. Habenular Neurons Expressing Mu Opioid Receptors Promote Negative Affect in a Projection-Specific Manner. Biol. Psychiatry 2022. preprint. [Google Scholar] [CrossRef]
- Banaei-Boroujeni, G.; Rezayof, A.; Alijanpour, S.; Nazari-Serenjeh, F. Targeting mediodorsal thalamic CB1 receptors to inhibit dextromethorphan-induced anxiety/exploratory-related behaviors in rats: The post-weaning effect of exercise and enriched environment on adulthood anxiety. J. Psychiatry Res. 2022, 157, 212–222. [Google Scholar] [CrossRef]
- Choudhary, D.; Sasibhushana, R.B.; Shankaranarayana Rao, B.S.; Srikumar, B.N. Mifepristone blocks the anxiolytic- and antidepressant-like effects of allopregnanolone in male rats. J. Psychol. Neurosci. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Kuo, J.Y.; Denman, A.J.; Beacher, N.J.; Glanzberg, J.T.; Zhang, Y.; Li, Y.; Lin, D.T. Using deep learning to study emotional behavior in rodent models. Front. Behav. Neurosci. 2022, 16, 1044492. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Chan, L.W.C.; Li, X.; Hu, R.; Yang, S. A high fat diet in glutamate 3-/Y mice causes changes in behavior that resemble human intellectual disability. Physiol. Behav. 2022, 259, 114050. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Xie, Q.; Li, Y.; Wang, Q. Neurobehavioral effects of cinnabar and the cinnabar-containing pediatric prescription, Yi-Nian-Jin, in juvenile rats. J. Trace Elem. Med. Biol. 2022, 76, 127112. [Google Scholar] [CrossRef]
- Buckingham, J.C. Corticotrophin releasing factor. Pharmacol. Rev. 1979, 31, 253–275. [Google Scholar] [PubMed]
- Amado, P.; Zegers, J.; Yarur, H.E.; Gysling, K. Transcriptional Regulation, Signaling Pathways, and Subcellular Localization of Corticotropin-Releasing Factor Receptors in the Central Nervous System. Mol. Pharmacol. 2022, 102, 280–287. [Google Scholar] [CrossRef]
- Buban, K.N.; Saperstein, S.E.; Oyola, M.G.; Rothwell, S.W.; John Wu, T. Alterations in the activation of corticotropin-releasing factor neurons in the paraventricular nucleus following a single or multiple days of sleep restriction. Neurosci. Lett. 2023, 792, 136940. [Google Scholar] [CrossRef]
- Vetrovoy, O.; Stratilov, V.; Lomert, E.; Tyulkova, E. Prenatal Hypoxia-Induced Adverse Reaction to Mild Stress is Associated with Depressive-Like Changes in the Glucocorticoid System of Rats. Neurochem. Res. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Wu, X.; Gu, J.; Zou, Z.; Yu, M.; Zhang, C.; Xiao, Q.; Chen, X.; Li, C. Suppressive Effects of Isofraxidin on Depressive-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sci. 2022, 12, 1376. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Shi, B.; Huang, F.; Gao, Y.; Miao, Z.; Ma, K.; Zhan, Z.; Zou, W.; Liu, M. Comparison of the chronic unpredictable mild stress and the maternal separation in mice postpartum depression modeling. Biochem. Biophys. Res. Commun. 2022, 632, 24–31. [Google Scholar] [CrossRef]
- Fiedler, D.; Pape, H.C.; Lange, M.D. Stress-induced impairment of fear extinction recall is associated with changes in neuronal activity patterns in PVT. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110338. [Google Scholar] [CrossRef]
- Kang, J.W.M.; Mor, D.; Keay, K.A. Nerve injury alters restraint-induced activation of the basolateral amygdala in male rats. Brain Struct. Funct. 2021, 226, 1209–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Chung, C. Brain-wide cellular mapping of acute stress-induced activation in male and female mice. FASEB 2021, 35, e22041. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, J.Y.; Kwon, H.J.; Han, P.L. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat. Commun. 2021, 12, 6682. [Google Scholar] [CrossRef] [PubMed]
- McNamara, E.H.; Tucker, L.B.; Liu, J.; Fu, A.H.; Kim, Y.; Vu, P.A.; McCabe, J.T. Limbic Responses Following Shock Wave Exposure in Male and Female Mice. Front. Behav. Neurosci. 2022, 16, 863195. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, K.; Gao, J.; Chen, S.; Zeng, S.; Zhao, Y. FG7142 combined with restraint stress induces anxiogenic-like effects via downregulation gamma-aminobutyric acid type A receptor subunit alpha1 and 5-hydroxytryptamine 1A receptors expression in the hippocampus. Neuroreport 2022, 33, 145–152. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Lee, S.; Hahm, D.H.; Lee, H.; Oh, C.T.; Han, H.J.; Ji, H.J.; Shim, I. Anti-stress effects of human placenta extract: Possible involvement of the oxidative stress system in rats. BMC Cam. 2018, 18, 149. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C.; Pennisi, M.; Topple, A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods 1985, 13, 139–143. [Google Scholar] [CrossRef]
- Savignac, H.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. The effects of repeated social interaction stress on behavioural and physiological parameters in a stress-sensitive mouse strain. Behav. Brain Res. 2011, 216, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Padovan, C.M.; Guimarães, F.S. Restraint-induced hypoactivity in an elevated plus-maze. Braz. J. Med. Biol. Res. 2000, 33, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Hamada, M.; Nishigawa, T.; Maesono, S.; Aso, K.; Ikeda, H.; Furuse, M. Decreased stress-induced depression-like behavior in lactating rats is associated with changes in the hypothalamic-pituitary-adrenal axis, brain monoamines, and brain amino acid metabolism. Stress 2019, 22, 482–491. [Google Scholar] [CrossRef]
- Mou, Z.; Huang, Q.; Chu, S.F.; Zhang, M.J.; Hu, J.F.; Chen, N.H.; Zhang, J.T. Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis. Biomed. Pharmacother. 2017, 92, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Kennett, G.A.; Dourish, C.T.; Curzon, G. Antidepressant-like action of 5-HT1A agonists and conventional antidepressants in an animal model of depression. Eur. J. Pharm. 1987, 134, 265–274. [Google Scholar] [CrossRef] [PubMed]
- McBlane, J.W.; Handley, S.L. Effects of two stressors on behaviour in the elevated X-maze: Preliminary investigation of their interaction with 8-OH-DPAT. Psychopharm 1994, 116, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, G. The effect of grounding the human body on mood. Psychol. Rep. 2015, 116, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oschman, J.L. Can electrons act as antioxidants? A review and commentary. J. Altern. Complement Med. 2007, 13, 955–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinatra, S.T.; Oschman, J.L.; Chevalier, G.; Sinatra, D. Electric Nutrition: The Surprising Health and Healing Benefits of Biological Grounding (Earthing). Altern. Ther. Health Med. 2017, 23, 8–16. [Google Scholar] [PubMed]
- Sokal, K.; Sokal, P. Earthing the human organism influences bioelectrical processes. J. Altern. Complement Med. 2012, 18, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokal, P.; Sokal, K. The neuromodulative role of earthing. Med. Hypotheses 2011, 77, 824–826. [Google Scholar] [CrossRef]
- Oschman, J.L.; Chevalier, G.; Brown, R. The effects of grounding (earthing) on inflammation, the immune response, wound healing, and prevention and treatment of chronic inflammatory and autoimmune diseases. J. Inflamm. Res. 2015, 8, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Angeles-Castellanos, M.; Aguilar-Roblero, R.; Escobar, C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R158–R165. [Google Scholar] [CrossRef] [Green Version]
- Senba, E.; Matsunaga, K.; Tohyama, M.; Noguchi, K. Stress-induced c-fos expression in the rat brain: Activation mechanism of sympathetic pathway. Brain Res. Bull. 1993, 31, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.D.; Gollock, M.J.; Gilmour, K.M. Social stress modulates the cortisol response to an acute stressor in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2014, 196, 8–16. [Google Scholar] [CrossRef]
- Arikawe, A.P.; Rorato, R.C.; Gomes, N.; Elias, L.L.; Anselmo-Franci, J. Hormonal and neural responses to restraint stress in an animal model of perimenopause in female rats. J. Neuroendocrinol. 2021, 33, e12976. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.P.; de Andrade, J.S.; Spina, M.; Chamon, J.V.; Silva, P.H.D.; Werder, A.K.; Ortolani, D.; Thomaz, L.D.S.C.; Romariz, S.; Ribeiro, D.A.; et al. Clozapine prevented social interaction deficits and reduced c-Fos immunoreactivity expression in several brain areas of rats exposed to acute restraint stress. PLoS ONE 2022, 17, e0262728. [Google Scholar] [CrossRef] [PubMed]
- Fóscolo, D.R.C.; Lima, P.M.A.; Rodovalho, G.V.; Coimbra, C.C. Early maternal separation alters the activation of stress-responsive brain areas in adulthood. Neurosci. Lett. 2022, 771, 136464. [Google Scholar] [CrossRef]
- Koureta, M.; Karaglani, M.; Panagopoulou, M.; Balgkouranidou, I.; Papadaki-Anastasopoulou, A.; Zarouchlioti, C.; Dekavallas, S.; Kolios, G.; Lambropoulou, M.; Baritaki, S.; et al. Corticotropin Releasing Factor Receptors in breast cancer: Expression and activity in hormone-dependent growth in vitro. Peptides 2020, 129, 170316. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, X.; Zhu, S. Effects of Shugan Hewei Granule on Depressive Behavior and Protein Expression Related to Visceral Sensitivity in a Rat Model of Nonerosive Reflux Disease. Evid. Based Complement Alternat. Med. 2019, 2019, 1505693. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.Y.; Liu, Y.; Wu, M.C.; Wang, H.W.; Chu, C.P.; Jin, H.; Li, Y.Z.; Qiu, D.L. Corticotrophin-Releasing Factor Modulates the Facial Stimulation-Evoked Molecular Layer Interneuron-Purkinje Cell Synaptic Transmission in vivo in Mice. Front. Cell Neurosci. 2020, 14, 563428. [Google Scholar] [CrossRef]
- Chevalier, G.; Sinatra, S.T.; Oschman, J.L.; Sokal, K.; Sokal, P. Earthing (Grounding) the Human Body Reduces Blood Viscosity—A Major Factor in Cardiovascular Disease. J. Environ. Public Health 2012. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.; Heckman, S. The local diurnal variation of cloud electrification and the global diurnal variation of negative charge on the Earth. J Geophys Res 1993, 98, 5221–5234. [Google Scholar] [CrossRef]
- Oschman, J.L. Charge transfer in the living matrix. J. Body Mov. Ther. 2009, 13, 215–228. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Jeong, W.; Yu, H.J.; Ye, M.; Hong, Y.; Kim, M.; Kim, J.Y.; Shim, I. The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat. Biomedicines 2023, 11, 57. https://doi.org/10.3390/biomedicines11010057
Park H-J, Jeong W, Yu HJ, Ye M, Hong Y, Kim M, Kim JY, Shim I. The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat. Biomedicines. 2023; 11(1):57. https://doi.org/10.3390/biomedicines11010057
Chicago/Turabian StylePark, Hyun-Jung, Woojin Jeong, Hyo Jeong Yu, Minsook Ye, Yunki Hong, Minji Kim, Ji Youn Kim, and Insop Shim. 2023. "The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat" Biomedicines 11, no. 1: 57. https://doi.org/10.3390/biomedicines11010057
APA StylePark, H.-J., Jeong, W., Yu, H. J., Ye, M., Hong, Y., Kim, M., Kim, J. Y., & Shim, I. (2023). The Effect of Earthing Mat on Stress-Induced Anxiety-like Behavior and Neuroendocrine Changes in the Rat. Biomedicines, 11(1), 57. https://doi.org/10.3390/biomedicines11010057







