A Novel TLR4-SYK Interaction Axis Plays an Essential Role in the Innate Immunity Response in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Total Tissue RNA Extraction and mRNA Expression Analysis
2.3. Tissue Total Protein Extraction and Protein Level Analysis
2.4. Isolation, Culture and Identification of Pathogenic Microorganisms in Milk
2.5. Isolation, Identification and Primary Culture of bMECs In Vitro
2.6. Lipopolysaccharide (LPS from E. coli) Stimulation Assay on bMECs In Vitro
2.7. Pathogenic Microorganism-Infected bMECs for In Vitro Validation
2.8. SYK Gene siRNA in bMECs
2.9. Immunofluorescence Colocalization for TLR4 and SYK in Bovine Mammary Gland Tissue and bMCEs
2.10. Coimmunoprecipitation (Co-IP) for TLR4 and SYK in Bovine Mammary Gland Tissue and bMCEs
2.11. Statistical Analysis Method
3. Results
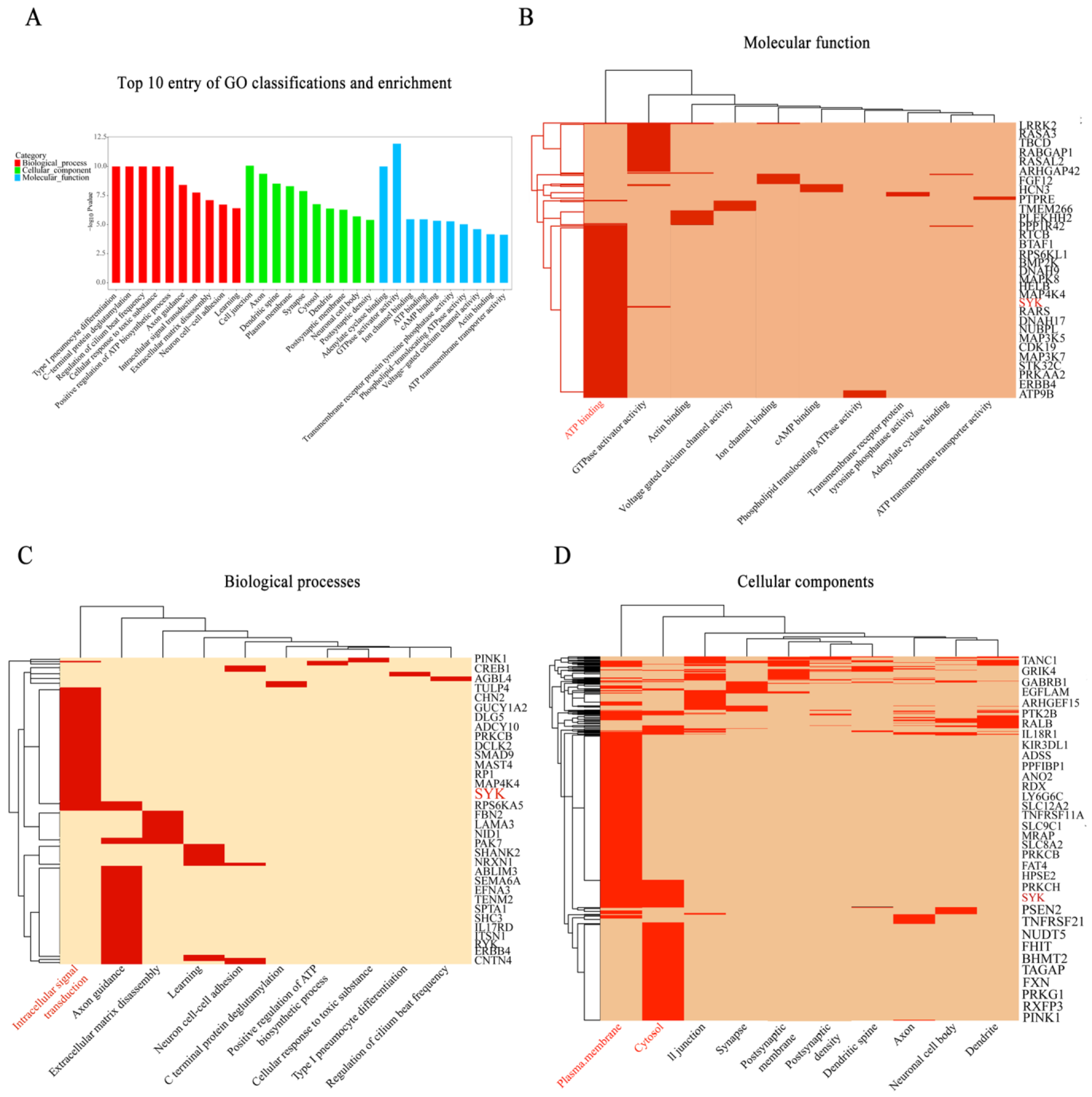
3.1. SYK Plays an Essential in Immune Response for Bovine Mastitis
3.2. SYK, TLR4, NLRP3 and IL-1β Gene Expressions in Mammary Gland Tissue of Chinese Holstein
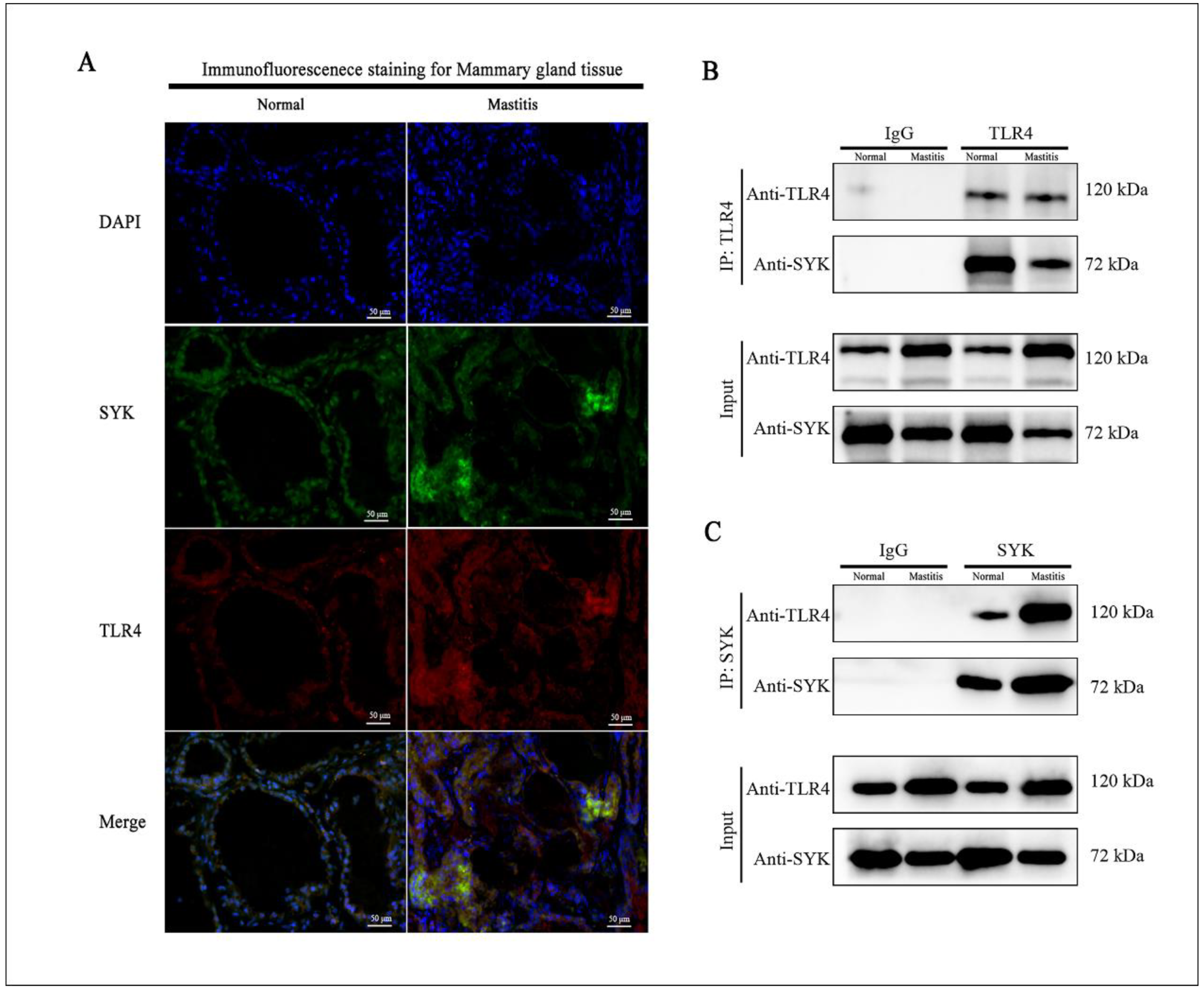
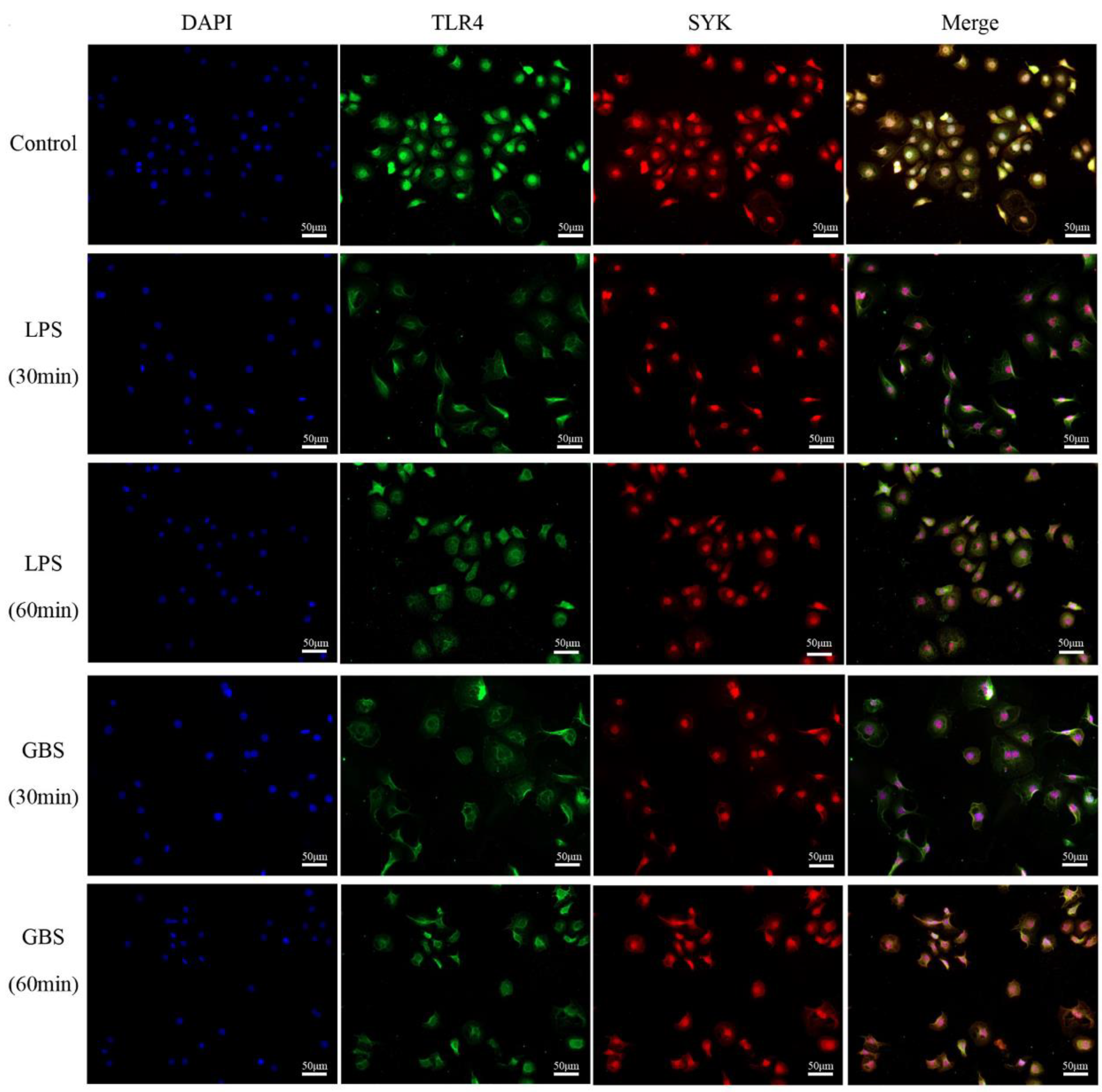
3.3. Colocalization of SYK and TLR4 in Bovine Mammary, Mastitis Tissues and bMECs
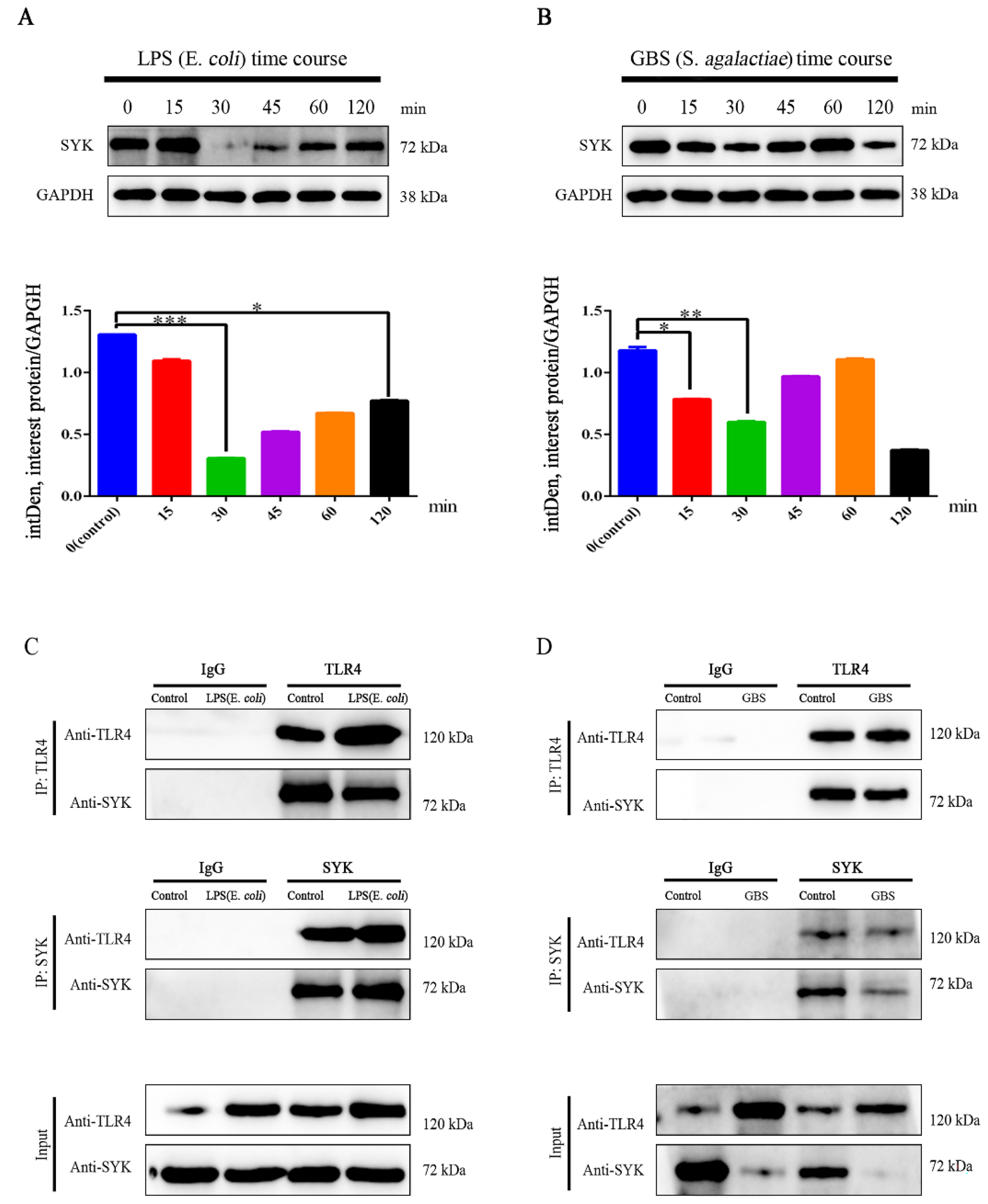
3.4. SYK Protein Levels Showed Time-Course Effect in bMECs with LPS Stimulation or GBS Infection
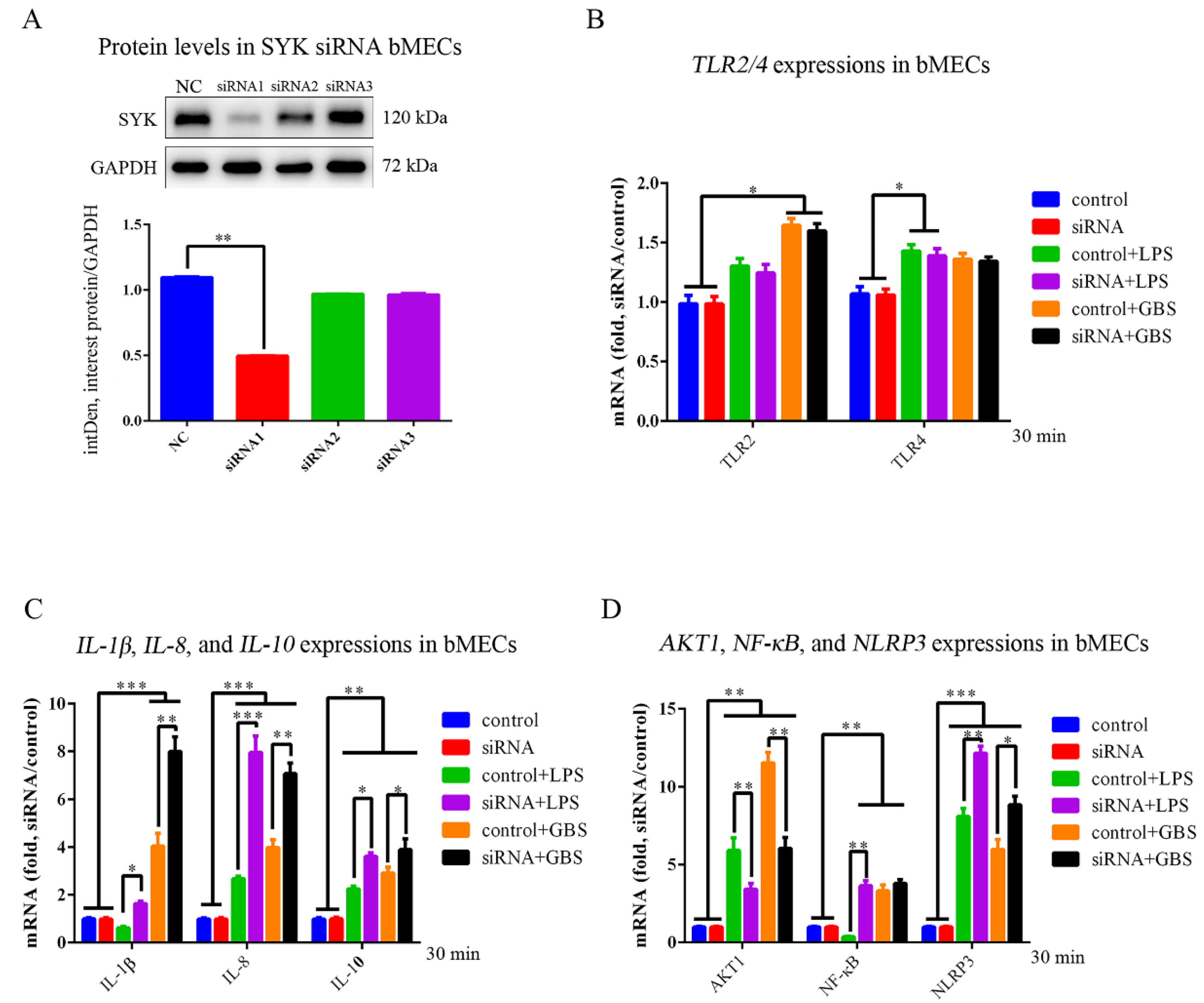
3.5. SYK, TLR4, NLRP3 and IL-1β Expressions in bMECs and SYK siRNA bMECs (bMECsSYK-) with LPS Stimulation or GBS Infection
3.6. SYK, TLR4, NLRP3 and IL-1β Protein Levels in bMECs and bMECsSYK- with LPS Stimulation or GBS Infection
4. Discussion
4.1. SYK Plays a Crucial Role in the Innate Immune Response of Bovine Mammary Gland Tissue and bMECs
4.2. The SYK mRNA Expression and Protein Level in bMECs Responded to LPS Stimulation or GBS Infection Presented Time-Dependent Properties
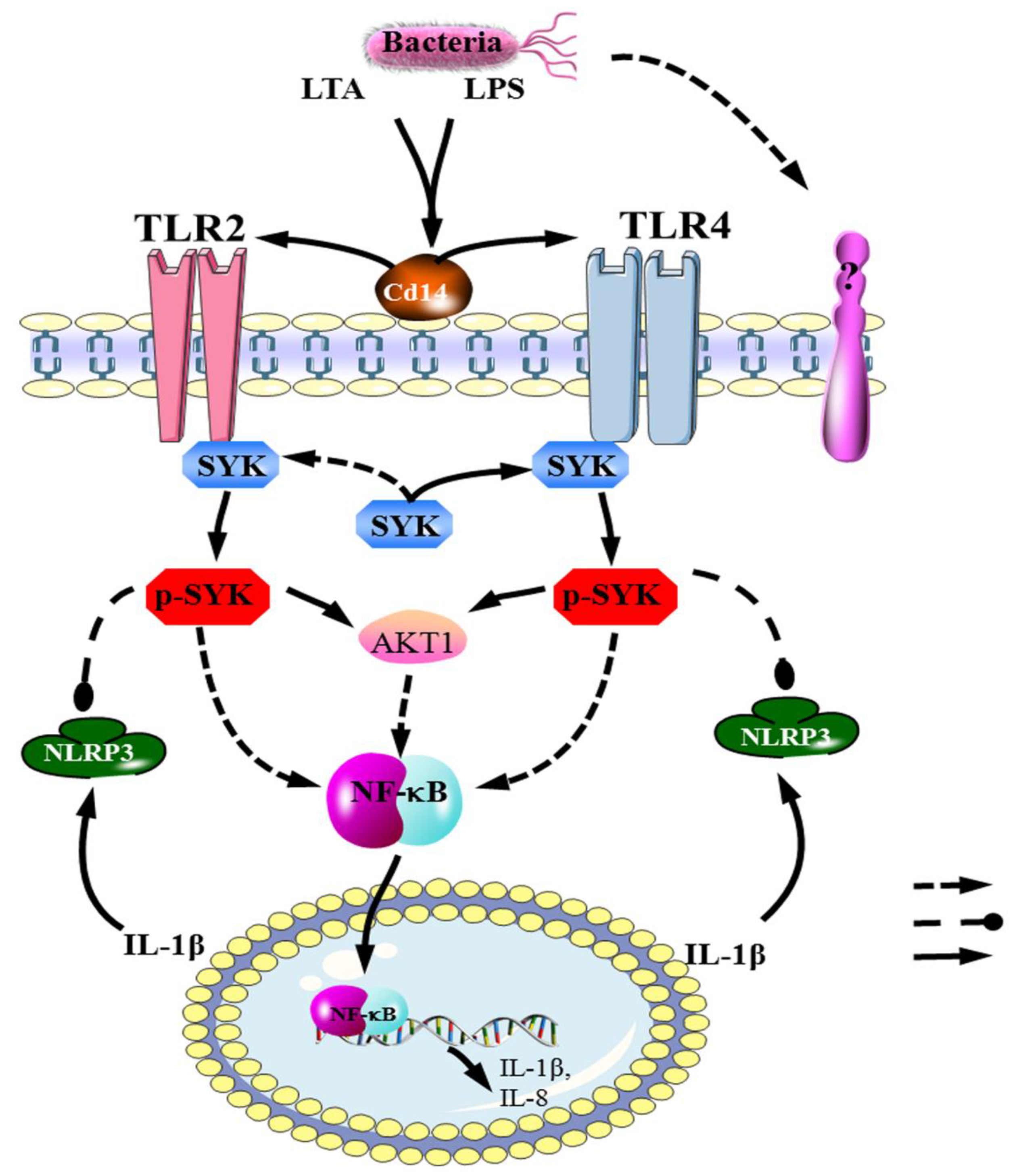
4.3. The TLR4–SYK Signaling Pathway Participates in the Immune Response of Bovine Mammary Gland Tissue and bMECs for Mastitis
4.4. SYK Affected AKT1, IL-1β, IL-8, IL-10, NF-κB and NLRP3 Expression in bMECs with LPS or GBS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogeveen, H.; Huijps, K.; Lam, T.J. Economic aspects of mastitis: New developments. New Zealand Vet. J. 2011, 59, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; Liski, E.; Heikkila, A.M.; Pyorala, S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J. Dairy Sci. 2017, 100, 493–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruet, P.; Maincent, P.; Berthelot, X.; Kaltsatos, V. Bovine mastitis and intramammary drug delivery: Review and perspectives. Adv. Drug Deliv. Rev. 2001, 50, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Petzl, W.; Zerbe, H.; Gunther, J.; Seyfert, H.M.; Hussen, J.; Schuberth, H.J. Pathogen-specific responses in the bovine udder. Models and immunoprophylactic concepts. Res. Vet. Sci. 2018, 116, 55–61. [Google Scholar] [CrossRef]
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Grohn, Y.T. Effects of pathogen-specific clinical mastitis on probability of conception in Holstein dairy cows. J. Dairy Sci. 2014, 97, 6942–6954. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L.M. Mammary Gland Immunobiology and Resistance to Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 507–523. [Google Scholar] [CrossRef]
- Lundberg, A.; Nyman, A.K.; Aspan, A.; Borjesson, S.; Unnerstad, H.E.; Waller, K.P. Udder infections with Staphylococcus aureus, Streptococcus dysgalactiae, and Streptococcus uberis at calving in dairy herds with suboptimal udder health. J. Dairy Sci. 2016, 99, 2102–2117. [Google Scholar] [CrossRef] [Green Version]
- de Greeff, A.; Zadoks, R.; Ruuls, L.; Toussaint, M.; Nguyen, T.K.; Downing, A.; Rebel, J.; Stockhofe-Zurwieden, N.; Smith, H. Early host response in the mammary gland after experimental Streptococcus uberis challenge in heifers. J. Dairy Sci. 2013, 96, 3723–3736. [Google Scholar] [CrossRef] [Green Version]
- Hughes, K. Development and Pathology of the Equine Mammary Gland. J. Mammary Gland Biol. Neoplasia 2021, 26, 121–134. [Google Scholar] [CrossRef]
- Merkin, J.; Russell, C.; Chen, P.; Burge, C.B. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science 2012, 338, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Humer, E.; Aditya, S.; Zebeli, Q. Innate immunity and metabolomic responses in dairy cows challenged intramammarily with lipopolysaccharide after subacute ruminal acidosis. Anim. Int. J. Anim. Biosci. 2018, 12, 2551–2560. [Google Scholar] [CrossRef]
- Isobe, N. Control mechanisms for producing antimicrobial factors in ruminant mammary gland. Anim. Sci. J. Nihon Chikusan Gakkaiho 2017, 88, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zerbe, H.; Petzl, W.; Brunner, R.M.; Gunther, J.; Draing, C.; von Aulock, S.; Schuberth, H.J.; Seyfert, H.M. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-kappaB in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008, 45, 1385–1397. [Google Scholar] [CrossRef]
- Stevens, M.G.; Peelman, L.J.; De Spiegeleer, B.; Pezeshki, A.; Van De Walle, G.R.; Duchateau, L.; Burvenich, C. Differential gene expression of the toll-like receptor-4 cascade and neutrophil function in early- and mid-lactating dairy cows. J. Dairy Sci. 2011, 94, 1277–1288. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Che, Y.; Zhang, M.; Ren, W.; Xia, X.; Liu, H.; Huang, T.; Huang, J.; Lei, L. IFN-gamma Activates the TLR4-CCL5 Signaling Through Reducing Arginine Level, Leading to Enhanced Susceptibility of Bovine Mammary Epithelial Cells to Staphylococcus aureus. Inflammation 2020, 43, 2209–2221. [Google Scholar] [CrossRef]
- Creamer, B.A.; Sakamoto, K.; Schmidt, J.W.; Triplett, A.A.; Moriggl, R.; Wagner, K.U. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol. Cell. Biol. 2010, 30, 2957–2970. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granie, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef] [Green Version]
- Gondaira, S.; Higuchi, H.; Iwano, H.; Nishi, K.; Nebu, T.; Nakajima, K.; Nagahata, H. Innate immune response of bovine mammary epithelial cells to Mycoplasma bovis. J. Vet. Sci. 2018, 19, 79–87. [Google Scholar] [CrossRef]
- Zhuang, C.; Liu, G.; Barkema, H.W.; Zhou, M.; Xu, S.; Ur Rahman, S.; Liu, Y.; Kastelic, J.P.; Gao, J.; Han, B. Selenomethionine Suppressed TLR4/NF-kappaB Pathway by Activating Selenoprotein S to Alleviate ESBL Escherichia coli-Induced Inflammation in Bovine Mammary Epithelial Cells and Macrophages. Front. Microbiol. 2020, 11, 1461. [Google Scholar] [CrossRef]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Xu, Y.; Lu, J.; Liu, M.; Bin, D.; Miao, J.; Yin, Y. Variant innate immune responses of mammary epithelial cells to challenge by Staphylococcus aureus, Escherichia coli and the regulating effect of taurine on these bioprocesses. Free Radic. Biol. Med. 2016, 96, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Mocsai, A.; Ruland, J.; Tybulewicz, V.L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.; Winther, S.; Natarajan, A.; Yang, H.; Jiang, M.; Thiru, P.; Shahsafaei, A.; Chavarria, T.E.; Lamming, D.W.; Sun, L.; et al. SYK kinase mediates brown fat differentiation and activation. Nat. Commun. 2017, 8, 2115. [Google Scholar] [CrossRef] [Green Version]
- Bukong, T.N.; Iracheta-Vellve, A.; Saha, B.; Ambade, A.; Satishchandran, A.; Gyongyosi, B.; Lowe, P.; Catalano, D.; Kodys, K.; Szabo, G. Inhibition of spleen tyrosine kinase activation ameliorates inflammation, cell death, and steatosis in alcoholic liver disease. Hepatology 2016, 64, 1057–1071. [Google Scholar] [CrossRef] [Green Version]
- Rosa, J.P.; Raslova, H.; Bryckaert, M. Filamin A: Key actor in platelet biology. Blood J. Am. Soc. Hematol. 2019, 134, 1279–1288. [Google Scholar] [CrossRef]
- Kiefer, F.; Brumell, J.; Al-Alawi, N.; Latour, S.; Cheng, A.; Veillette, A.; Grinstein, S.; Pawson, T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol. Cell. Biol. 1998, 18, 4209–4220. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Zhu, J.; Zikherman, J.; Parameswaran, R.; Kadlecek, T.A.; Wang, Q.; Au-Yeung, B.; Ploegh, H.; Kuriyan, J.; Das, J.; et al. Monovalent and multivalent ligation of the B cell receptor exhibit differential dependence upon Syk and Src family kinases. Sci. Signal. 2013, 6, ra1. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, S.; Miyazaki, Y.; Yoshii, C.; Nakaya, M.; Ozaki, N.; Toda, S.; Kuroda, E.; Ishibashi, K.; Yasuda, T.; Natsuaki, Y.; et al. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat. Commun. 2014, 5, 3755. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Zhao, M.; Gao, L.; Sen, R.; MacGlashan, D., Jr. Identifying regulatory pathways of spleen tyrosine kinase expression in human basophils. J. Allergy Clin. Immunol. 2019, 145, 947–957. [Google Scholar] [CrossRef]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef]
- Falker, K.; Klarstrom-Engstrom, K.; Bengtsson, T.; Lindahl, T.L.; Grenegard, M. The toll-like receptor 2/1 (TLR2/1) complex initiates human platelet activation via the src/Syk/LAT/PLCgamma2 signalling cascade. Cell. Signal. 2014, 26, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, B.; Buhren, S.; Bock, B.; Alves, F.; Vogel, W.F.; Kiefer, F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell. Mol. Life Sci. CMLS 2011, 68, 3757–3770. [Google Scholar] [CrossRef]
- Yang, W.S.; Kim, J.S.; Han, N.J.; Lee, M.J.; Park, S.K. Toll-like receptor 4/spleen tyrosine kinase complex in high glucose signal transduction of proximal tubular epithelial cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 2309–2319. [Google Scholar] [CrossRef]
- Hou, X.; Lin, L.; Xing, W.; Yang, Y.; Duan, X.; Li, Q.; Gao, X.; Lin, Y. Spleen tyrosine kinase regulates mammary epithelial cell proliferation in mammary glands of dairy cows. J. Dairy Sci. 2016, 99, 3858–3868. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Zhou, H.; Kang, Y.; Zhang, X.; Duan, X.; Alnabhan, R.; Liang, S.; Scott, D.A.; Lamont, R.J.; Shang, J.; et al. Syk negatively regulates TLR4-mediated IFNbeta and IL-10 production and promotes inflammatory responses in dendritic cells. Biochim. Et Biophys. Acta 2016, 1860, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Bae, Y.S. The SYK side of TLR4: Signalling mechanisms in response to LPS and minimally oxidized LDL. Br. J. Pharmacol. 2012, 167, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Jedrzejczak, M.; Szatkowska, I. Bovine mammary epithelial cell cultures for the study of mammary gland functions. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 389–398. [Google Scholar] [CrossRef]
- Basirico, L.; Morera, P.; Dipasquale, D.; Troscher, A.; Serra, A.; Mele, M.; Bernabucci, U. Conjugated linoleic acid isomers strongly improve the redox status of bovine mammary epithelial cells (BME-UV1). J. Dairy Sci. 2015, 98, 7071–7082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, T.; Barash, I. Characterization of an epithelial cell line from bovine mammary gland. Vitr. Cell. Dev. Biol. Anim. 2002, 38, 282–292. [Google Scholar] [CrossRef]
- Yang, F.; Chen, F.; Li, L.; Yan, L.; Badri, T.; Lv, C.; Yu, D.; Zhang, M.; Jang, X.; Li, J.; et al. Three Novel Players: PTK2B, SYK, and TNFRSF21 Were Identified to Be Involved in the Regulation of Bovine Mastitis Susceptibility via GWAS and Post-transcriptional Analysis. Front. Immunol. 2019, 10, 1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunert, T.; Stessl, B.; Wolf, F.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Distinct phenotypic traits of Staphylococcus aureus are associated with persistent, contagious bovine intramammary infections. Sci. Rep. 2018, 8, 15968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurz, J.P.; Yang, Z.; Weiss, R.B.; Wilson, D.J.; Rood, K.A.; Liu, G.E.; Wang, Z. A genome-wide association study for mastitis resistance in phenotypically well-characterized Holstein dairy cattle using a selective genotyping approach. Immunogenetics 2019, 71, 35–47. [Google Scholar] [CrossRef]
- Blum, S.E.; Goldstone, R.J.; Connolly, J.P.R.; Reperant-Ferter, M.; Germon, P.; Inglis, N.F.; Krifucks, O.; Mathur, S.; Manson, E.; McLean, K.; et al. Postgenomics Characterization of an Essential Genetic Determinant of Mammary Pathogenic Escherichia coli. MBio 2018, 9, e00423-18. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Wang, J.; Wang, Y.; Wang, C.; Liu, X.; Han, Z.; Fu, Y.; Yang, Z. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in vitro. Front. Immunol. 2019, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Alva-Murillo, N.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. Sodium Octanoate Modulates the Innate Immune Response of Bovine Mammary Epithelial Cells through the TLR2/P38/JNK/ERK1/2 Pathway: Implications during Staphylococcus aureus Internalization. Front. Cell. Infect. Microbiol. 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tang, J.; Yang, D.; Tang, C.; Chen, J. Staphylococcal enterotoxin M induced inflammation and impairment of bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8350–8359. [Google Scholar] [CrossRef]
- Shahid, M.; Wang, J.; Gu, X.; Chen, W.; Ali, T.; Gao, J.; Han, D.; Yang, R.; Fanning, S.; Han, B. Prototheca zopfii Induced Ultrastructural Features Associated with Apoptosis in Bovine Mammary Epithelial Cells. Front. Cell. Infect. Microbiol. 2017, 7, 299. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-kappaB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. J. Dairy Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef] [Green Version]
- Enger, B.D.; Nickerson, S.C.; Tucker, H.L.M.; Parsons, C.L.M.; Akers, R.M. Apoptosis and proliferation in Staphylococcus aureus-challenged, nonlactating mammary glands stimulated to grow rapidly and develop with estradiol and progesterone. J. Dairy Sci. 2019, 102, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Brenaut, P.; Lefevre, L.; Rau, A.; Laloe, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhou, E.; Liu, Z.; Li, F.; Liang, D.; Liu, B.; Song, X.; Zhao, F.; Fen, X.; Li, D.; et al. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2013, 155, 245–252. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huang, D.Y.; Chu, C.L.; Lin, Y.L.; Lin, W.W. The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci. Signal. 2013, 6, ra71. [Google Scholar] [CrossRef]
- Boulougouris, X.; Rogiers, C.; Van Poucke, M.; De Spiegeleer, B.; Peelman, L.; Duchateau, L.; Burvenich, C. Methylation of selected CpG islands involved in the transcription of myeloperoxidase and superoxide dismutase 2 in neutrophils of periparturient and mid-lactation cows. J. Dairy Sci. 2019, 102, 7421–7434. [Google Scholar] [CrossRef]
- Chen, C.C.; Boxer, R.B.; Stairs, D.B.; Portocarrero, C.P.; Horton, R.H.; Alvarez, J.V.; Birnbaum, M.J.; Chodosh, L.A. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res. BCR 2010, 12, R72. [Google Scholar] [CrossRef] [Green Version]
- Arranz, A.; Doxaki, C.; Vergadi, E.; Martinez de la Torre, Y.; Vaporidi, K.; Lagoudaki, E.D.; Ieronymaki, E.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA 2012, 109, 9517–9522. [Google Scholar] [CrossRef] [Green Version]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Boulanger, D.; Bureau, F.; Mélotte, D.; Mainil, J.; Lekeux, P. Increased nuclear factor kappaB activity in milk cells of mastitis-affected cows. J Dairy Sci 2003, 86, 1259–1267. [Google Scholar] [CrossRef]
- Connelly, L.; Barham, W.; Pigg, R.; Saint-Jean, L.; Sherrill, T.; Cheng, D.S.; Chodosh, L.A.; Blackwell, T.S.; Yull, F.E. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J. Cell. Physiol. 2010, 222, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Mo, X.; Yu, J.; Huang, Z. Alpinetin attenuates inflammatory responses by interfering toll-like receptor 4/nuclear factor kappa B signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2013, 17, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ershun, Z.; Yunhe, F.; Zhengkai, W.; Yongguo, C.; Naisheng, Z.; Zhengtao, Y. Cepharanthine attenuates lipopolysaccharide-induced mice mastitis by suppressing the NF-κB signaling pathway. Inflammation 2014, 37, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Li, F.; Liang, D.; Yang, Z.; Wang, T.; Wang, W.; Song, X.; Guo, M.; Zhou, E.; Li, D.; Cao, Y.; et al. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. Int. Immunopharmacol. 2013, 17, 478–482. [Google Scholar] [CrossRef]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yuan, L.; Xiang, M.; Jiang, Q.; Zhang, M.; Chen, F.; Tong, J.; Huang, J.; Cai, Y. A Novel TLR4-SYK Interaction Axis Plays an Essential Role in the Innate Immunity Response in Bovine Mammary Epithelial Cells. Biomedicines 2023, 11, 97. https://doi.org/10.3390/biomedicines11010097
Yang F, Yuan L, Xiang M, Jiang Q, Zhang M, Chen F, Tong J, Huang J, Cai Y. A Novel TLR4-SYK Interaction Axis Plays an Essential Role in the Innate Immunity Response in Bovine Mammary Epithelial Cells. Biomedicines. 2023; 11(1):97. https://doi.org/10.3390/biomedicines11010097
Chicago/Turabian StyleYang, Fan, Lu Yuan, Minghui Xiang, Qiang Jiang, Manling Zhang, Fanghui Chen, Jie Tong, Jinming Huang, and Yafei Cai. 2023. "A Novel TLR4-SYK Interaction Axis Plays an Essential Role in the Innate Immunity Response in Bovine Mammary Epithelial Cells" Biomedicines 11, no. 1: 97. https://doi.org/10.3390/biomedicines11010097






