COVID-19 Related Myocarditis and Myositis in a Patient with Undiagnosed Antisynthetase Syndrome
Abstract
:1. Introduction
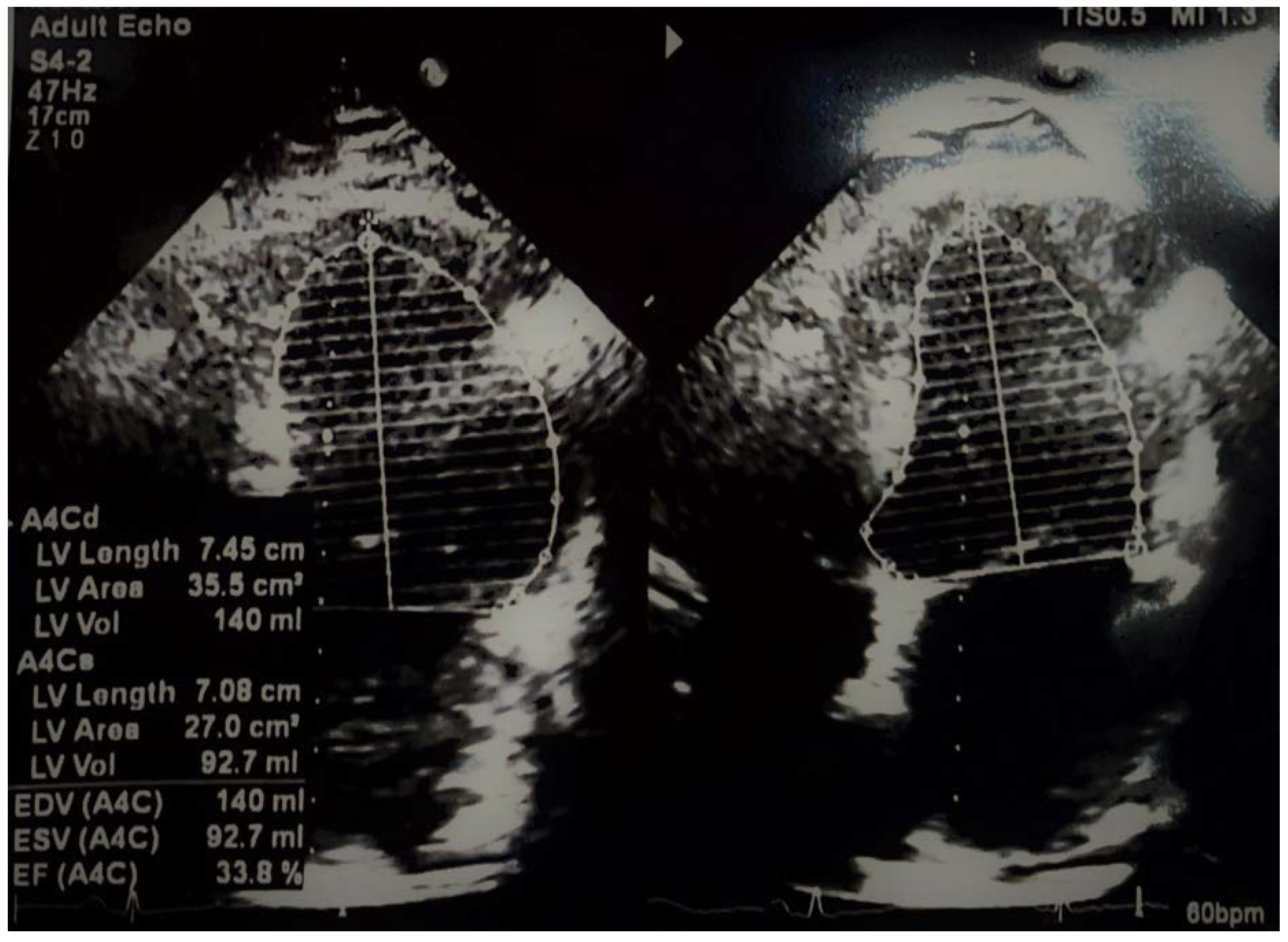
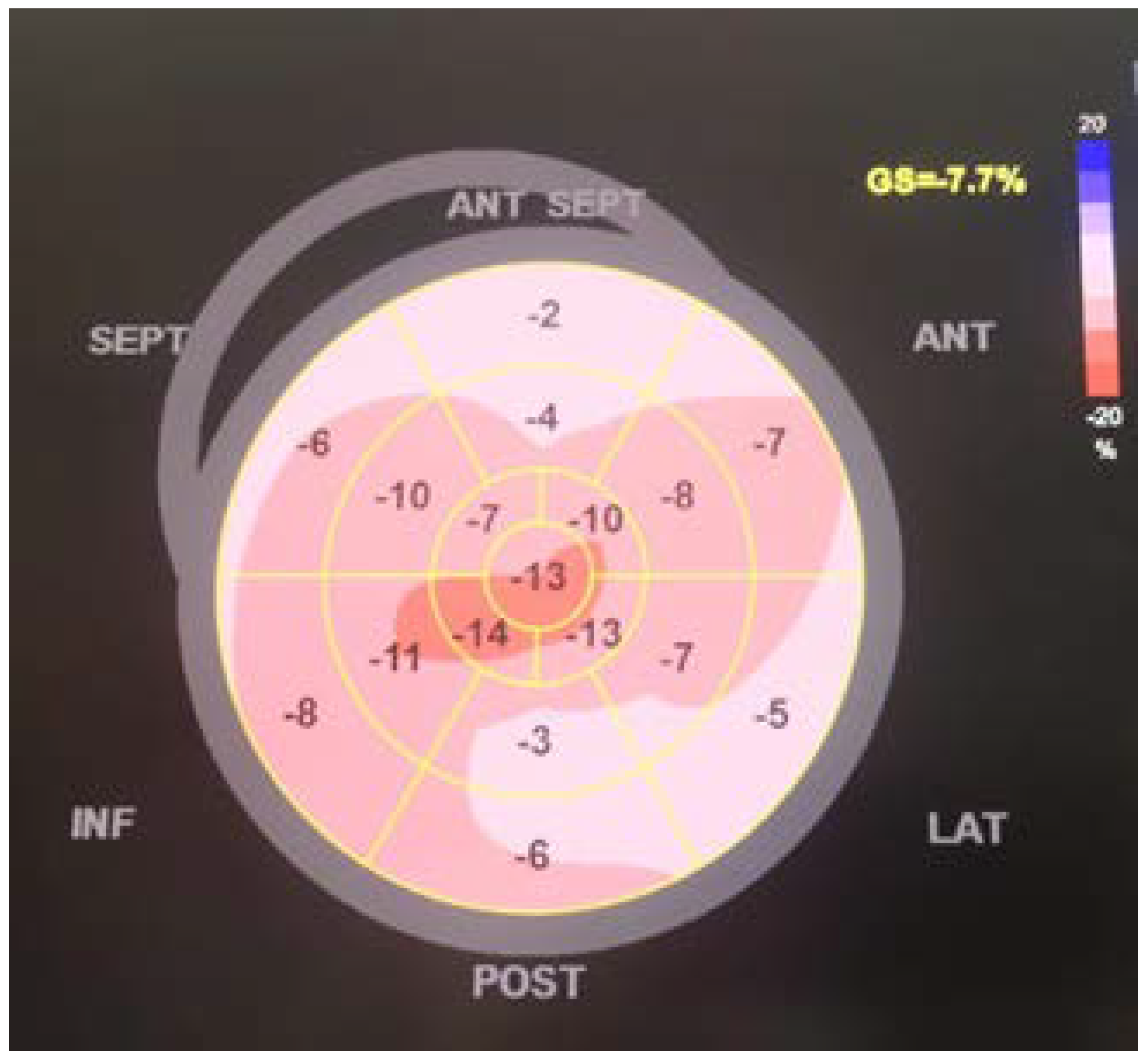
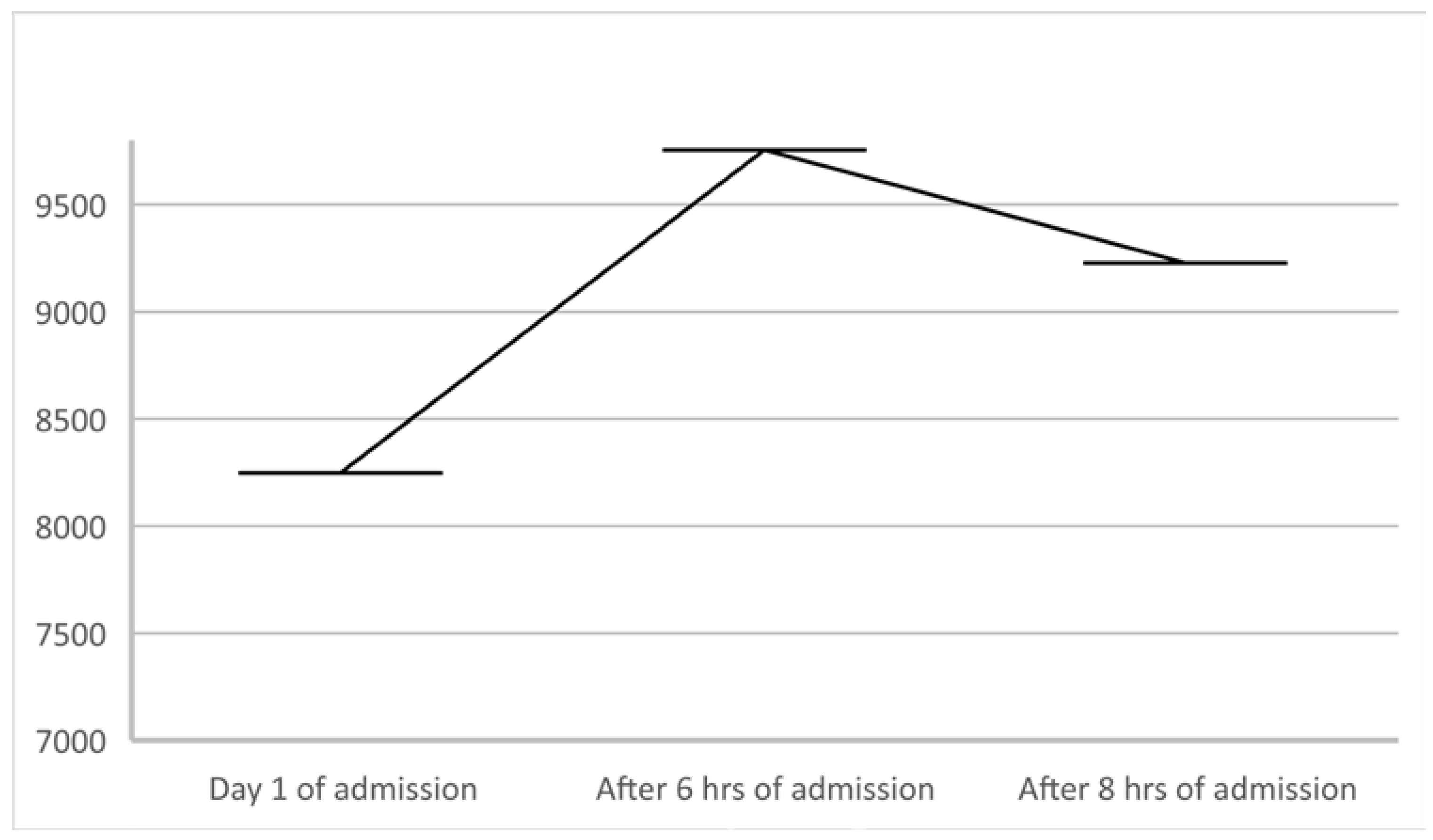
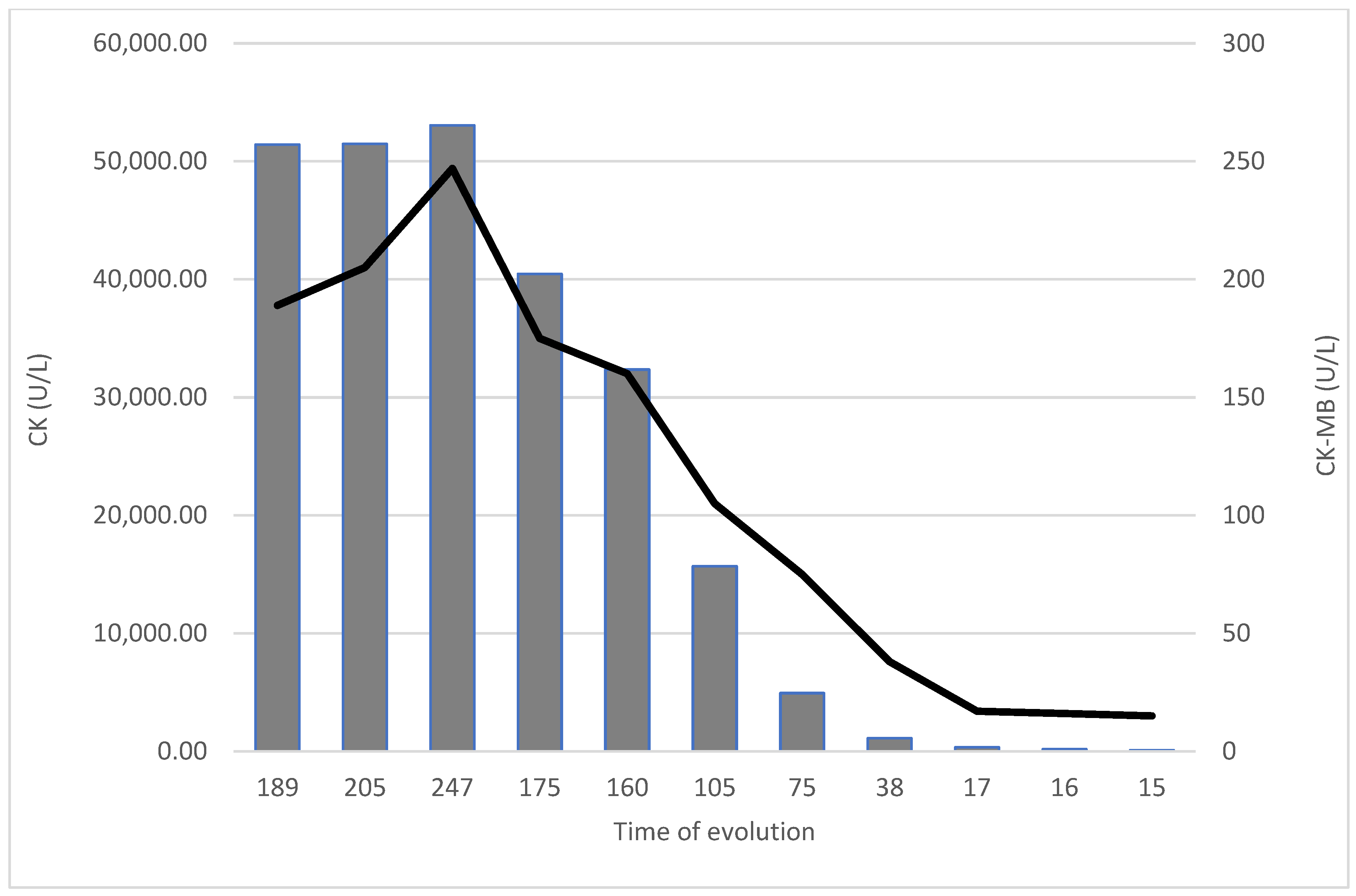
2. Case Details
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, S.C.; Lu, C.C.; Bau, D.T.; Chiu, Y.J.; Yen, Y.T.; Hsu, Y.M.; Fu, C.W.; Kuo, S.C.; Lo, Y.S.; Chiu, H.Y.; et al. Approaches towards fighting the COVID-19 pandemic (Review). Int. J. Mol. Med. 2021, 47, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Sreepadmanabh, M.; Sahu, A.K.; Chande, A. COVID-19, Advances in diagnostic tools, treatment strategies, and vaccine development. J. Biosci. 2020, 45, 148. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Tunescu, M.; Christodorescu, R.; Sharma, A.; Barsac, C.R.; Rogobete, A.F.; Crisan, D.C.; Popovici, S.E.; Kundnani, N.R.; Sandesc, D.; Bedreag, O. The preoperative evaluation of post-COVID-19 patients scheduled for elective surgery-What is important not to miss! Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7607–7615. [Google Scholar]
- Mocanu, V.; Bhagwani, D.; Sharma, A.; Borza, C.; Rosca, C.I.; Stelian, M.; Bhagwani, S.; Haidar, L.; Kshtriya, L.; Kundnani, N.R.; et al. COVID-19 and the Human Eye: Conjunctivitis, a Lone COVID-19 Finding-A Case-Control Study. Med. Princ. Pract. 2022, 31, 66–73. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19, Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Horga, N.G.; Cirnatu, D.; Kundnani, N.R.; Ciurariu, E.; Parvu, S.; Ignea, A.L.; Borza, C.; Sharma, A.; Morariu, S. Evaluation of Non-Pharmacological Measures Implemented in the Management of the COVID-19 Pandemic in Romania. Healthcare 2022, 10, 1756. [Google Scholar] [CrossRef]
- Soumya, R.S.; Unni, T.G.; Raghu, K.G. Impact of COVID-19 on the Cardiovascular System: A Review of Available Reports. Cardiovasc. Drugs Ther. 2021, 35, 411–425. [Google Scholar] [CrossRef]
- Basu-Ray, I.; Almaddah, N.K.; Adeboye, A.; Soos, M.P. Cardiac Manifestations of Coronavirus (COVID-19). In Treasure Island; StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Chung, M.K.; Zidar, D.A.; Bristow, M.R.; Cameron, S.J.; Chan, T.; Harding, C.V., 3rd; Kwon, D.H.; Singh, T.; Tilton, J.C.; Tsai, E.J.; et al. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res. 2021, 128, 1214–1236. [Google Scholar] [CrossRef]
- Siripanthong, B.; Asatryan, B.; Hanff, T.C.; Chatha, S.R.; Khanji, M.Y.; Ricci, F.; Muser, D.; Ferrari, V.A.; Nazarian, S.; Santangeli, P.; et al. The Pathogenesis and Long-Term Consequences of COVID-19 Cardiac Injury. JACC Basic Transl. Sci. 2022, 7, 294–308. [Google Scholar] [CrossRef]
- Blake, T.; Noureldin, B. Anti-PL-7 antisynthetase syndrome presenting as COVID-19. Rheumatology 2021, 60, e252–e254. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Zhang, J.; Hao, Y.; Ou, W.; Ming, F.; Liang, G.; Qian, Y.; Cai, Q.; Dong, S.; Hu, S.; Wang, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study. J. Transl. Med. 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Camm, C.F.; Elkington, A.; Tilling, L.; Stirrup, J.; Chan, A.; Bull, S. Myopericarditis and myositis in a patient with COVID-19, a case report. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dushyant Pawar, K.P.; Nandini, N. A case of myocarditis and myositis following COVID19 vaccine. J. Am. Coll. Cardiol. 2022, 79, 2693. [Google Scholar] [CrossRef]
- Durucan, I.; Guner, S.; Kilickiran Avci, B.; Unverengil, G.; Melikoglu, M.; Ugurlu, S. Post Covıd-19 Vaccınatıon Inflammatory Syndrome: A Case Report. Mod. Rheumatol. Case Rep. 2022, 12, rxac041. [Google Scholar] [CrossRef] [PubMed]
- Beydon, M.; Chevalier, K.; Al Tabaa, O.; Hamroun, S.; Delettre, A.S.; Thomas, M.; Herrou, J.; Riviere, E.; Mariette, X. Myositis as a manifestation of SARS-CoV-2. Ann. Rheum. Dis. 2021, 80, e42. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Matsumoto, H.; Sasajima, T.; Suzuki, T.; Okubo, Y.; Fujita, Y.; Temmoku, J.; Yoshida, S.; Asano, T.; Ohira, H.; et al. New-onset dermatomyositis following COVID-19, A case report. Front. Immunol. 2022, 13, 1002329. [Google Scholar] [CrossRef]
- Vertui, V.; Zanframundo, G.; Castañeda, S.; Biglia, A.; Palermo, B.L.; Cavazzana, I.; Meloni, F.; Cavagna, L. Clinical evolution of antisynthetase syndrome after SARS-CoV2 infection: A 6-month follow-up analysis. Clin. Rheumatol. 2022, 41, 2601–2604. [Google Scholar] [CrossRef]
- Momin, E.; Nagori, M. Unusual case of anti-synthetase syndrome correlated with exposure to covid-19 infection or vaccine. Chest 2022, 162, A2165. [Google Scholar] [CrossRef]
- Swartzman, I.; Gu, J.J.; Toner, Z.; Grover, R.; Suresh, L.; Ullman, L.E. Prevalence of Myositis-Specific Autoantibodies and Myositis-Associated Autoantibodies in COVID-19 Patients: A Pilot Study and Literature Review. Cureus 2022, 14, e29752. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; Martin, J.; Rhys-Dillon, C. Correction to: Increased incidence of anti-synthetase syndrome during COVID-19 pandemic. Rheumatology 2022, 61, 3875. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Seiman, D.; Kundnani, N.R.; Dugaci, D.; Man, D.E.; Velimirovici, D.; Dragan, S.R. COVID-19 Related Myocarditis and Myositis in a Patient with Undiagnosed Antisynthetase Syndrome. Biomedicines 2023, 11, 95. https://doi.org/10.3390/biomedicines11010095
Duda-Seiman D, Kundnani NR, Dugaci D, Man DE, Velimirovici D, Dragan SR. COVID-19 Related Myocarditis and Myositis in a Patient with Undiagnosed Antisynthetase Syndrome. Biomedicines. 2023; 11(1):95. https://doi.org/10.3390/biomedicines11010095
Chicago/Turabian StyleDuda-Seiman, Daniel, Nilima Rajpal Kundnani, Daniela Dugaci, Dana Emilia Man, Dana Velimirovici, and Simona Ruxanda Dragan. 2023. "COVID-19 Related Myocarditis and Myositis in a Patient with Undiagnosed Antisynthetase Syndrome" Biomedicines 11, no. 1: 95. https://doi.org/10.3390/biomedicines11010095
APA StyleDuda-Seiman, D., Kundnani, N. R., Dugaci, D., Man, D. E., Velimirovici, D., & Dragan, S. R. (2023). COVID-19 Related Myocarditis and Myositis in a Patient with Undiagnosed Antisynthetase Syndrome. Biomedicines, 11(1), 95. https://doi.org/10.3390/biomedicines11010095






