FT3 to FT4 Conversion Ratio May Be an Independent Prognostic Factor in Pancreatic Cancer Patients
Abstract
:1. Introduction
2. Material and Methods
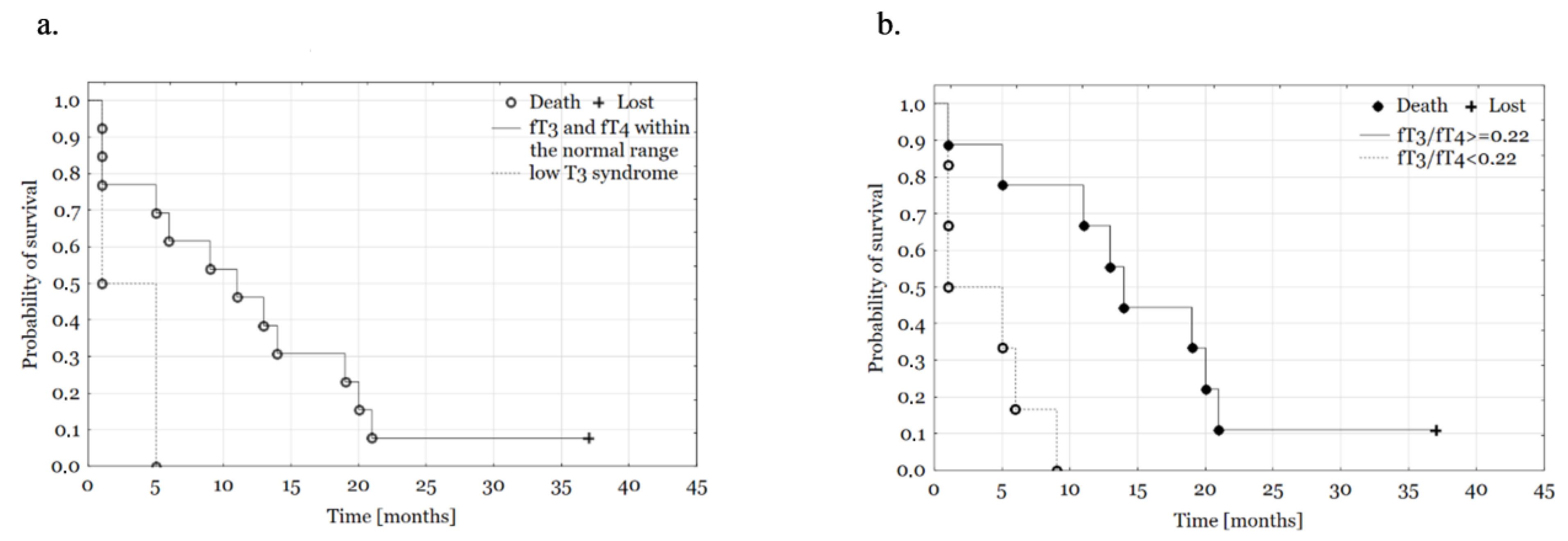
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Köhrle, J. Thyroid Hormones and Derivatives: Endogenous Thyroid Hormones and Their Targets. Methods Mol. Biol. 2018, 1801, 85–104. [Google Scholar] [PubMed]
- Aranda, A.; Alonso-Merino, E.; Zambrano, A. Receptors of thyroid hormones. Pediatr. Endocrinol. Rev. 2013, 11, 2–13. [Google Scholar] [PubMed]
- Nappi, A.; De Stefano, M.A.; Dentice, M.; Salvatore, D. Deiodinases and Cancer. Endocrinology 2021, 162, bqab016. [Google Scholar] [CrossRef] [PubMed]
- Pappa, T.A.; Vagenakis, A.G.; Alevizaki, M. The nonthyroidal illness syndrome in the non–critically ill patient. Eur. J. Clin. Investig. 2011, 41, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Führer, D. Thyroid hormone, thyroid hormone receptors, and cancer: A clinical perspectiveness. Endocr. Relat. Cancer 2013, 20, R19–R29. [Google Scholar] [CrossRef] [Green Version]
- Ness, R.B.; Grisso, J.A.; Cottreau, C.; Klapper, J.; Vergona, R.; Wheeler, J.E.; Morgan, M.; Schlesselman, J.J. Factors related to inflammation of the ovarian epithelium and risk of ovarian cancer. Epidemiology 2000, 11, 111–117. [Google Scholar] [CrossRef]
- Lehrer, S.; Diamond, E.J.; Bajwa, A.M.; Kornreich, R.; Stagger, S.; Stone, N.N.; Stock, R.G. Association between serum triiodothyronine (T3) level and risk of disease recurrence in men with localized prostate cancer. Prostate Cancer Prostatic Dis. 2001, 4, 232–234. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, S.; Diamond, E.J.; Stone, N.N.; Droller, M.J.; Stock, R.G. Serum triiodothyronine is increased in men with prostate cancer and benign prostatic hyperplasia. J. Urol. 2002, 168, 2431–2433. [Google Scholar] [CrossRef]
- Ristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Cancer 2005, 103, 1122–1128. [Google Scholar] [CrossRef]
- Puhr, H.C.; Wolf, P.; Berghoff, A.S.; Schoppmann, S.F.; Preusser, M.; Ilhan-Mutlu, A. Elevated Free Thyroxine Levels Are Associated with Poorer Overall Survival in Patients with Gastroesophageal Cancer: A Retrospective Single Center Analysis. HORM CANC 2020, 11, 42–51. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Ambrosio, R.; Dentice, M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol. Cell Endocrinol. 2017, 459, 84. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Tu, H.M.; Harney, J.W.; Venihaki, M.; Butte, A.J.; Kozakewich, H.P.; Larsen, P.R. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N. Engl. J. Med. 2000, 343, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Antonini, D.; Salvatore, D. Type 3 deiodinase and solid tumors: An intriguing pair. Expert Opin. Ther. Targets. 2013, 17, 1369–1379. [Google Scholar] [CrossRef]
- Wu, C.C.; Islam, M.M.; Nguyen, P.A.; Poly, T.N.; Wang, C.H.; Iqbal, U.; Li, Y.J.; Yang, H.C. Risk of cancer in long-term levothyroxine users: Retrospective population-based study. Cancer Sci. 2021, 112, 2533–2541. [Google Scholar] [CrossRef]
- Hercbergs, A.A.; Goyal, L.K.; Suh, J.H.; Lee, S.; Reddy, C.A.; Cohen, B.H.; Stevens, G.H.; Reddy, S.K.; Peereboom, D.M.; Elson, P.J.; et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003, 23, 617–626. [Google Scholar] [PubMed]
- Nelson, M.; Hercbergs, A.; Rybicki, L.; Strome, M. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1041–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Docter, R.; Krenning, E.P.; de Jong, M.; Hennemann, G. The sick euthyroid syndrome: Changes in thyroid hormone serum parameters and hormone metabolism. Clin. Endocrinol. 1993, 39, 499–518. [Google Scholar] [CrossRef]
- Gangemi, E.N.; Garino, F.; Berchialla, P.; Martinese, M.; Arecco, F.; Orlandi, F.; Stella, M. Low triiodothyronine serum levels as a predictor of poor prognosis in burn patients. Burns 2008, 34, 817–824. [Google Scholar] [CrossRef]
- Du, J.B.; Da, C.H.; Zhao, Y.; Guo, Y.; Guo, G.; Ju, T.F.; Xu, Y.P. The role of brain natriuretic peptide and serum triiodothyronine in the diagnosis and prognosis of chronic heart failure. Acta Cardiol. 2012, 67, 291–296. [Google Scholar] [CrossRef]
- Agiasotelli, D.; Alexopoulou, A.; Vasilieva, L.; Dourakis, S.P. Low free T3 levels are related to early mortality in patients with decompensated cirrhosis and acute-on chronic liver failure. J. Hepatol. 2014, 61, 1446–1447. [Google Scholar] [CrossRef]
- Gao, R.; Chen, R.Z.; Xia, Y.; Liang, J.H.; Wang, L.; Zhu, H.Y.; Zhu, W.J.; Fan, L.; Li, J.Y.; Yang, T.; et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int. J. Cancer 2018, 143, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Q.; Jian, Y.; Zhang, Y.; Zhang, W.; Chen, Z.; Yang, Y.; Liu, A.; Wang, G. The Association Between Low T3 Syndrome and Survival in Patients With Newly Diagnosed Multiple Myeloma: A Retrospective Study. Technol. Cancer Res. Treat. 2022, 21, 15330338221094422. [Google Scholar] [CrossRef] [PubMed]
- Yasar, Z.A.; Kirakli, C.; Yilmaz, U.; Ucar, Z.Z.; Talay, F. Can non-thyroid illness syndrome predict mortality in lung cancer patients? A prospective cohort study. Horm. Cancer 2014, 5, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Deltuva, V.; Tamasauskas, S.; Tamasauskas, A.; Laws, E.R.; Bunevicius, R. Low triiodothyronine syndrome as a predictor of poor outcomes in patients undergoing brain tumor surgery: A pilot study: Clinical article. J. Neurosurg. 2013, 118, 1279–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huang, Y.; Mo, G.; Zhou, T.; Hou, Q.; Shi, C.; Yu, J.; Lv, Y. Combined prognostic value of preoperative serum thyrotrophin and thyroid hormone concentration in papillary thyroid cancer. J. Clin. Lab. Anal. 2022, 6, e24503. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Z.; Shen, Y.; Xia, S.F. The Roles of Thyroid and Thyroid Hormone in Pancreas: Physiology and Pathology. Int. J. Endocrinol. 2018, 14, 2861034. [Google Scholar] [CrossRef] [PubMed]
- Strzałka, A.; Hogendorf, P.; Skulimowski, A.; Spychalski, M.; Strzelczyk, J.; Durczyński, A. Thyroid hormones concentration in portal and peripheral blood in patients with pancreatic cancer: Preliminary study. Cancer Biomark. 2020, 29, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Durczynski, A.; Skulimowski, A.; Hogendorf, P.; Szymanski, D.; Kumor, A.; Marski, K.; Ovenerg Juliebo, S.; Poznańska, G.; Strzelczyk, J. The concentration of D-dimers in portal blood positively correlates with overall survival in patients with non-resectable pancreatic cancer. World J. Surg. Oncol. 2017, 15, 223. [Google Scholar] [CrossRef] [Green Version]
- Durczynski, A.; Strzelczyk, J. Portal, but not peripheral, blood D-dimer level may help to differentiate malignant from benign pancreatic tumors. Blood Coagul. Fibrinolysis 2015, 26, 115–116. [Google Scholar] [CrossRef]
- Durczynski, A.; Kumor, A.; Hogendorf, P.; Szymanski, D.; Grzelak, P.; Strzelczyk, J. Preoperative high level of D-dimers predicts unresectability of pancreatic head cancer. World J. Gastroenterol. 2014, 20, 13167–13171. [Google Scholar] [CrossRef]
- Tibaldi, J.M.; Surks, M.I. Animal models of nonthyroidal disease. Endocr. Rev. 1985, 6, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Kauffmann, E.; Cacace, C.; Menonna, F.; Caramella, D.; Cappelli, C.; Boggi, U. Factors predicting survival in patients with locally advanced pancreatic cancer undergoing pancreatectomy with arterial resection. Updates Surg. 2021, 73, 233–249. [Google Scholar] [CrossRef] [PubMed]


| Thyroid Hormones Concentrations | ||
|---|---|---|
| Median (IQR) | Min.–Max. | |
| Age | 60 (54–67) | 52–73 |
| TSH | 1.41 (0.51–2.14) | 0.08–4.73 |
| fT3 [pg/dL] | 2.68 (2.11–3.11) | 1.32–3.37 |
| fT4 [pg/dL] | 11.1 (10.1–13.0) | 8.90–16.30 |
| fT3/fT4 ratio | 0.23 (0.20–0.27) | 0.08–0.29 |
| Clinical features; n (%) | ||
| Sex, n (%) | ||
| Women | 8 (53.3) | |
| Men | 7 (46.7) | |
| Grade | ||
| 1 | 2 (13.3) | |
| 2 | 11 (73.3) | |
| Not known | 2 (13.3) | |
| Stage | ||
| IA | 1 (6.7) | |
| IIA | 4 (26.7) | |
| IIB | 6 (40.0) | |
| III | 4 (26.7) | |
| Type of surgery, n (%) | ||
| Whipple’s procedure | 12 (80) | |
| Distal pancreatectomy | 3 (20) | |
| Survival, n (%) | ||
| <6 months | 5 (33.3) | |
| 6–12 months | 4 (26.7) | |
| >12 months | 6 (40.0) | |
| Sick euthyroid syndrome, n (%) | ||
| Low T3 syndrome | 2 (13.3) | |
| fT3 and fT4 within the normal range | 13 (86.7) | |
| TSH | fT3 | fT4 | fT3/fT4 Ratio | ||
|---|---|---|---|---|---|
| Sex, (n, %) | |||||
| Women | 8 (53) | 1.79 (0.56–3.07) | 2.78 (2.44–2.97) | 11.05 (10.15–12.35) | 0.25 (0.21–0.26) |
| Men | 7 (47) | 1.86 (0.51–2.11) | 2.16 (1.45–3.23) | 11.10 (9.6–14.6) | 0.21 (0.14–0.28) |
| Stage (n, %) | |||||
| I–II | 9 (50) | 1.41 (0.51–2.14) | 2.77 (2.16–3.11) | 11.10 (10.20–13.00) | 0.24 (0.20–0.27) |
| III | 9 (50) | 1.33 (0.32–3.04) | 1.82 (1.39–2.76) | 10.90 (9.40–14.10) | 0.19 (0.12–0.25) |
| Grade, n (%) | |||||
| 1 | 2 (16) | 1.51 (0.87–2.14) | 2.97 (2.83–3.11) | 12.85 (11.70–14.00) | 0.23 (0.20–0.27) |
| 2 | 11 (84) | 1.86 (0.51–3.97) | 2.68 (2.16–3.23) | 10.90 (9.90–11.90) | 0.24 (0.21–0.27) |
| Type of surgery, n (%) | |||||
| Whipple’s procedure | 12 (67) | 1.38 (0.52–3.06) | 2.47 (2.09–2.81) | 11.00 (10.15–12.85) | 0.22 (0.18–0.26) |
| Distal pancreatectomy | 2 (11) | 1.41 (0.08–2.11) | 3.32 (2.19–3.37) | 11.90 (9.90–13.00) | 0.26 (0.22–0.28) |
| OS (n, %) | * | ||||
| <6 months | 5 (33) | 1.41 (0.55–2.11) | 2.11 (1.45–2.25) | 13.00 (10.60–14.60) | 0.16 (0.14–0.21) |
| 6–12 months | 4 (27) | 1.38 (0.88–2.93) | 2.76 (2.38–3.03) | 11.15 (10.60–12.60) | 0.22 (0.20–0.27) |
| >12 months | 6 (40) | 1.31 (0.25–2.14) | 2.78 (2.19–3.11) | 10.55 (9.90–11.70) | 0.26 (0.23–0.27) |
| Sick euthyroid syndrome (n,%) | ** | *** | |||
| Low T3 syndrome | 2 (13) | 2.26 (0.55–3.97) | 1.39 (1.32–1.45) | 12.60 (8.90–16.30) | 0.12 (0.08–0.16) |
| fT3 and fT4 within the normal range | 13 (87) | 1.41 (0.51–2.11) | 2.77 (2.19–3.11) | 11.10 (10.20–11.90) | 0.24 (0.21–0.27) |
| Univariate | ||
| HR | p | |
| Age | 0.99 (0.92–1.06) | 0.768 |
| CEA | 0.92 (0.78–1.09) | 0.34 |
| CA-125 | 1.01 (0.97–1.06) | 0.622 |
| CA-19.9 | 1.00 (1.00–1.00) | 0.605 |
| CA-15.3 | 1.02 (0.97–1.09) | 0.34 |
| Sex (male) | 1.65 (0.57–4.78) | 0.36 |
| Type of procedure (Whipple’s procedure) | 0.87 (0.23–3.24) | 0.837 |
| Stage (I–II) | ||
| fT3/fT4 ≥0.22 | 0.71 (0.21–2.37) | 0.579 |
| 0.13 (0.03–0.70) | 0.017 * | |
| Multivariate | ||
| Model | ||
| Age | ||
| CEA | 0.93 (0.78–1.10) | 0.37 |
| CA-125 | 0.77 (0.57–1.06) | 0.105 |
| CA-19.9 | 1.00 (0.90–1.10) | 0.923 |
| CA-15.3 | 1.00 (1.00–1.00) | 0.481 |
| 1.18 (1.02–1.36) | 0.025 * | |
| Sex (male) | ||
| Type of procedure (Whipple’s procedure) | 3.96 (0.75–20.78) | 0.104 |
| Stage (I–II) | 0.01 (0.00–0.48) | 0.023 * |
| fT3/fT4 ≥ 0.22 | ||
| 4.06 (0.31–53.72) | 0.287 | |
| 0.01 (0.00–0.25) | 0.004 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majos, A.; Sewerynek, E.; Grząsiak, O.; Ciesielski, W.; Hogendorf, P.; Hołyński, J.; Strzelczyk, J.; Durczyński, A. FT3 to FT4 Conversion Ratio May Be an Independent Prognostic Factor in Pancreatic Cancer Patients. Biomedicines 2023, 11, 77. https://doi.org/10.3390/biomedicines11010077
Majos A, Sewerynek E, Grząsiak O, Ciesielski W, Hogendorf P, Hołyński J, Strzelczyk J, Durczyński A. FT3 to FT4 Conversion Ratio May Be an Independent Prognostic Factor in Pancreatic Cancer Patients. Biomedicines. 2023; 11(1):77. https://doi.org/10.3390/biomedicines11010077
Chicago/Turabian StyleMajos, Alicja, Ewa Sewerynek, Oliwia Grząsiak, Wojciech Ciesielski, Piotr Hogendorf, Jarosław Hołyński, Janusz Strzelczyk, and Adam Durczyński. 2023. "FT3 to FT4 Conversion Ratio May Be an Independent Prognostic Factor in Pancreatic Cancer Patients" Biomedicines 11, no. 1: 77. https://doi.org/10.3390/biomedicines11010077