Vasopressin as Possible Treatment Option in Autism Spectrum Disorder
Abstract
1. Introduction
2. Major Symptoms of Autism Spectrum Disorder
Symptoms in Animal Models
| Symptoms in Humans | Behavioral Test | Main Parameters in Rodents | References | |
|---|---|---|---|---|
| Impaired social interaction | Three-chamber sociability test | Time and fr. near stimulus animal | [70] | |
| Social interaction test | Time and fr. of social interactions | |||
| Deficient communication | MS-USV | USV | [71] | |
| Repetitive behavior | MBT | Number of buried marbles, time of digging | [73] | |
| Self-grooming | Grooming time and fr. in OFT | [74] | ||
| Comorbidity | Motor functions | Rotarod | Latency to fall | [75] |
| Erasmus Ladder | Number of missteps | [76] | ||
| DigiGait | Stance stride length and steps/sec | [77] | ||
| Delay eyeblink conditioning test | Successful conditioned response, blink amplitude and blinking speed | [77] | ||
| Anxiety | OFT | Total distance, time in center | [72] | |
| EPM | Time and fr. in arms | [72] | ||
| Pain sensitivity | Hot plate test | Withdraw latency | [78] | |
| Tail flick test | Withdraw latency | [78] | ||
3. Vasopressin
3.1. Vasopressin Receptors
3.2. Vasopressin and Autism
3.2.1. Peripheral Vasopressin Function and Autism
3.2.2. Social Behavior and Vasopressin with Implication in Autism
| Model | Major Problems | References | ||
|---|---|---|---|---|
| Type | Name/Implicated Molecule | |||
| Genetic models | KO | OTR | soc. | [57] |
| CNTNAP2 | soc., com. | [128] | ||
| MAGEL2 | soc. | [129] | ||
| OPRM1 | soc. | [130,131] | ||
| Klf7 | soc., rep. | [132] | ||
| Fragile X | FMR1 | soc., rep., motor problem, mood | [40] | |
| Rett syndrome | MECP2 | soc., com. | [133] | |
| Tuberous sclerosis | TSC1, TSC2 | soc., rep.; cerebellum; V2 antagonist | [134] | |
| Indirect evidence | NLGN mutations | soc., rest., com. | [103,135] | |
| TSHZ3 KO | soc., rep., narrowness of the field of interest | [68,105] | ||
| GLUT3 KO | soc., rep., com., memory problems | [111,112] | ||
| parvalbumin KO | soc., rep., com. | [136,137] | ||
| GAP43 | soc., resistance to change | [138,139] | ||
| SERT variants | soc., rep. | [140,141,142,143] | ||
| Environmental models | Drugs | VPA | soc., rep., com. | [144,145] |
| Maternal infection and inflammation | poly I:C | soc., rep. | [146,147] | |
| LPS | soc. | [148,149] | ||
| MIA | soc. | [147] | ||
3.2.3. Motor Signs: Repetitive Behavior and Convulsions
Grooming
Marble Burying
Epilepsy—A Comorbidity
Stress in Autism—The Third “Hit”
- The Autonomic Nervous System
- Vasopressin in Thermoregulation with Implication in Autism
- The Hypothalamic–Pituitary–Adrenocortical Axis
- Mood Disorders and Autism—A Comorbidity
- Anxiety
- Depression and Serotonin
Sleep Disturbances in Autism—Another Third “Hit”?
Vasopressinergic Pain Regulation with Implication in Autism
3.3. Brain Areas as Possible Links between Vasopressin and Autism
3.3.1. Social Behavioral Network
3.3.2. Motor Behavior and Vasopressin
3.3.3. Vasopressinergic Link to Stress, and Related Disorder
3.3.4. Circadian Rhythm and Vasopressin
4. Sex Differences in Autism with Focus on Vasopressin
5. Vasopressin-Related Possible Therapies in Autism
5.1. Available Therapies with Possible Vasopressinergic Contribution
5.2. Influencing the Vasopressinergic System in Autism-Related Problems
5.2.1. Intranasal Vasopressin Application
5.2.2. Vasopressin Antagonist Treatment
5.2.3. Oxytocin Treatment
5.2.4. Contradiction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cakir, J.; Frye, R.E.; Walker, S.J. The lifetime social cost of autism: 1990–2029. Res. Autism Spectr. Disord. 2020, 72, 101505. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Rasoulpoor, S.; Rasoulpoor, S.; Shohaimi, S.; Jafarpour, S.; Abdoli, N.; Khaledi-Paveh, B.; Mohammadi, M. The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 2022, 48, 112. [Google Scholar] [CrossRef]
- Wing, L.; Potter, D. The epidemiology of autistic spectrum disorders: Is the prevalence rising? Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 151–161. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Bearman, P. Diagnostic change and the increased prevalence of autism. Int. J. Epidemiol. 2009, 38, 1224–1234. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Newschaffer, C.J.; Lee, L.C.; Cunniff, C.M.; Daniels, J.L.; Kirby, R.S.; Leavitt, L.; Miller, L.; Zahorodny, W.; et al. Advanced parental age and the risk of autism spectrum disorder. Am. J. Epidemiol. 2008, 168, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Meaney, F.J.; Levy, S.E.; DiGuiseppi, C.; Nicholas, J.S.; Kirby, R.S.; Pinto-Martin, J.A.; Schieve, L.A. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE 2010, 5, e11551. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef]
- Leblond, C.S.; Le, T.L.; Malesys, S.; Cliquet, F.; Tabet, A.C.; Delorme, R.; Rolland, T.; Bourgeron, T. Operative list of genes associated with autism and neurodevelopmental disorders based on database review. Mol. Cell. Neurosci. 2021, 113, 103623. [Google Scholar] [CrossRef]
- Ueoka, I.; Pham, H.T.N.; Matsumoto, K.; Yamaguchi, M. Autism Spectrum Disorder-Related Syndromes: Modeling with Drosophila and Rodents. Int. J. Mol. Sci. 2019, 20, 4071. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Quinnies, K.M.; Eschendroeder, A.; Didrick, P.M.; Eugster, E.A.; Rissman, E.F. Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice. Psychoneuroendocrinology 2015, 51, 271–281. [Google Scholar] [CrossRef]
- Tick, B.; Bolton, P.; Happe, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child. Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Makowska-Zubrycka, M.; Czarzasta, K.; Kasarello, K.; Aggarwal, V.; Bialy, M.; Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A. Common Genetic Variants Link the Abnormalities in the Gut-Brain Axis in Prematurity and Autism. Cerebellum 2019, 18, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ferreira, H.; Martins, J.; Goncalves, J.; Castelo-Branco, M. Male sex bias in early and late onset neurodevelopmental disorders: Shared aspects and differences in Autism Spectrum Disorder, Attention Deficit/hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics 2011, 128, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.N.; Tsimis, M.; Burd, I. Infections and Brain Development. Obstet. Gynecol. Surv. 2015, 70, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, D.Q.; Stevens, H.E.; Margolis, K.G.; Van de Water, J. Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Curr. Pharm. Des. 2019, 25, 4331–4343. [Google Scholar] [CrossRef]
- Vohr, B.R.; Poggi Davis, E.; Wanke, C.A.; Krebs, N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 2017, 139 (Suppl. S1), S38–S49. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Jaber, M. Genetic and environmental mouse models of autism reproduce the spectrum of the disease. J. Neural Transm. 2023, 130, 425–432. [Google Scholar] [CrossRef]
- Tartaglione, A.M.; Schiavi, S.; Calamandrei, G.; Trezza, V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 2019, 159, 107477. [Google Scholar] [CrossRef]
- Coiro, V.; Chiodera, P. Inhibition by sodium valproate of the arginine vasopressin and adrenocorticotropin responses to angiotensin II in normal men. Brain Res. 1989, 491, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Chiodera, P.; Gnudi, A.; Volpi, R.; Marchesi, C.; Marchesi, M.; Davoli, D.; Capretti, L.; Coiro, V. Effects of the GABAergic agent sodium valproate on the arginine vasopressin responses to hypertonic stimulation and upright posture in man. Clin. Endocrinol. 1989, 30, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Murch, S.H.; Anthony, A.; Linnell, J.; Casson, D.M.; Malik, M.; Berelowitz, M.; Dhillon, A.P.; Thomson, M.A.; Harvey, P.; et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998, 351, 637–641. [Google Scholar] [CrossRef]
- Taylor, L.E.; Swerdfeger, A.L.; Eslick, G.D. Vaccines are not associated with autism: An evidence-based meta-analysis of case-control and cohort studies. Vaccine 2014, 32, 3623–3629. [Google Scholar] [CrossRef]
- Maglione, M.A.; Das, L.; Raaen, L.; Smith, A.; Chari, R.; Newberry, S.; Shanman, R.; Perry, T.; Goetz, M.B.; Gidengil, C. Safety of vaccines used for routine immunization of U.S. children: A systematic review. Pediatrics 2014, 134, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, O.; Fresno-Rodriguez, A.; Spencer-Contreras, R.E.; Tarraga-Minguez, R.; Gonzalez-Fernandez, D.; Sepulveda-Opazo, F. Research Mapping of Trauma Experiences in Autism Spectrum Disorders: A Bibliometric Analysis. Healthcare 2023, 11, 1267. [Google Scholar] [CrossRef]
- Makris, G.; Agorastos, A.; Chrousos, G.P.; Pervanidou, P. Stress System Activation in Children and Adolescents with Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 756628. [Google Scholar] [CrossRef]
- Thoen, A.; Steyaert, J.; Alaerts, K.; Evers, K.; Van Damme, T. A Systematic Review of Self-Reported Stress Questionnaires in People on the Autism Spectrum. Rev. J. Autism Dev. Disord. 2023, 10, 295–318. [Google Scholar] [CrossRef]
- de Bruin, E.I.; Ferdinand, R.F.; Meester, S.; de Nijs, P.F.; Verheij, F. High rates of psychiatric co-morbidity in PDD-NOS. J. Autism Dev. Disord. 2007, 37, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Lukmanji, S.; Manji, S.A.; Kadhim, S.; Sauro, K.M.; Wirrell, E.C.; Kwon, C.S.; Jette, N. The co-occurrence of epilepsy and autism: A systematic review. Epilepsy Behav. 2019, 98 Pt A, 238–248. [Google Scholar] [CrossRef]
- Leyfer, O.T.; Folstein, S.E.; Bacalman, S.; Davis, N.O.; Dinh, E.; Morgan, J.; Tager-Flusberg, H.; Lainhart, J.E. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J. Autism Dev. Disord. 2006, 36, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Pezzimenti, F.; Han, G.T.; Vasa, R.A.; Gotham, K. Depression in Youth with Autism Spectrum Disorder. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Postorino, V.; Kerns, C.M.; Vivanti, G.; Bradshaw, J.; Siracusano, M.; Mazzone, L. Anxiety Disorders and Obsessive-Compulsive Disorder in Individuals with Autism Spectrum Disorder. Curr. Psychiatry Rep. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Berenguer-Forner, C.; Miranda-Casas, A.; Pastor-Cerezuela, G.; Rosello-Miranda, R. Comorbidity of autism spectrum disorder and attention deficit with hyperactivity. A review study. Rev. Neurol. 2015, 60 (Suppl. S1), S37–S43. [Google Scholar]
- Francis, S.M.; Sagar, A.; Levin-Decanini, T.; Liu, W.; Carter, C.S.; Jacob, S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014, 1580, 199–218. [Google Scholar] [CrossRef]
- Frye, R.E. Social Skills Deficits in Autism Spectrum Disorder: Potential Biological Origins and Progress in Developing Therapeutic Agents. CNS Drugs 2018, 32, 713–734. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Perrone, R.; Margari, L.; Leopoldo, M. Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J. Med. Chem. 2017, 60, 9114–9141. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.; Dawson, G.; Osterling, J.; Dinno, N. Brief report: Recognition of autism spectrum disorder before one year of age: A retrospective study based on home videotapes. J. Autism Dev. Disord. 2000, 30, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Osterling, J.; Dawson, G. Early recognition of children with autism: A study of first birthday home videotapes. J. Autism Dev. Disord. 1994, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Cook, E.H.; Leventhal, B.L.; Amaral, D.G. Autism spectrum disorders. Neuron 2000, 28, 355–363. [Google Scholar] [CrossRef]
- Kelley, M.E.; Nadler, C.B.; Rey, C.; Cowie, S.; Podlesnik, C.A. Noncontingent reinforcement competes with response performance. J. Exp. Anal. Behav. 2017, 107, 343–353. [Google Scholar] [CrossRef] [PubMed]
- De Giacomo, A.; Fombonne, E. Parental recognition of developmental abnormalities in autism. Eur. Child. Adolesc. Psychiatry 1998, 7, 131–136. [Google Scholar] [CrossRef]
- Rutter, M. Concepts of autism: A review of research. J. Child. Psychol. Psychiatry 1968, 9, 1–25. [Google Scholar] [CrossRef]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- Rapoport, J.; Chavez, A.; Greenstein, D.; Addington, A.; Gogtay, N. Autism spectrum disorders and childhood-onset schizophrenia: Clinical and biological contributions to a relation revisited. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 10–18. [Google Scholar] [CrossRef]
- Trevisan, D.A.; Foss-Feig, J.H.; Naples, A.J.; Srihari, V.; Anticevic, A.; McPartland, J.C. Autism Spectrum Disorder and Schizophrenia Are Better Differentiated by Positive Symptoms Than Negative Symptoms. Front. Psychiatry 2020, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Sala, M.; Braida, D.; Lentini, D.; Busnelli, M.; Bulgheroni, E.; Capurro, V.; Finardi, A.; Donzelli, A.; Pattini, L.; Rubino, T.; et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biol. Psychiatry 2011, 69, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, R.; Kooy, R.F. Mouse models of fragile X-related disorders. Dis. Model. Mech. 2023, 16, dmm049485. [Google Scholar] [CrossRef]
- Ergaz, Z.; Weinstein-Fudim, L.; Ornoy, A. Genetic and non-genetic animal models for autism spectrum disorders (ASD). Reprod. Toxicol. 2016, 64, 116–140. [Google Scholar] [CrossRef]
- Bucknor, M.C.; Gururajan, A.; Dale, R.C.; Hofer, M.J. A comprehensive approach to modeling maternal immune activation in rodents. Front. Neurosci. 2022, 16, 1071976. [Google Scholar] [CrossRef]
- Heuer, E.; Kazama, A.; Bachevalier, J. Acoustic startle and prepulse inhibition deficits in adult monkeys with neonatal lesions of the hippocampus, amygdala and orbital frontal cortex. Behav. Brain Res. 2023, 438, 114170. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Pagani, J.H.; Zhao, M.; Cui, Z.; Avram, S.K.; Caruana, D.A.; Dudek, S.M.; Young, W.S. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 2015, 20, 490–499. [Google Scholar] [CrossRef]
- Stevenson, E.L.; Caldwell, H.K. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 2012, 61, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Csillag, A.; Adam, A.; Zachar, G. Avian models for brain mechanisms underlying altered social behavior in autism. Front. Physiol. 2022, 13, 1032046. [Google Scholar] [CrossRef] [PubMed]
- Kareklas, K.; Teles, M.C.; Nunes, A.R.; Oliveira, R.F. Social zebrafish: Danio rerio as an emerging model in social neuroendocrinology. J. Neuroendocrinol. 2023, in press. [CrossRef]
- Tayanloo-Beik, A.; Hamidpour, S.K.; Abedi, M.; Shojaei, H.; Tavirani, M.R.; Namazi, N.; Larijani, B.; Arjmand, B. Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective. Front. Psychiatry 2022, 13, 911770. [Google Scholar] [CrossRef] [PubMed]
- Roubertoux, P.L.; Tordjman, S.; Caubit, X.; di Cristopharo, J.; Ghata, A.; Fasano, L.; Kerkerian-Le Goff, L.; Gubellini, P.; Carlier, M. Construct Validity and Cross Validity of a Test Battery Modeling Autism Spectrum Disorder (ASD) in Mice. Behav. Genet. 2020, 50, 26–40. [Google Scholar] [CrossRef]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011, 8, 8–26. [Google Scholar] [CrossRef]
- Penagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Angoa-Perez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.E.; Fuccillo, M.V.; Maxeiner, S.; Hayton, S.J.; Gokce, O.; Lim, B.K.; Fowler, S.C.; Malenka, R.C.; Sudhof, T.C. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 2014, 158, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Ten Brinke, M.M.; Stedehouder, J.; Reinelt, C.M.; Wu, B.; Zhou, H.; Zhou, K.; Boele, H.J.; Kushner, S.A.; Lee, M.G.; et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 2016, 7, 12627. [Google Scholar] [CrossRef]
- Piochon, C.; Kloth, A.D.; Grasselli, G.; Titley, H.K.; Nakayama, H.; Hashimoto, K.; Wan, V.; Simmons, D.H.; Eissa, T.; Nakatani, J.; et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 2014, 5, 5586. [Google Scholar] [CrossRef]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef]
- Cuevas, J. Neurotransmitters and Their Life Cycle. Ref. Modul. Biomed. Sci. 2019, 1–7. [Google Scholar] [CrossRef]
- Acher, R. Neurohypophysial peptide systems: Processing machinery, hydroosmotic regulation, adaptation and evolution. Regul. Pept. 1993, 45, 1–13. [Google Scholar] [CrossRef]
- Acher, R.; Chauvet, J.; Chauvet, M.T. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1995, 395, 615–627. [Google Scholar] [PubMed]
- Grimmelikhuijzen, C.J.; Graff, D. Arg-Phe-amide-like peptides in the primitive nervous systems of coelenterates. Peptides 1985, 6 (Suppl. S3), 477–483. [Google Scholar] [CrossRef]
- Ocampo Daza, D.; Bergqvist, C.A.; Larhammar, D. The Evolution of Oxytocin and Vasotocin Receptor Genes in Jawed Vertebrates: A Clear Case for Gene Duplications Through Ancestral Whole-Genome Duplications. Front. Endocrinol. 2021, 12, 792644. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Correction: Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221 Pt 19, jeb151092. [Google Scholar] [CrossRef]
- Gwee, P.C.; Tay, B.H.; Brenner, S.; Venkatesh, B. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: Origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Kochman, K. Neurohormones: Oxytocin, vasopressin and related peptides—Structure, genes, receptors, and evolution. J. Anim. Feed. Sci. 2013, 22, 283–294. [Google Scholar] [CrossRef][Green Version]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Hurtado-Alvarado, G.; Soto-Tinoco, E. Vasopressin: An output signal from the suprachiasmatic nucleus to prepare physiology and behaviour for the resting phase. J. Neuroendocrinol. 2021, 33, e12998. [Google Scholar] [CrossRef]
- Danziger, J.; Zeidel, M.L. Osmotic homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 852–862. [Google Scholar] [CrossRef]
- McKay, E.C.; Counts, S.E. Oxytocin Receptor Signaling in Vascular Function and Stroke. Front. Neurosci. 2020, 14, 574499. [Google Scholar] [CrossRef]
- Zhou, X.B.; Lutz, S.; Steffens, F.; Korth, M.; Wieland, T. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: Implications for myometrial contractility. Mol. Endocrinol. 2007, 21, 740–752. [Google Scholar] [CrossRef] [PubMed]
- de Wied, D. Neuropeptides as psychotropic drugs. Acta Neuropsychiatr. 1992, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leboyer, M.; Bouvard, M.P.; Launay, J.M.; Tabuteau, F.; Waller, D.; Dugas, M.; Kerdelhue, B.; Lensing, P.; Panksepp, J. Brief report: A double-blind study of naltrexone in infantile autism. J. Autism Dev. Disord. 1992, 22, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, M.P.; Leboyer, M.; Launay, J.M.; Recasens, C.; Plumet, M.H.; Waller-Perotte, D.; Tabuteau, F.; Bondoux, D.; Dugas, M.; Lensing, P.; et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: A double-blind, placebo-controlled study. Psychiatry Res. 1995, 58, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Garner, J.P.; Hyde, S.A.; Libove, R.A.; Berquist, S.W.; Hornbeak, K.B.; Jackson, L.P.; Sumiyoshi, R.D.; Howerton, C.L.; Hannah, S.L.; et al. Arginine Vasopressin Is a Blood-Based Biomarker of Social Functioning in Children with Autism. PLoS ONE 2015, 10, e0132224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Dai, Y.C.; Wu, J.; Jia, M.X.; Zhang, J.S.; Shou, X.J.; Han, S.P.; Zhang, R.; Han, J.S. Plasma Oxytocin and Arginine-Vasopressin Levels in Children with Autism Spectrum Disorder in China: Associations with Symptoms. Neurosci. Bull. 2016, 32, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Shou, X.J.; Li, J.; Jia, M.X.; Zhang, J.S.; Guo, Y.; Wei, Q.Y.; Zhang, X.T.; Han, S.P.; Zhang, R.; et al. Mothers of autistic children: Lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS ONE 2013, 8, e74849. [Google Scholar] [CrossRef]
- Voinsky, I.; Bennuri, S.C.; Svigals, J.; Frye, R.E.; Rose, S.; Gurwitz, D. Peripheral Blood Mononuclear Cell Oxytocin and Vasopressin Receptor Expression Positively Correlates with Social and Behavioral Function in Children with Autism. Sci. Rep. 2019, 9, 13443. [Google Scholar] [CrossRef]
- von Gontard, A.; Hussong, J.; Yang, S.S.; Chase, J.; Franco, I.; Wright, A. Neurodevelopmental disorders and incontinence in children and adolescents: Attention-deficit/hyperactivity disorder, autism spectrum disorder, and intellectual disability-A consensus document of the International Children’s Continence Society. Neurourol. Urodyn. 2022, 41, 102–114. [Google Scholar] [CrossRef]
- Gubbiotti, M.; Elisei, S.; Bedetti, C.; Marchiafava, M.; Giannantoni, A. Urinary and bowel disfunction in autism spectrum disorder: A prospective, observational study. Psychiatr. Danub. 2019, 31 (Suppl. S3), 475–478. [Google Scholar]
- Clothier, J.; Absoud, M. Autism spectrum disorder and kidney disease. Pediatr. Nephrol. 2021, 36, 2987–2995. [Google Scholar] [CrossRef]
- Miot, S.; Akbaraly, T.; Michelon, C.; Couderc, S.; Crepiat, S.; Loubersac, J.; Picot, M.C.; Pernon, E.; Gonnier, V.; Jeandel, C.; et al. Comorbidity Burden in Adults with Autism Spectrum Disorders and Intellectual Disabilities-A Report From the EFAAR (Frailty Assessment in Ageing Adults with Autism Spectrum and Intellectual Disabilities) Study. Front. Psychiatry 2019, 10, 617. [Google Scholar] [CrossRef]
- Calahorro, F.; Alejandre, E.; Ruiz-Rubio, M. Osmotic avoidance in Caenorhabditis elegans: Synaptic function of two genes, orthologues of human NRXN1 and NLGN1, as candidates for autism. J. Vis. Exp. 2009, 34, e1616. [Google Scholar]
- Hornberg, H.; Perez-Garci, E.; Schreiner, D.; Hatstatt-Burkle, L.; Magara, F.; Baudouin, S.; Matter, A.; Nacro, K.; Pecho-Vrieseling, E.; Scheiffele, P. Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 2020, 584, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, I.; Magalhaes, P.; Ranjzad, P.; Fatmi, A.; Richard, F.; Manh, T.P.V.; Saurin, A.J.; Feuillet, G.; Denis, C.; Woolf, A.S.; et al. Haploinsufficiency of the mouse Tshz3 gene leads to kidney defects. Hum. Mol. Genet. 2021, 31, 1921–1945. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, N.L.; Lolait, S.J.; Bradley, D.J.; O’Carroll, A.M.; Brownstein, M.J.; Young, W.S., 3rd. Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinol. 1992, 131, 533–535. [Google Scholar] [CrossRef]
- Frizzo, M.E. Putative role of glycogen as a peripheral biomarker of GSK3beta activity. Med. Hypotheses 2013, 81, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O. Potential opposite roles of the extracellular signal-regulated kinase (ERK) pathway in autism spectrum and bipolar disorders. Neurosci. Biobehav. Rev. 2012, 36, 2206–2213. [Google Scholar] [CrossRef]
- Arciniegas Ruiz, S.M.; Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Front. Mol. Neurosci. 2021, 14, 792364. [Google Scholar] [CrossRef]
- Aoyagi, T.; Birumachi, J.; Hiroyama, M.; Fujiwara, Y.; Sanbe, A.; Yamauchi, J.; Tanoue, A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 2007, 148, 2075–2084. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Maher, F.; Koehler, E.; Simpson, I.A. Altered expression of GLUT-1 and GLUT-3 glucose transporters in neurohypophysis of water-deprived or diabetic rats. Am. J. Physiol. 1994, 267, E605–E611. [Google Scholar] [CrossRef]
- Zhao, Y.; Fung, C.; Shin, D.; Shin, B.C.; Thamotharan, S.; Sankar, R.; Ehninger, D.; Silva, A.; Devaskar, S.U. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol. Psychiatry 2010, 15, 286–299. [Google Scholar] [CrossRef]
- Pais, R.; Rievaj, J.; Meek, C.; De Costa, G.; Jayamaha, S.; Alexander, R.T.; Reimann, F.; Gribble, F. Role of enteroendocrine L-cells in arginine vasopressin-mediated inhibition of colonic anion secretion. J. Physiol. 2016, 594, 4865–4878. [Google Scholar] [CrossRef]
- Nouri, Z.; Zhang, X.Y.; Khakisahneh, S.; Degen, A.A.; Wang, D.H. The microbiota-gut-kidney axis mediates host osmoregulation in a small desert mammal. NPJ Biofilms Microbiomes 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.W.; Engelmann, M.; Tobin, V.A.; Meddle, S.L.; Ludwig, M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Ann. N. Y. Acad. Sci. 2011, 1220, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Tobin, V.A.; Hashimoto, H.; Wacker, D.W.; Takayanagi, Y.; Langnaese, K.; Caquineau, C.; Noack, J.; Landgraf, R.; Onaka, T.; Leng, G.; et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 2010, 464, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Harty, S.; Johnson, K.V.; Moeller, A.H.; Carmody, R.N.; Lehto, S.M.; Erdman, S.E.; Dunbar, R.I.M.; Burnet, P.W.J. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tang, X.; Feng, C.; Lin, J.; Zhang, H.; Liu, Q.; Zheng, Q.; Zhuang, H.; Liu, X.; Li, H.; et al. A Systematic Investigation of Complement and Coagulation-Related Protein in Autism Spectrum Disorder Using Multiple Reaction Monitoring Technology. Neurosci. Bull. 2023, in press. [CrossRef]
- Coban, N.; Gokcen, C.; Akbayram, S.; Calisgan, B. Evaluation of Platelet Parameters in Children with Autism Spectrum Disorder: Elongated Collagen-Adenosine Diphosphate and Collagen-Epinephrine Closure Times. Autism Res. 2019, 12, 1069–1076. [Google Scholar] [CrossRef]
- Chandrakumaran, P.; Hews-Girard, J.; Poon, M.C. Desmopressin (DDAVP) use in patients with von Willebrand disease: A single-centre retrospective review of test response and clinical outcomes. Haemophilia 2023, 29, 1095–1103. [Google Scholar] [CrossRef]
- Preijers, T.; Schutte, L.M.; Kruip, M.; Cnossen, M.H.; Leebeek, F.W.G.; van Hest, R.M.; Mathot, R.A.A. Strategies for Individualized Dosing of Clotting Factor Concentrates and Desmopressin in Hemophilia A and B. Ther. Drug Monit. 2019, 41, 192–212. [Google Scholar] [CrossRef]
- Song, T.J.; Lan, X.Y.; Wei, M.P.; Zhai, F.J.; Boeckers, T.M.; Wang, J.N.; Yuan, S.; Jin, M.Y.; Xie, Y.F.; Dang, W.W.; et al. Altered Behaviors and Impaired Synaptic Function in a Novel Rat Model with a Complete Shank3 Deletion. Front. Cell Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Young, L.J.; Flanagan-Cato, L.M. Editorial comment: Oxytocin, vasopressin and social behavior. Horm. Behav. 2012, 61, 227–229. [Google Scholar] [CrossRef]
- Albers, H.E. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 2012, 61, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Baran, N.M.; Peck, S.C.; Kim, T.H.; Goldstein, M.H.; Adkins-Regan, E. Early life manipulations of vasopressin-family peptides alter vocal learning. Proc. Biol. Sci. 2017, 284, 20171114. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Allchorne, A.J.; Zhang, M.; Tsuji, C.; Tobin, V.A.; Pineda, R.; Raftogianni, A.; Stern, J.E.; Grinevich, V.; Leng, G.; et al. Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 2017, 595, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Hume, C.; Allchorne, A.; Grinevich, V.; Leng, G.; Ludwig, M. Effects of optogenetic stimulation of vasopressinergic retinal afferents on suprachiasmatic neurones. J. Neuroendocrinol. 2019, 31, e12806. [Google Scholar] [CrossRef]
- Penagarikano, O.; Lazaro, M.T.; Lu, X.H.; Gordon, A.; Dong, H.; Lam, H.A.; Peles, E.; Maidment, N.T.; Murphy, N.P.; Yang, X.W.; et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015, 7, 271ra8. [Google Scholar] [CrossRef]
- Borie, A.M.; Theofanopoulou, C.; Andari, E. The promiscuity of the oxytocin-vasopressin systems and their involvement in autism spectrum disorder. Handb. Clin. Neurol. 2021, 182, 121–140. [Google Scholar]
- Tanaka, H.; Nishina, K.; Shou, Q.; Takahashi, H.; Sakagami, M.; Matsuda, T.; Inoue-Murayama, M.; Takagishi, H. Association between arginine vasopressin receptor 1A (AVPR1A) polymorphism and inequity aversion. Proc. Biol. Sci. 2023, 290, 20230378. [Google Scholar] [CrossRef]
- Garbugino, L.; Centofante, E.; D’Amato, F.R. Early Social Enrichment Improves Social Motivation and Skills in a Monogenic Mouse Model of Autism, the Oprm1 (-/-) Mouse. Neural Plast. 2016, 2016, 5346161. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, Y.; Guo, M.; Wang, Y.; Wang, R.; Wang, C.; Chen, X.; Tian, W. Kruppel-like factor 7 deficiency causes autistic-like behavior in mice via regulating Clock gene. Cell Biosci. 2022, 12, 166. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, E.; Martin-Sanchez, A.; Kul, E.; Bose, A.; Martinez-Martinez, F.J.; Stork, O.; Martinez-Garcia, F.; Lanuza, E.; Santos, M.; Agustin-Pavon, C. Male-specific features are reduced in Mecp2-null mice: Analyses of vasopressinergic innervation, pheromone production and social behaviour. Brain Struct. Funct. 2020, 225, 2219–2238. [Google Scholar] [CrossRef]
- Guerra-Torres, X.E. A Case Report of Tuberous Sclerosis and Autosomal Dominant Polycystic Kidney Disease in the Era of Tolvaptan. Curr. Rev. Clin. Exp. Pharmacol. 2023, 18, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Wohr, M.; Orduz, D.; Gregory, P.; Moreno, H.; Khan, U.; Vorckel, K.J.; Wolfer, D.P.; Welzl, H.; Gall, D.; Schiffmann, S.N.; et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl. Psychiatry 2015, 5, e525. [Google Scholar] [CrossRef] [PubMed]
- Hammock, E.A.; Levitt, P. Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR). Neuroscience 2012, 222, 20–28. [Google Scholar] [CrossRef]
- Feng, Z.; Ou, Y.; Zhou, M.; Wu, G.; Ma, L.; Zhang, Y.; Liu, Y.; Qi, S. Functional ectopic neural lobe increases GAP-43 expression via PI3K/AKT pathways to alleviate central diabetes insipidus after pituitary stalk lesion in rats. Neurosci. Lett. 2018, 673, 1–6. [Google Scholar] [CrossRef]
- Zaccaria, K.J.; Lagace, D.C.; Eisch, A.J.; McCasland, J.S. Resistance to change and vulnerability to stress: Autistic-like features of GAP43-deficient mice. Genes. Brain Behav. 2010, 9, 985–996. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Pham, M.; Roth, A.; Cachat, J.; Green, J.; Gaikwad, S.; Kalueff, A.V. Alterations in grooming activity and syntax in heterozygous SERT and BDNF knockout mice: The utility of behavior-recognition tools to characterize mutant mouse phenotypes. Brain Res. Bull. 2012, 89, 168–176. [Google Scholar] [CrossRef]
- Veenstra-VanderWeele, J.; Muller, C.L.; Iwamoto, H.; Sauer, J.E.; Owens, W.A.; Shah, C.R.; Cohen, J.; Mannangatti, P.; Jessen, T.; Thompson, B.J.; et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 5469–5474. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Moller-Leimkuhler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef]
- Morrison, T.R.; Melloni, R.H., Jr. The role of serotonin, vasopressin, and serotonin/vasopressin interactions in aggressive behavior. Curr. Top. Behav. Neurosci. 2014, 17, 189–228. [Google Scholar]
- Zhou, B.; Zheng, X.; Chen, Y.; Yan, X.; Peng, J.; Liu, Y.; Zhang, Y.; Tang, L.; Wen, M. The Changes of Amygdala Transcriptome in Autism Rat Model After Arginine Vasopressin Treatment. Front. Neurosci. 2022, 16, 838942. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Shang, S.; Su, Y. The arginine vasopressin V1b receptor gene and prosociality: Mediation role of emotional empathy. Psych. J. 2015, 4, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.Y.; Gu, Y.Y.; Li, M.J.; Song, T.J.; Zhai, F.J.; Zhang, Y.; Zhan, J.S.; Bockers, T.M.; Yue, X.N.; Wang, J.N.; et al. Poly(I:C)-induced maternal immune activation causes elevated self-grooming in male rat offspring: Involvement of abnormal postpartum static nursing in dam. Front. Cell Dev. Biol. 2023, 11, 1054381. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Felice, D.; Golubeva, A.V.; Moloney, G.; Dinan, T.G.; Cryan, J.F. Strain differences in the susceptibility to the gut-brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behav. Pharmacol. 2018, 29, 181–198. [Google Scholar] [CrossRef]
- Whylings, J.; Rigney, N.; Peters, N.V.; de Vries, G.J.; Petrulis, A. Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain Behav. Immun. 2020, 83, 68–77. [Google Scholar] [CrossRef]
- Taylor, P.V.; Veenema, A.H.; Paul, M.J.; Bredewold, R.; Isaacs, S.; de Vries, G.J. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol. Sex. Differ. 2012, 3, 15. [Google Scholar] [CrossRef]
- Fetter-Pruneda, I.; Hart, T.; Ulrich, Y.; Gal, A.; Oxley, P.R.; Olivos-Cisneros, L.; Ebert, M.S.; Kazmi, M.A.; Garrison, J.L.; Bargmann, C.I.; et al. An oxytocin/vasopressin-related neuropeptide modulates social foraging behavior in the clonal raider ant. PLoS Biol. 2021, 19, e3001305. [Google Scholar] [CrossRef]
- Gemmer, A.; Mirkes, K.; Anneser, L.; Eilers, T.; Kibat, C.; Mathuru, A.; Ryu, S.; Schuman, E. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci. Rep. 2022, 12, 4322. [Google Scholar] [CrossRef]
- Tomaszycki, M.L.; Atchley, D. Pairing Increases Activation of V1aR, but not OTR, in Auditory Regions of Zebra Finches: The Importance of Signal Modality in Nonapeptide-Social Behavior Relationships. Integr. Comp. Biol. 2017, 57, 878–890. [Google Scholar] [CrossRef]
- Albers, H.E.; Pollock, J.; Simmons, W.H.; Ferris, C.F. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J. Neurosci. 1986, 6, 2085–2089. [Google Scholar] [CrossRef]
- Irvin, R.W.; Szot, P.; Dorsa, D.M.; Potegal, M.; Ferris, C.F. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol. Behav. 1990, 48, 693–699. [Google Scholar] [CrossRef]
- Wang, Z.; Ferris, C.F.; De Vries, G.J. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. USA 1994, 91, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Musullulu, H.; Raymond, J.S.; Wilson, B.; Langguth, M.; Bowen, M.T. Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice. Neuropharmacology 2019, 156, 107573. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Douglas, A.J.; Solano, E.; Blois, S.M.; Hagen, E.; Klapp, B.F.; Clark, D.A.; Arck, P.C. Neutralization of LPS or blockage of TLR4 signaling prevents stress-triggered fetal loss in murine pregnancy. J. Mol. Med. 2011, 89, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.J.; Neumann, I.D. Vasopressin released within the central amygdala promotes maternal aggression. Eur. J. Neurosci. 2010, 31, 883–891. [Google Scholar] [CrossRef]
- Torok, B.; Csikota, P.; Fodor, A.; Balazsfi, D.; Ferenczi, S.; Demeter, K.; Toth, Z.E.; Konczol, K.; Perna, J.C.; Farkas, I.; et al. Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats. Int. J. Mol. Sci. 2022, 23, 1357. [Google Scholar] [CrossRef]
- Zelena, D.; Demeter, K.; Haller, J.; Balazsfi, D. Considerations for the use of virally delivered genetic tools for in-vivo circuit analysis and behavior in mutant mice: A practical guide to optogenetics. Behav. Pharmacol. 2017, 28, 598–609. [Google Scholar] [CrossRef]
- Csikota, P.; Fodor, A.; Balazsfi, D.; Pinter, O.; Mizukami, H.; Weger, S.; Heilbronn, R.; Engelmann, M.; Zelena, D. Vasopressinergic control of stress-related behavior: Studies in Brattleboro rats. Stress 2016, 19, 349–361. [Google Scholar] [CrossRef]
- Sipos, E.; Torok, B.; Barna, I.; Engelmann, M.; Zelena, D. Vasopressin and post-traumatic stress disorder. Stress 2020, 23, 732–745. [Google Scholar] [CrossRef]
- Zelena, D.; Pinter, O.; Balazsfi, D.G.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin signaling at brain level controls stress hormone release: The vasopressin-deficient Brattleboro rat as a model. Amino Acids 2015, 47, 2245–2253. [Google Scholar] [CrossRef]
- Feifel, D.; Mexal, S.; Melendez, G.; Liu, P.Y.; Goldenberg, J.R.; Shilling, P.D. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology 2009, 34, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Fazekas, C.L.; Szabo, A.; Zelena, D. Epigenetic Modulation of Vasopressin Expression in Health and Disease. Int. J. Mol. Sci. 2021, 22, 9415. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Fodor, A.; Klausz, B.; Varga, J.; Zelena, D. Ameliorating schizophrenia-like symptoms in vasopressin deficient male Brattleboro rat by chronic antipsychotic treatment. Eur. J. Pharmacol. 2021, 909, 174383. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Fodor, A.; Klausz, B.; Zelena, D. Anxiogenic role of vasopressin during the early postnatal period: Maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 2015, 47, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; McFarlane, H.G.; Zhodzishsky, V.; Caldwell, H.K.; Young, W.S.; Ricceri, L.; Crawley, J.N. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav. Brain Res. 2008, 187, 371–378. [Google Scholar] [CrossRef]
- Torok, B.; Fodor, A.; Zsebok, S.; Sipos, E.; Zelena, D. The Effect of Vasopressin Antagonists on Maternal-Separation-Induced Ultrasonic Vocalization and Stress-Hormone Level Increase during the Early Postnatal Period. Brain Sci. 2021, 11, 444. [Google Scholar] [CrossRef]
- Fodor, A.; Klausz, B.; Pinter, O.; Daviu, N.; Rabasa, C.; Rotllant, D.; Balazsfi, D.; Kovacs, K.B.; Nadal, R.; Zelena, D. Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Horm. Behav. 2012, 62, 539–551. [Google Scholar] [CrossRef]
- Levy, G.; Oppenheim, D.; Koren-Karie, N.; Ariav-Paraira, I.; Gal, N.; Yirmiya, N. Disrupted maternal communication and attachment disorganization in children with autism spectrum disorder. Attach. Hum. Dev. 2020, 22, 568–581. [Google Scholar] [CrossRef]
- Avinun, R.; Ebstein, R.P.; Knafo, A. Human maternal behaviour is associated with arginine vasopressin receptor 1A gene. Biol. Lett. 2012, 8, 894–896. [Google Scholar] [CrossRef]
- Wu, J.; Dai, Y.C.; Lan, X.Y.; Zhang, H.F.; Bai, S.Z.; Hu, Y.; Han, S.P.; Han, J.S.; Zhang, R. Postnatal AVP treatments prevent social deficit in adolescence of valproic acid-induced rat autism model. Peptides 2021, 137, 170493. [Google Scholar] [CrossRef]
- Freeman, A.R.; Hare, J.F.; Anderson, W.G.; Caldwell, H.K. Effects of arginine vasopressin on Richardson’s ground squirrel social and vocal behavior. Behav. Neurosci. 2018, 132, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, A.; Kekesi, K.A.; Juhasz, G.; Szekely, A.D.; Lovas, G.; Kovacs, Z. Receptors of peptides as therapeutic targets in epilepsy research. Curr. Med. Chem. 2014, 21, 764–787. [Google Scholar] [CrossRef] [PubMed]
- Madlon-Kay, S.; Montague, M.J.; Brent, L.J.N.; Ellis, S.; Zhong, B.; Snyder-Mackler, N.; Horvath, J.E.; Skene, J.H.P.; Platt, M.L. Weak effects of common genetic variation in oxytocin and vasopressin receptor genes on rhesus macaque social behavior. Am. J. Primatol. 2018, 80, e22873. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Walton, J.C.; McCann, K.E.; Norvelle, A.; Liu, Q.; Vander Velden, J.W.; Borland, J.M.; Hart, M.; Jin, C.; Huhman, K.L.; et al. CRISPR-Cas9 editing of the arginine-vasopressin V1a receptor produces paradoxical changes in social behavior in Syrian hamsters. Proc. Natl. Acad. Sci. USA 2022, 119, e2121037119. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, D.S.; Kaczmarek, V.; Jurek, B.; van den Burg, E.H.; Neumann, I.D.; Gassner, B.M.; Klampfl, S.M.; Bosch, O.J. Antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats. Horm. Behav. 2016, 79, 18–27. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Aulino, E.A.; Rodriguez, K.M.; Witchey, S.K.; Yaw, A.M. Social Context, Stress, Neuropsychiatric Disorders, and the Vasopressin 1b Receptor. Front. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef]
- Cataldo, I.; Azhari, A.; Esposito, G. A Review of Oxytocin and Arginine-Vasopressin Receptors and Their Modulation of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef]
- Tansey, K.E.; Hill, M.J.; Cochrane, L.E.; Gill, M.; Anney, R.J.; Gallagher, L. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): Implications for autism. Mol. Autism 2011, 2, 3. [Google Scholar] [CrossRef]
- Yang, S.Y.; Cho, S.C.; Yoo, H.J.; Cho, I.H.; Park, M.; Yoe, J.; Kim, S.A. Family-based association study of microsatellites in the 5′ flanking region of AVPR1A with autism spectrum disorder in the Korean population. Psychiatry Res. 2010, 178, 199–201. [Google Scholar] [CrossRef]
- Meisenberg, G. Short-term behavioral effects of posterior pituitary peptides in mice. Peptides 1981, 2, 1–8. [Google Scholar] [CrossRef]
- Meisenberg, G. Short-term behavioural effects of neurohypophyseal hormones: Pharmacological characteristics. Neuropharmacology 1982, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, G.; Simmons, W.H. Behavioral effects of intracerebroventricularly administered neurohypophyseal hormone analogs in mice. Pharmacol. Biochem. Behav. 1982, 16, 819–825. [Google Scholar] [CrossRef]
- Boakes, R.J.; Ednie, J.M.; Edwardson, J.A.; Keith, A.B.; Sahgal, A.; Wright, C. Abnormal behavioural changes associated with vasopressin-induced barrel rotations. Brain Res. 1985, 326, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Van Wimersma Greidanus, T.B.; Kroodsma, J.M.; Pot, M.L.; Stevens, M.; Maigret, C. Neurohypophyseal hormones and excessive grooming behaviour. Eur. J. Pharmacol. 1990, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cormier, H.C.; Della-Maggiore, V.; Karatsoreos, I.N.; Koletar, M.M.; Ralph, M.R. Suprachiasmatic vasopressin and the circadian regulation of voluntary locomotor behavior. Eur. J. Neurosci. 2015, 41, 79–88. [Google Scholar] [CrossRef]
- Mlynarik, M.; Zelena, D.; Bagdy, G.; Makara, G.B.; Jezova, D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm. Behav. 2007, 51, 395–405. [Google Scholar] [CrossRef]
- Fodor, A.; Kovacs, K.B.; Balazsfi, D.; Klausz, B.; Pinter, O.; Demeter, K.; Daviu, N.; Rabasa, C.; Rotllant, D.; Nadal, R.; et al. Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol. Behav. 2016, 158, 100–111. [Google Scholar] [CrossRef]
- Winslow, J.; Insel, T.R. Vasopressin modulates male squirrel monkeys′ behavior during social separation. Eur. J. Pharmacol. 1991, 200, 95–101. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Drago, F.; Prange, A.J., Jr.; Pedersen, C.A. A comparison of grooming behavior potencies of neurohypophyseal nonapeptides. Regul. Pept. 1986, 14, 261–271. [Google Scholar] [CrossRef]
- Gaffori, O.; de Wied, D. Further evidence for a dissociation of peripheral and central effects of vasopressin. Psychoneuroendocrinology 1985, 10, 439–444. [Google Scholar] [CrossRef]
- Tendis, S.; Klingberg, F.; Schaeker, W. Arginine-vasopressin and lysine-vasopressin have different effects on spontaneous behaviour of rats. Biomed. Biochim. Acta 1987, 46, 719–725. [Google Scholar] [PubMed]
- Taylor, G.T.; Lerch, S.; Chourbaji, S. Marble burying as compulsive behaviors in male and female mice. Acta Neurobiol. Exp. 2017, 77, 254–260. [Google Scholar] [CrossRef]
- Egashira, N.; Tanoue, A.; Matsuda, T.; Koushi, E.; Harada, S.; Takano, Y.; Tsujimoto, G.; Mishima, K.; Iwasaki, K.; Fujiwara, M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 2007, 178, 123–127. [Google Scholar] [CrossRef]
- Balazsfi, D.; Pinter, O.; Klausz, B.; Kovacs, K.B.; Fodor, A.; Torok, B.; Engelmann, M.; Zelena, D. Restoration of peripheral V2 receptor vasopressin signaling fails to correct behavioral changes in Brattleboro rats. Psychoneuroendocrinology 2015, 51, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Schatz, K.C.; Kyne, R.F.; Parmeter, S.L.; Paul, M.J. Investigation of social, affective, and locomotor behavior of adolescent Brattleboro rats reveals a link between vasopressin’s actions on arousal and social behavior. Horm. Behav. 2018, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Assuah, F.B.; Emanuel, B.; Lacasse, B.M.; Beggs, J.; Lou, J.; Motta, F.C.; Nemzer, L.R.; Worth, R.; Cravens, G.D. A Literature Review of Similarities Between and Among Patients with Autism Spectrum Disorder and Epilepsy. Cureus 2023, 15, e33946. [Google Scholar] [CrossRef]
- Brang, D.; Ramachandran, V.S. Olfactory bulb dysgenesis, mirror neuron system dysfunction, and autonomic dysregulation as the neural basis for autism. Med. Hypotheses 2010, 74, 919–921. [Google Scholar] [CrossRef]
- Croiset, G.; De Wied, D. Proconvulsive effect of vasopressin; mediation by a putative V2 receptor subtype in the central nervous system. Brain Res. 1997, 759, 18–23. [Google Scholar] [CrossRef]
- Clynen, E.; Swijsen, A.; Raijmakers, M.; Hoogland, G.; Rigo, J.M. Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 2014, 50, 626–646. [Google Scholar] [CrossRef]
- Wurpel, J.N.; Dundore, R.L.; Barbella, Y.R.; Balaban, C.D.; Keil, L.C.; Severs, W.B. Barrel rotation evoked by intracerebroventricular vasopressin injections in conscious rats. II. Visual/vestibular interactions and efficacy of antiseizure drugs. Brain Res. 1986, 365, 30–41. [Google Scholar] [CrossRef]
- Krause, K.H.; Rascher, W.; Berlit, P. Plasma arginine vasopressin concentrations in epileptics under monotherapy. J. Neurol. 1983, 230, 193–196. [Google Scholar] [CrossRef]
- Javadian, N.; Rahimi, N.; Javadi-Paydar, M.; Doustimotlagh, A.H.; Dehpour, A.R. The modulatory effect of nitric oxide in pro- and anti-convulsive effects of vasopressin in PTZ-induced seizures threshold in mice. Epilepsy Res. 2016, 126, 134–140. [Google Scholar] [CrossRef]
- Fehr, F.S.; Stern, J.A. Peripheral physiological variables and emotion: The James-Lange theory revisited. Psychol. Bull. 1970, 74, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Villar de Araujo, T.; Ruesch, A.; Bankwitz, A.; Rufer, M.; Kleim, B.; Olbrich, S. Autism spectrum disorders in adults and the autonomic nervous system: Heart rate variability markers in the diagnostic procedure. J. Psychiatr. Res. 2023, 164, 235–242. [Google Scholar] [CrossRef]
- Chiu, H.T.; Ip, I.N.; Ching, F.N.Y.; Wong, B.P.; Lui, W.H.; Tse, C.S.; Wong, S.W.H. Resting Heart Rate Variability and Emotion Dysregulation in Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.M.; Amelynck, S.; Nystrom, P.; van Esch, L.; Van Lierde, T.; Warreyn, P.; Roeyers, H.; Noens, I.; Naulaers, G.; Boets, B.; et al. Investigating the development of the autonomic nervous system in infancy through pupillometry. J. Neural Transm. 2023, 130, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 14414. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress and the “extended” autonomic system. Auton. Neurosci. 2021, 236, 102889. [Google Scholar] [CrossRef]
- Barbier, A.; Chen, J.H.; Huizinga, J.D. Autism Spectrum Disorder in Children Is Not Associated with Abnormal Autonomic Nervous System Function: Hypothesis and Theory. Front. Psychiatry 2022, 13, 830234. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Vargas, N.T. Regulation of Body Temperature by Autonomic and Behavioral Thermoeffectors. Exerc. Sport. Sci. Rev. 2019, 47, 116–126. [Google Scholar] [CrossRef]
- Yuan, H.L.; Lai, C.Y.Y.; Wong, M.N.K.; Kwong, T.C.; Choy, Y.S.; Mung, S.W.Y.; Chan, C.C.H. Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review. Children 2022, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Curran, L.K.; Newschaffer, C.J.; Lee, L.C.; Crawford, S.O.; Johnston, M.V.; Zimmerman, A.W. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 2007, 120, e1386–e1392. [Google Scholar] [CrossRef] [PubMed]
- Good, P. Simplifying study of fever’s dramatic relief of autistic behavior. Clin. Nutr. ESPEN 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kasting, N.W.; Carr, D.B.; Martin, J.B.; Blume, H.; Bergland, R. Changes in cerebrospinal fluid and plasma vasopressin in the febrile sheep. Can. J. Physiol. Pharmacol. 1983, 61, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Sharples, P.M.; Seckl, J.R.; Human, D.; Lightman, S.L.; Dunger, D.B. Plasma and cerebrospinal fluid arginine vasopressin in patients with and without fever. Arch. Dis. Child. 1992, 67, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Richmond, C.A. The role of arginine vasopressin in thermoregulation during fever. J. Neurosci. Nurs. 2003, 35, 281–286. [Google Scholar] [CrossRef]
- Zelena, D. Vasopressin in health and disease with a focus on affective disorders. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 286–303. [Google Scholar] [CrossRef]
- Cooper, K.E.; Blahser, S.; Malkinson, T.J.; Merker, G.; Roth, J.; Zeisberger, E. Changes in body temperature and vasopressin content of brain neurons, in pregnant and non-pregnant guinea pigs, during fevers produced by Poly I:Poly C. Pflug. Arch. 1988, 412, 292–296. [Google Scholar] [CrossRef]
- Milton, N.G.; Hillhouse, E.W.; Milton, A.S. Does endogenous peripheral arginine vasopressin have a role in the febrile responses of conscious rabbits? J. Physiol. 1993, 469, 525–534. [Google Scholar] [CrossRef]
- Marinovic-Curin, J.; Marinovic-Terzic, I.; Bujas-Petkovic, Z.; Zekan, L.; Skrabic, V.; Dogas, Z.; Terzic, J. Slower cortisol response during ACTH stimulation test in autistic children. Eur. Child. Adolesc. Psychiatry 2008, 17, 39–43. [Google Scholar] [CrossRef]
- Corbett, B.A.; Simon, D. Adolescence, Stress and Cortisol in Autism Spectrum Disorders. OA Autism 2014, 1, 2. [Google Scholar] [PubMed]
- Jacobson, L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr. Physiol. 2014, 4, 715–738. [Google Scholar] [PubMed]
- Hamza, R.T.; Hewedi, D.H.; Ismail, M.A. Basal and adrenocorticotropic hormone stimulated plasma cortisol levels among Egyptian autistic children: Relation to disease severity. Ital. J. Pediatr. 2010, 36, 71. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef]
- Landgraf, R.; Kessler, M.S.; Bunck, M.; Murgatroyd, C.; Spengler, D.; Zimbelmann, M.; Nussbaumer, M.; Czibere, L.; Turck, C.W.; Singewald, N.; et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: Focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007, 31, 89–102. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef]
- Bleickardt, C.J.; Mullins, D.E.; Macsweeney, C.P.; Werner, B.J.; Pond, A.J.; Guzzi, M.F.; Martin, F.D.; Varty, G.B.; Hodgson, R.A. Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology 2009, 202, 711–718. [Google Scholar] [CrossRef]
- Lago, T.R.; Brownstein, M.J.; Page, E.; Beydler, E.; Manbeck, A.; Beale, A.; Roberts, C.; Balderston, N.; Damiano, E.; Pineles, S.L.; et al. The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: A randomized proof-of-concept study. Psychopharmacology 2021, 238, 2393–2403. [Google Scholar] [CrossRef]
- Simon, N.G.; Guillon, C.; Fabio, K.; Heindel, N.D.; Lu, S.F.; Miller, M.; Ferris, C.F.; Brownstein, M.J.; Garripa, C.; Koppel, G.A. Vasopressin antagonists as anxiolytics and antidepressants: Recent developments. Recent. Pat. CNS Drug Discov. 2008, 3, 77–93. [Google Scholar] [CrossRef]
- Griebel, G.; Beeske, S.; Stahl, S.M. The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: Results from 4 randomized, double-blind, placebo-controlled studies. J. Clin. Psychiatry 2012, 73, 1403–1411. [Google Scholar] [CrossRef]
- Griebel, G.; Holsboer, F. Neuropeptide receptor ligands as drugs for psychiatric diseases: The end of the beginning? Nat. Rev. Drug Discov. 2012, 11, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Aman, M.G.; Arnold, L.E. Neurochemical correlates of autistic disorder: A review of the literature. Res. Dev. Disabil. 2006, 27, 254–289. [Google Scholar] [CrossRef] [PubMed]
- Rodnyy, A.Y.; Kondaurova, E.M.; Tsybko, A.S.; Popova, N.K.; Kudlay, D.A.; Naumenko, V.S. The brain serotonin system in autism. Rev. Neurosci. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Delville, Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology 1994, 19, 593–601. [Google Scholar] [CrossRef]
- Landry, M.; Frasier, M.; Chen, Z.; Van De Kar, L.D.; Zhang, Y.; Garcia, F.; Battaglia, G. Fluoxetine treatment of prepubescent rats produces a selective functional reduction in the 5-HT2A receptor-mediated stimulation of oxytocin. Synapse 2005, 58, 102–109. [Google Scholar] [CrossRef]
- Ferris, C.F.; Stolberg, T.; Delville, Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus). Behav. Neurosci. 1999, 113, 804–815. [Google Scholar] [CrossRef]
- Couturier, J.L.; Speechley, K.N.; Steele, M.; Norman, R.; Stringer, B.; Nicolson, R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: Prevalence, severity, and pattern. J. Am. Acad. Child. Adolesc. Psychiatry 2005, 44, 815–822. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.L. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. J. Sleep. Res. 2008, 17, 197–206. [Google Scholar] [CrossRef]
- Delahaye, J.; Kovacs, E.; Sikora, D.; Hall, T.A.; Orlich, F.; Clemons, T.E.; van der Weerd, E.; Glick, L.; Kuhlthau, K. The relationship between Health-Related Quality of Life and sleep problems in children with Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2014, 8, 292–303. [Google Scholar] [CrossRef]
- Hoshino, K. Problems in the Development of the Sleep-Wake Rhythm Influence Neurodevelopmental Disorders in Children. Diagnostics 2023, 13, 1859. [Google Scholar] [CrossRef]
- Reghunandanan, V. Vasopressin in circadian function of SCN. J. Biosci. 2020, 45, 1–11. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Massoni, L.; Battaglini, S.; Cremone, I.M.; Carmassi, C.; Carpita, B. Biological correlates of altered circadian rhythms, autonomic functions and sleep problems in autism spectrum disorder. Ann. Gen. Psychiatry 2022, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Barassin, S.; Kalsbeek, A.; Saboureau, M.; Bothorel, B.; Vivien-Roels, B.; Malan, A.; Buijs, R.M.; Pevet, P. Potentiation effect of vasopressin on melatonin secretion as determined by trans-pineal microdialysis in the Rat. J. Neuroendocrinol. 2000, 12, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Rotarus, R.; Wivall, I.L.; Coculescu, M.; Brismar, K. The influence of vasopressin deficiency and acute desmopressin administration on melatonin secretion in patients with central diabetes insipidus. J. Endocrinol. Investig. 2004, 27, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.D.; Gerdes, M.B.; Williams, Z.J.; Moore, D.J.; Cascio, C.J. Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain. Rep. 2020, 5, e861. [Google Scholar] [CrossRef]
- Zheng, H.; Lim, J.Y.; Kim, Y.; Jung, S.T.; Hwang, S.W. The role of oxytocin, vasopressin, and their receptors at nociceptors in peripheral pain modulation. Front. Neuroendocrinol. 2021, 63, 100942. [Google Scholar] [CrossRef]
- Yang, F.J.; Ma, L.; Yang, J.; Zhu, Z.L.; Wang, C.H. Intranasal Vasopressin Relieves Orthopedic Pain After Surgery. Pain. Manag. Nurs. 2019, 20, 126–132. [Google Scholar] [CrossRef]
- Schorscher-Petcu, A.; Sotocinal, S.; Ciura, S.; Dupre, A.; Ritchie, J.; Sorge, R.E.; Crawley, J.N.; Hu, S.B.; Nishimori, K.; Young, L.J.; et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. 2010, 30, 8274–8284. [Google Scholar] [CrossRef]
- Legrain, V.; Iannetti, G.D.; Plaghki, L.; Mouraux, A. The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol. 2011, 93, 111–124. [Google Scholar] [CrossRef]
- Yang, J.; Song, C.Y.; Liu, W.Y.; Lin, B.C. Only through the brain nuclei, arginine vasopressin regulates antinociception in the rat. Peptides 2006, 27, 3341–3346. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.M.; Liu, W.Y.; Song, C.Y.; Lin, B.C. Through V2, not V1 receptor relating to endogenous opiate peptides, arginine vasopressin in periaqueductal gray regulates antinociception in the rat. Regul. Pept. 2006, 137, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Oztan, O.; Garner, J.P.; Partap, S.; Sherr, E.H.; Hardan, A.Y.; Farmer, C.; Thurm, A.; Swedo, S.E.; Parker, K.J. Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann. Neurol. 2018, 84, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.W. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.K.; Albers, H.E. Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr. Top. Behav. Neurosci. 2016, 27, 51–103. [Google Scholar] [PubMed]
- Bamshad, M.; Albers, H.E. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus). J. Comp. Neurol. 1996, 369, 252–263. [Google Scholar] [CrossRef]
- Keller, D.; Láng, T.; Cservenák, M.; Puska, G.; Barna, J.; Csillag, V.; Farkas, I.; Zelena, D.; Dóra, F.; Barteczko, L.; et al. A thalamo-preoptic pathway promoting social touch. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bosch, O.J.; Pfortsch, J.; Beiderbeck, D.I.; Landgraf, R.; Neumann, I.D. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J. Neuroendocrinol. 2010, 22, 420–429. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.B.; Ren, X.; Terwilliger, E.F.; Young, L.J. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron 2005, 47, 503–513. [Google Scholar] [CrossRef]
- Gabor, C.S.; Phan, A.; Clipperton-Allen, A.E.; Kavaliers, M.; Choleris, E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012, 126, 97–109. [Google Scholar] [CrossRef]
- Liu, Y.; Curtis, J.T.; Wang, Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 2001, 115, 910–919. [Google Scholar] [CrossRef]
- Wang, Z.; Young, L.J.; De Vries, G.J.; Insel, T.R. Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog. Brain Res. 1998, 119, 483–499. [Google Scholar] [PubMed]
- Insel, T.R.; Gelhard, R.; Shapiro, L.E. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience 1991, 43, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Borie, A.M.; Dromard, Y.; Guillon, G.; Olma, A.; Manning, M.; Muscatelli, F.; Desarmenien, M.G.; Jeanneteau, F. Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J. Clin. Investig. 2021, 131, e144450. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; Li, T.; Chen, X.; Gautam, P.; Haroon, E.; Thompson, R.R. Arginine Vasopressin Effects on Subjective Judgments and Neural Responses to Same and Other-Sex Faces in Men and Women. Front. Endocrinol. 2017, 8, 200. [Google Scholar] [CrossRef]
- Rood, B.D.; De Vries, G.J. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol. 2011, 519, 2434–2474. [Google Scholar] [CrossRef]
- Li, Y.; Mathis, A.; Grewe, B.F.; Osterhout, J.A.; Ahanonu, B.; Schnitzer, M.J.; Murthy, V.N.; Dulac, C. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 2017, 171, 1176–1190 e17. [Google Scholar] [CrossRef]
- Zink, C.F.; Stein, J.L.; Kempf, L.; Hakimi, S.; Meyer-Lindenberg, A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J. Neurosci. 2010, 30, 7017–7022. [Google Scholar] [CrossRef]
- Shou, X.J.; Xu, X.J.; Zeng, X.Z.; Liu, Y.; Yuan, H.S.; Xing, Y.; Jia, M.X.; Wei, Q.Y.; Han, S.P.; Zhang, R.; et al. A Volumetric and Functional Connectivity MRI Study of Brain Arginine-Vasopressin Pathways in Autistic Children. Neurosci. Bull. 2017, 33, 130–142. [Google Scholar] [CrossRef]
- Avino, T.A.; Barger, N.; Vargas, M.V.; Carlson, E.L.; Amaral, D.G.; Bauman, M.D.; Schumann, C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. USA 2018, 115, 3710–3715. [Google Scholar] [CrossRef]
- Walker, D.L.; Davis, M. Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008, 213, 29–42. [Google Scholar] [CrossRef]
- Paul, M.J.; Terranova, J.I.; Probst, C.K.; Murray, E.K.; Ismail, N.I.; de Vries, G.J. Sexually dimorphic role for vasopressin in the development of social play. Front. Behav. Neurosci. 2014, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.N.; Clauss, J.A.; Blackford, J.U. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology 2016, 41, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Bruzsik, B.; Biro, L.; Sarosdi, K.R.; Zelena, D.; Sipos, E.; Szebik, H.; Torok, B.; Mikics, E.; Toth, M. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol. Stress 2021, 15, 100415. [Google Scholar] [CrossRef]
- Clauss, J.A.; Avery, S.N.; Benningfield, M.M.; Blackford, J.U. Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety 2019, 36, 666–675. [Google Scholar] [CrossRef]
- Murray, E.K.; Varnum, M.M.; Fernandez, J.L.; de Vries, G.J.; Forger, N.G. Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J. Neuroendocrinol. 2011, 23, 906–914. [Google Scholar] [CrossRef][Green Version]
- Mohapatra, A.N.; Wagner, S. The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front. Psychiatry 2023, 14, 1205199. [Google Scholar] [CrossRef] [PubMed]
- Sailer, L.; Duclot, F.; Wang, Z.; Kabbaj, M. Consequences of prenatal exposure to valproic acid in the socially monogamous prairie voles. Sci. Rep. 2019, 9, 2453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yan, X.; Yang, L.; Zheng, X.; Chen, Y.; Liu, Y.; Ren, Y.; Peng, J.; Zhang, Y.; Huang, J.; et al. Effects of arginine vasopressin on the transcriptome of prefrontal cortex in autistic rat model. J. Cell. Mol. Med. 2022, 26, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Koob, G.F.; Bluthe, R.M.; Le Moal, M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988, 457, 143–147. [Google Scholar] [CrossRef]
- de Vries, G.J.; Buijs, R.M.; Swaab, D.F. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981, 218, 67–78. [Google Scholar] [CrossRef]
- Engelmann, M.; Landgraf, R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol. Behav. 1994, 55, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010, 58, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Compaan, J.C.; Buijs, R.M.; Pool, C.W.; De Ruiter, A.J.; Koolhaas, J.M. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res. Bull. 1993, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.P.; Jensen, C.L.; Franks, B.; Champagne, F.A. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 2012, 61, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Albers, H.E.; Wesolowski, S.M.; Goldman, B.D.; Luman, S.E. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science 1984, 224, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, A.C.; Whitman, D.C.; Albers, H.E. Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus). Brain Res. 1992, 569, 136–140. [Google Scholar] [CrossRef]
- Islam, M.T.; Maejima, T.; Matsui, A.; Mieda, M. Paraventricular hypothalamic vasopressin neurons induce self-grooming in mice. Mol. Brain 2022, 15, 47. [Google Scholar] [CrossRef]
- Verevkina, S.V. Some mechanisms of action of vasopressin on animal behavior. Neurosci. Behav. Physiol. 1988, 18, 306–310. [Google Scholar] [CrossRef]
- Elkabir, D.R.; Wyatt, M.E.; Vellucci, S.V.; Herbert, J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul. Pept. 1990, 28, 199–214. [Google Scholar] [CrossRef]
- Willcox, B.J.; Poulin, P.; Veale, W.L.; Pittman, Q.J. Vasopressin-induced motor effects: Localization of a sensitive site in the amygdala. Brain Res. 1992, 596, 58–64. [Google Scholar] [CrossRef]
- Maiti, A.; Shahid Salles, K.; Grassi, S.; Abood, L.G. Barrel rotation and prostration by vasopressin and nicotine in the vestibular cerebellum. Pharmacol. Biochem. Behav. 1986, 25, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus paper: Pathological role of the cerebellum in autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.; Courchesne, E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 2001, 49, 655–664. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, A.M.; Stoodley, C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Reith, R.M.; McKenna, J.; Wu, H.; Hashmi, S.S.; Cho, S.H.; Dash, P.K.; Gambello, M.J. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2013, 51, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, S.; Van ‘t Veer, A.E.; Witteman, J.; Meijer, W.M.; Van, I.M.H.; Bakermans-Kranenburg, M.J. Effects of vasopressin on neural processing of infant crying in expectant fathers. Horm. Behav. 2018, 103, 19–27. [Google Scholar] [CrossRef]
- Zhang, L.; Hernandez, V.S. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience 2013, 228, 139–162. [Google Scholar] [CrossRef]
- Rood, B.D.; Beck, S.G. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 2014, 260, 205–216. [Google Scholar] [CrossRef]
- Keck, M.E.; Welt, T.; Muller, M.B.; Uhr, M.; Ohl, F.; Wigger, A.; Toschi, N.; Holsboer, F.; Landgraf, R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology 2003, 28, 235–243. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. Corticotropin-releasing hormone and arginine vasopressin in depression focus on the human postmortem hypothalamus. Vitam. Horm. 2010, 82, 339–365. [Google Scholar]
- Wang, S.S.; Kamphuis, W.; Huitinga, I.; Zhou, J.N.; Swaab, D.F. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: The presence of multiple receptor imbalances. Mol. Psychiatry 2008, 13, 786–799, 741. [Google Scholar] [CrossRef]
- Grzeda, E.; Ziarniak, K.; Sliwowska, J.H. The paraventricular nucleus of the hypothalamus—The concertmaster of autonomic control. Focus on blood pressure regulation. Acta Neurobiol. Exp. 2023, 83, 34–44. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; de Zavalia, N.; Belforte, N.; Amir, S. In utero Exposure to Valproic-Acid Alters Circadian Organisation and Clock-Gene Expression: Implications for Autism Spectrum Disorders. Front. Behav. Neurosci. 2021, 15, 711549. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Fliers, E.; Hofman, M.A.; Swaab, D.F.; Buijs, R.M. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 2010, 22, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.D.; Burton, K.J.; Zhang, C.; Hu, S.B.; Zhou, Q.Y. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R824–R830. [Google Scholar] [CrossRef]
- Song, C.K.; Bartness, T.J.; Petersen, S.L.; Bittman, E.L. Co-expression of melatonin (MEL1a) receptor and arginine vasopressin mRNAs in the Siberian hamster suprachiasmatic nucleus. J. Neuroendocrinol. 2000, 12, 627–634. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Rikkers, M.; Vivien-Roels, B.; Pevet, P. Vasopressin and vasoactive intestinal peptide infused in the paraventricular nucleus of the hypothalamus elevate plasma melatonin levels. J. Pineal Res. 1993, 15, 46–52. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevention, Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Dworzynski, K.; Ronald, A.; Bolton, P.; Happe, F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Jacquemont, S. The effects of sex on prevalence and mechanisms underlying neurodevelopmental disorders. Handb. Clin. Neurol. 2020, 173, 327–339. [Google Scholar] [PubMed]
- Aulino, E.A.; Caldwell, H.K. Subtle sex differences in vasopressin mRNA expression in the embryonic mouse brain. J. Neuroendocrinol. 2020, 32, e12835. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.; Caffe, A.R.; De Vries, G.J. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: Sex differences and the influence of androgens. Brain Res. 1985, 325, 391–394. [Google Scholar] [CrossRef]
- DiBenedictis, B.T.; Nussbaum, E.R.; Cheung, H.K.; Veenema, A.H. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol. 2017, 525, 2549–2570. [Google Scholar] [CrossRef]
- Veenema, A.H.; Bredewold, R.; De Vries, G.J. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav. 2012, 61, 50–56. [Google Scholar] [CrossRef]
- Walsh, M.J.M.; Wallace, G.L.; Gallegos, S.M.; Braden, B.B. Brain-based sex differences in autism spectrum disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 2021, 31, 102719. [Google Scholar] [CrossRef]
- Smith, R.E.W.; Avery, J.A.; Wallace, G.L.; Kenworthy, L.; Gotts, S.J.; Martin, A. Sex Differences in Resting-State Functional Connectivity of the Cerebellum in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Dong, H.W.; Swanson, L.W. Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 2006, 494, 142–178. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Chung, W.C.; Kruijver, F.P.; Hofman, M.A.; Hestiantoro, A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol. Aging 2003, 24 (Suppl. S1), S1–S16; discussion S17–S19. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.J.; Best, W.; Sluiter, A.A. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983, 284, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.J.; Vanzo, R.J. Progesterone treatment of adult male rats suppresses arginine vasopressin expression in the bed nucleus of the stria terminalis and the centromedial amygdala. J. Neuroendocrinol. 2006, 18, 187–194. [Google Scholar] [CrossRef]
- Miller, M.; Bales, K.L.; Taylor, S.L.; Yoon, J.; Hostetler, C.M.; Carter, C.S.; Solomon, M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. 2013, 6, 91–102. [Google Scholar] [CrossRef]
- Khan, A.; Brodhead, A.E.; Schwartz, K.A.; Kolts, R.L.; Brown, W.A. Sex differences in antidepressant response in recent antidepressant clinical trials. J. Clin. Psychopharmacol. 2005, 25, 318–324. [Google Scholar] [CrossRef]
- Winslow, J.T.; Hastings, N.; Carter, C.S.; Harbaugh, C.R.; Insel, T.R. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 1993, 365, 545–548. [Google Scholar] [CrossRef]
- Insel, T.R.; Hulihan, T.J. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 1995, 109, 782–789. [Google Scholar] [CrossRef]
- Cho, M.M.; DeVries, A.C.; Williams, J.R.; Carter, C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 1999, 113, 1071–1079. [Google Scholar] [CrossRef]
- Jacob, D. Types of Medication for Autism. 2022. Available online: https://www.rxlist.com/types_of_medication_for_autism/drugs-condition.htm (accessed on 1 August 2023).
- Semple, B.D.; Raghupathi, R. A Pro-social Pill? The Potential of Pharmacological Treatments to Improve Social Outcomes After Pediatric Traumatic Brain Injury. Front. Neurol. 2021, 12, 714253. [Google Scholar] [CrossRef]
- Yeung, P.P.; Johnson, K.A.; Riesenberg, R.; Orejudos, A.; Riccobene, T.; Kalluri, H.V.; Malik, P.R.; Varughese, S.; Findling, R.L. Cariprazine in Pediatric Patients with Autism Spectrum Disorder: Results of a Pharmacokinetic, Safety and Tolerability Study. J. Child. Adolesc. Psychopharmacol. 2023, 33, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Appenrodt, E.; Bojanowska, E.; Janus, J.; Stempniak, B.; Guzek, J.W.; Schwarzberg, H. Effects of methylphenidate on oxytocin and vasopressin levels in pinealectomized rats during light-dark cycle. Pharmacol. Biochem. Behav. 1997, 58, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Appenrodt, E.; Schwarzberg, H. Methylphenidate-induced motor activity in rats: Modulation by melatonin and vasopressin. Pharmacol. Biochem. Behav. 2003, 75, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kawasaki, H.; Takasaki, K. Intracerebroventricular treatment with vasopressin V1-receptor antagonist inhibits centrally-mediated pressor response to clonidine in conscious rats. Jpn. J. Pharmacol. 1993, 63, 447–453. [Google Scholar] [CrossRef]
- Brown, G.M.; Mazurek, M.; Allen, D.; Szechtman, B.; Cleghorn, J.M. Dose-response profiles of plasma growth hormone and vasopressin after clonidine challenge in man. Psychiatry Res. 1990, 31, 311–320. [Google Scholar] [CrossRef]
- Alexander, S.L.; Irvine, C.H. The effect of the alpha-2-adrenergic agonist, clonidine, on secretion patterns and rates of adrenocorticotropic hormone and its secretagogues in the horse. J. Neuroendocrinol. 2000, 12, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.R.; Clarke, G.; Wakerley, J.B. Inhibition of supraoptic vasopressin neurones following systemic clonidine. Neuropharmacology 1994, 33, 211–214. [Google Scholar] [CrossRef]
- Pujol, C.N.; Pellissier, L.P.; Clement, C.; Becker, J.A.J.; Le Merrer, J. Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl. Psychiatry 2018, 8, 197. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, M.X.; Zhang, J.S.; Xu, X.J.; Shou, X.J.; Zhang, X.T.; Li, L.; Li, N.; Han, S.P.; Han, J.S. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: A prospective single-blinded controlled study. Res. Dev. Disabil. 2012, 33, 1136–1146. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child. Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Donovan, M.; Mackey, C.S.; Lynch, M.D.J.; Platt, G.N.; Brown, A.N.; Washburn, B.K.; Trickey, D.J.; Curtis, J.T.; Liu, Y.; Charles, T.C.; et al. Limosilactobacillus reuteri administration alters the gut-brain-behavior axis in a sex-dependent manner in socially monogamous prairie voles. Front. Microbiol. 2023, 14, 1015666. [Google Scholar] [CrossRef] [PubMed]
- Felix-Ortiz, A.C.; Febo, M. Gestational valproate alters BOLD activation in response to complex social and primary sensory stimuli. PLoS ONE 2012, 7, e37313. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Intorre, A.A.; French, J.A. Vasopressin and Oxytocin Reduce Food Sharing Behavior in Male, but Not Female Marmosets in Family Groups. Front. Endocrinol. 2017, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.C.; Balland, J.F.; Dhauna, J.; Yang, S.Y.; Traina, J.L.; Vazquez, J.; Bales, K.L. Early Intranasal Vasopressin Administration Impairs Partner Preference in Adult Male Prairie Voles (Microtus ochrogaster). Front. Endocrinol. 2017, 8, 145. [Google Scholar] [CrossRef]
- Jarcho, M.R.; Mendoza, S.P.; Mason, W.A.; Yang, X.; Bales, K.L. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes. Brain Behav. 2011, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Mohsin, N.; Karhson, D.S.; Sumiyoshi, R.D.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 2019, 11, eaau7356. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.A.; Teed, A.R.; Castle, E.; Dutcher, J.M.; Meyer, M.L.; Bryan, R.; Irwin, M.R.; Lieberman, M.D.; Eisenberger, N.I. Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: Evidence from a randomized controlled trial. Psychoneuroendocrinology 2019, 107, 124–132. [Google Scholar] [CrossRef]
- Ludwig, M.; Tobin, V.A.; Callahan, M.F.; Papadaki, E.; Becker, A.; Engelmann, M.; Leng, G. Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. J. Neuroendocrinol. 2013, 25, 655–667. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zheng, X.; Becker, B.; Lei, W.; Xu, X.; Kendrick, K.M. Intranasal vasopressin like oxytocin increases social attention by influencing top-down control, but additionally enhances bottom-up control. Psychoneuroendocrinology 2021, 133, 105412. [Google Scholar] [CrossRef]
- Price, D.; Burris, D.; Cloutier, A.; Thompson, C.B.; Rilling, J.K.; Thompson, R.R. Dose-Dependent and Lasting Influences of Intranasal Vasopressin on Face Processing in Men. Front. Endocrinol. 2017, 8, 220. [Google Scholar] [CrossRef]
- Wu, X.; Xu, P.; Luo, Y.J.; Feng, C. Differential Effects of Intranasal Vasopressin on the Processing of Adult and Infant Cues: An ERP Study. Front. Hum. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Coccaro, E.F.; Cremers, H.; McCarron, R.; Lu, S.F.; Brownstein, M.J.; Simon, N.G. A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: An fMRI study. Front. Syst. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Brunnlieb, C.; Munte, T.F.; Tempelmann, C.; Heldmann, M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013, 1499, 29–42. [Google Scholar] [CrossRef]
- Silver, J.M.; Anderson, K. Vasopressin treats the persistent feeling of coldness after brain injury. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Orr, S.P.; Lasko, N.B. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993, 48, 107–117. [Google Scholar] [CrossRef]
- Perras, B.; Pannenborg, H.; Marshall, L.; Pietrowsky, R.; Born, J.; Lorenz Fehm, H. Beneficial treatment of age-related sleep disturbances with prolonged intranasal vasopressin. J. Clin. Psychopharmacol. 1999, 19, 28–36. [Google Scholar] [CrossRef]
- Perras, B.; Droste, C.; Born, J.; Fehm, H.L.; Pietrowsky, R. Verbal memory after three months of intranasal vasopressin in healthy old humans. Psychoneuroendocrinology 1997, 22, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Baska, F.; Bozo, E.; Patocs, T. Vasopressin receptor antagonists: A patent summary (2018–2022). Expert. Opin. Ther. Pat. 2023, 33, 385–395. [Google Scholar] [CrossRef]
- Umbricht, D.; Del Valle Rubido, M.; Hollander, E.; McCracken, J.T.; Shic, F.; Scahill, L.; Noeldeke, J.; Boak, L.; Khwaja, O.; Squassante, L.; et al. A Single Dose, Randomized, Controlled Proof-of-Mechanism Study of a Novel Vasopressin 1a Receptor Antagonist (RG7713) in High-Functioning Adults with Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 1914–1923. [Google Scholar] [CrossRef]
- Bolognani, F.; Rubido, M.d.V.; Squassante, L.; Wandel, C.; Derks, M.; Murtagh, L.; Sevigny, J.; Khwaja, O.; Umbricht, D.; Fontoura, P. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 2019, 11, eaat7838. [Google Scholar] [CrossRef]
- Schnider, P.; Bissantz, C.; Bruns, A.; Dolente, C.; Goetschi, E.; Jakob-Roetne, R.; Kunnecke, B.; Mueggler, T.; Muster, W.; Parrott, N.; et al. Discovery of Balovaptan, a Vasopressin 1a Receptor Antagonist for the Treatment of Autism Spectrum Disorder. J. Med. Chem. 2020, 63, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; Jacob, S.; Jou, R.; McNamara, N.; Sikich, L.; Tobe, R.; Smith, J.; Sanders, K.; Squassante, L.; Murtagh, L.; et al. Balovaptan vs. Placebo for Social Communication in Childhood Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Veenstra-VanderWeele, J.; Murphy, D.; McCracken, J.; Smith, J.; Sanders, K.; Meyenberg, C.; Wiese, T.; Deol-Bhullar, G.; Wandel, C.; et al. Efficacy and safety of balovaptan for socialisation and communication difficulties in autistic adults in North America and Europe: A phase 3, randomised, placebo-controlled trial. Lancet Psychiatry 2022, 9, 199–210. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, Z.; Yan, H.; Guo, W. Efficacy of Treatments Targeting Hypothalamic-Pituitary-Adrenal Systems for Major Depressive Disorder: A Meta-Analysis. Front. Pharmacol. 2021, 12, 732157. [Google Scholar] [CrossRef]
- Chaki, S. Vasopressin V1B Receptor Antagonists as Potential Antidepressants. Int. J. Neuropsychopharmacol. 2021, 24, 450–463. [Google Scholar] [CrossRef]
- Inatani, S.; Mizuno-Yasuhira, A.; Kamiya, M.; Nishino, I.; Sabia, H.D.; Endo, H. Prediction of a clinically effective dose of THY1773, a novel V(1B) receptor antagonist, based on preclinical data. Biopharm. Drug Dispos. 2021, 42, 204–217. [Google Scholar] [CrossRef]
- Kamiya, M.; Sabia, H.D.; Marella, J.; Fava, M.; Nemeroff, C.B.; Umeuchi, H.; Iijima, M.; Chaki, S.; Nishino, I. Efficacy and safety of TS-121, a novel vasopressin V(1B) receptor antagonist, as adjunctive treatment for patients with major depressive disorder: A randomized, double-blind, placebo-controlled study. J. Psychiatr. Res. 2020, 128, 43–51. [Google Scholar] [CrossRef]
- Katz, D.A.; Locke, C.; Greco, N.; Liu, W.; Tracy, K.A. Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized Phase 1b trial. Brain Behav. 2017, 7, e00628. [Google Scholar] [CrossRef]
- Watanabe, T.; Abe, O.; Kuwabara, H.; Yahata, N.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; Takao, H.; Kawakubo, Y.; et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: A randomized trial. JAMA Psychiatry 2014, 71, 166–175. [Google Scholar] [CrossRef]
- Hu, L.; Du, X.; Jiang, Z.; Song, C.; Liu, D. Oxytocin treatment for core symptoms in children with autism spectrum disorder: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 1357–1363. [Google Scholar] [CrossRef]
- Carson, D.S.; Howerton, C.L.; Garner, J.P.; Hyde, S.A.; Clark, C.L.; Hardan, A.Y.; Penn, A.A.; Parker, K.J. Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides 2014, 61, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zelena, D. Adenoviruses for better rain function. Integr. Physiol. 2021, 2, 254–260. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Li, B.; Wu, X.; Li, T.; Wang, L.; Zhang, Y.; Lin, W.; Qu, C.; Feng, C. Intranasal vasopressin modulates resting state brain activity across multiple neural systems: Evidence from a brain imaging machine learning study. Neuropharmacology 2021, 190, 108561. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Hadizadeh, M.; Cheong, J.P.G. Global Trends in Physical-Activity Research of Autism: Bibliometric Analysis Based on the Web of Science Database (1980–2021). Int. J. Environ. Res. Public Health 2022, 19, 7278. [Google Scholar] [CrossRef]
- Zuvekas, S.H.; Grosse, S.D.; Lavelle, T.A.; Maenner, M.J.; Dietz, P.; Ji, X. Healthcare Costs of Pediatric Autism Spectrum Disorder in the United States, 2003–2015. J. Autism. Dev. Disord. 2021, 51, 2950–2958. [Google Scholar] [CrossRef]
- Liptak, G.S.; Stuart, T.; Auinger, P. Health care utilization and expenditures for children with autism: Data from U.S. national samples. J. Autism. Dev. Disord. 2006, 36, 871–879. [Google Scholar] [CrossRef]
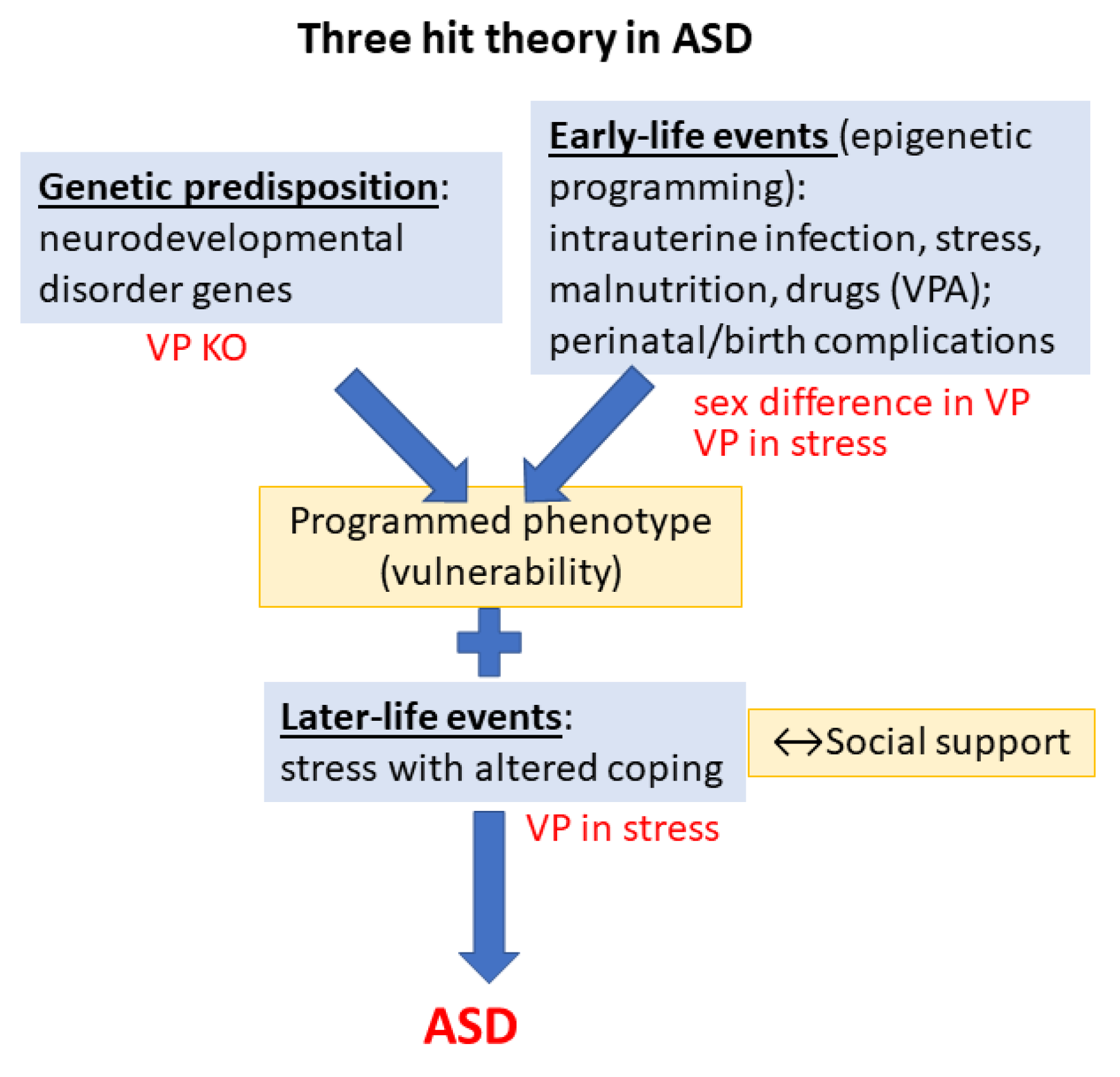
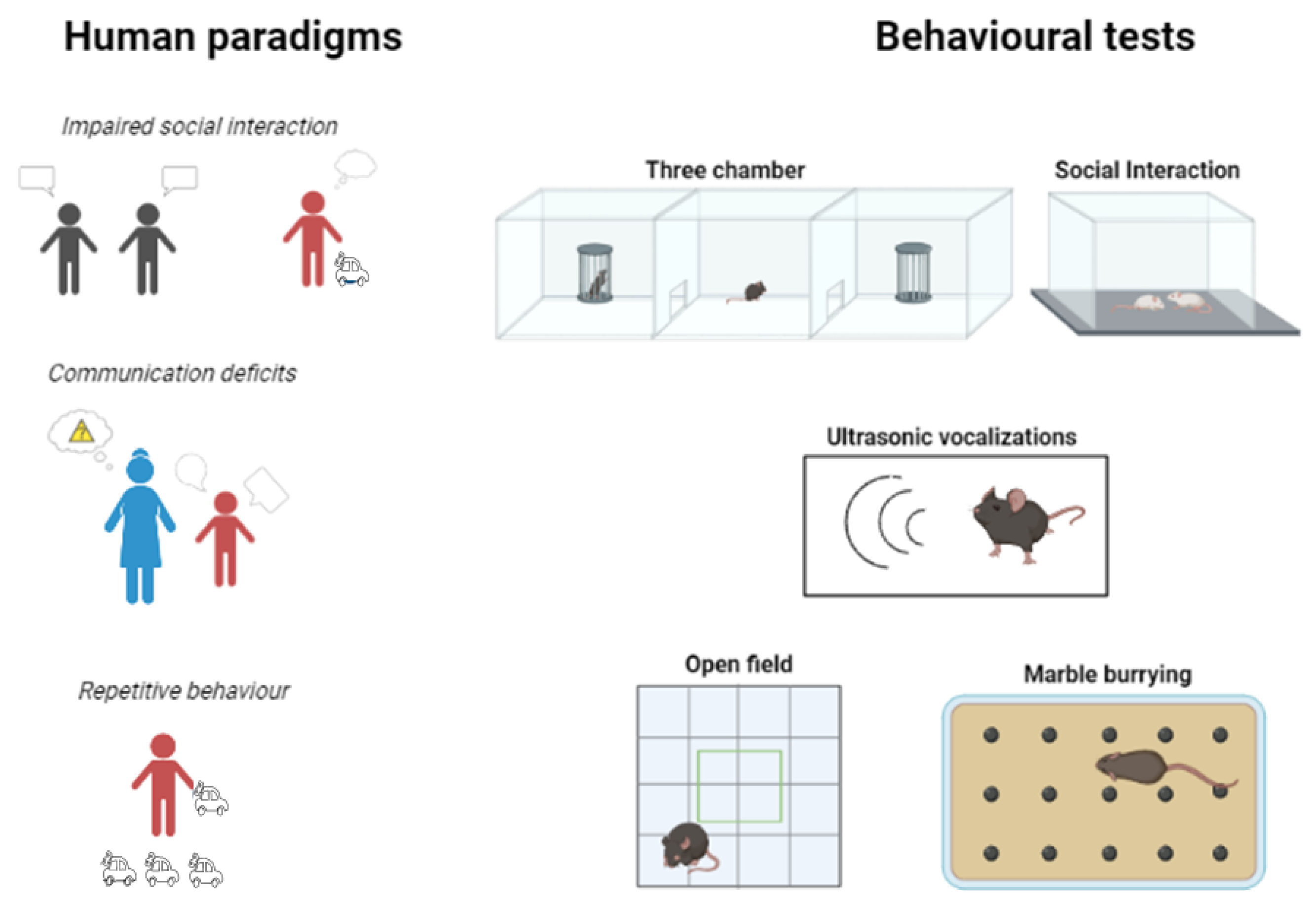
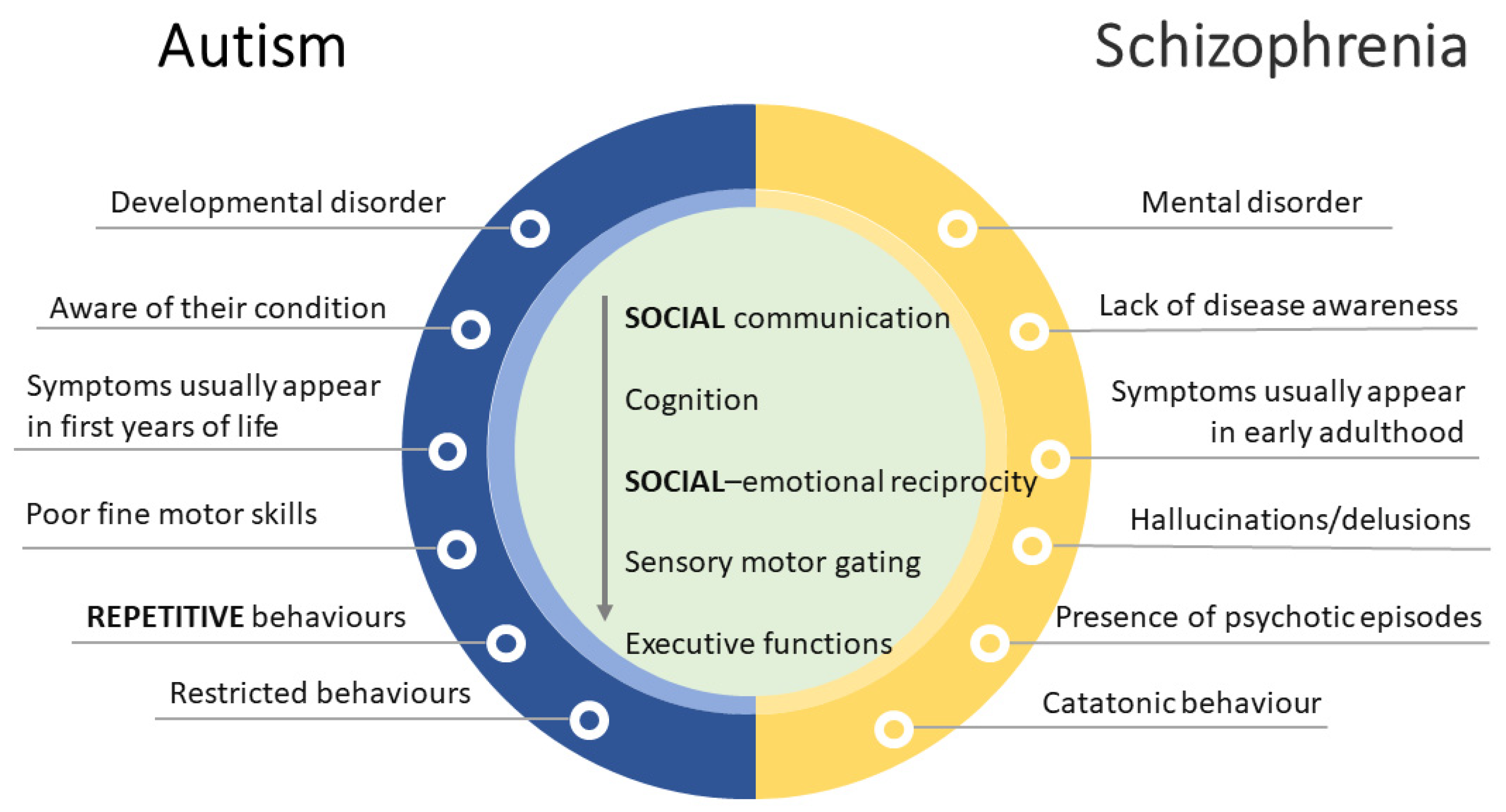
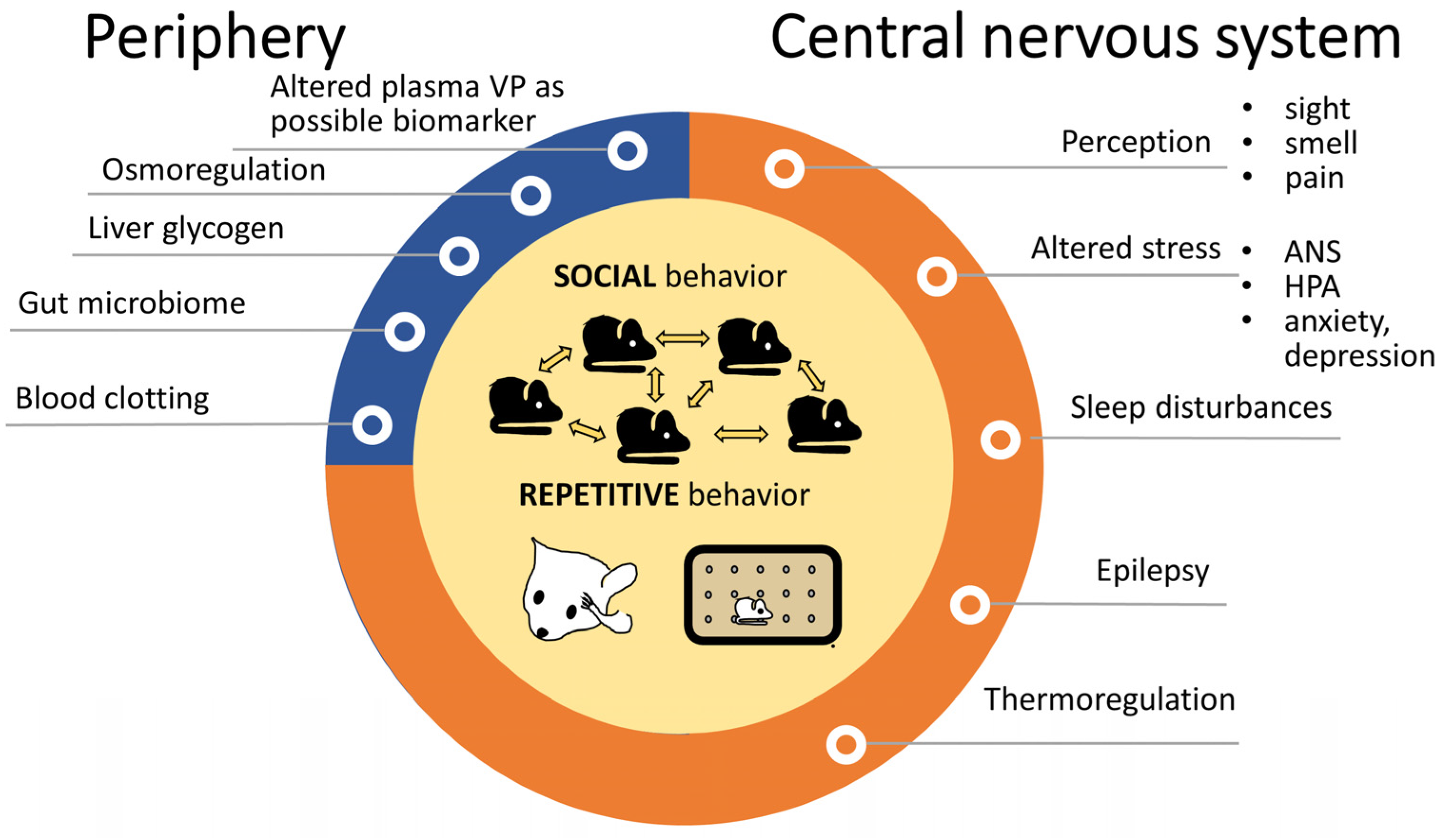
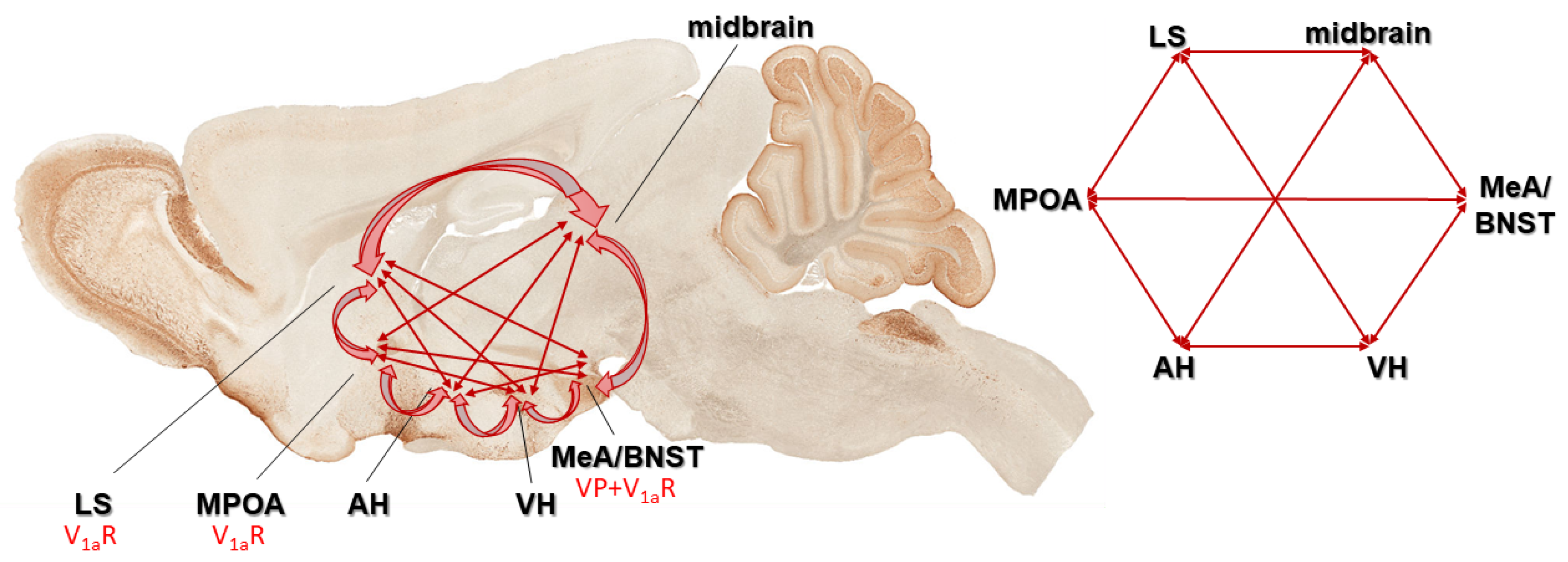
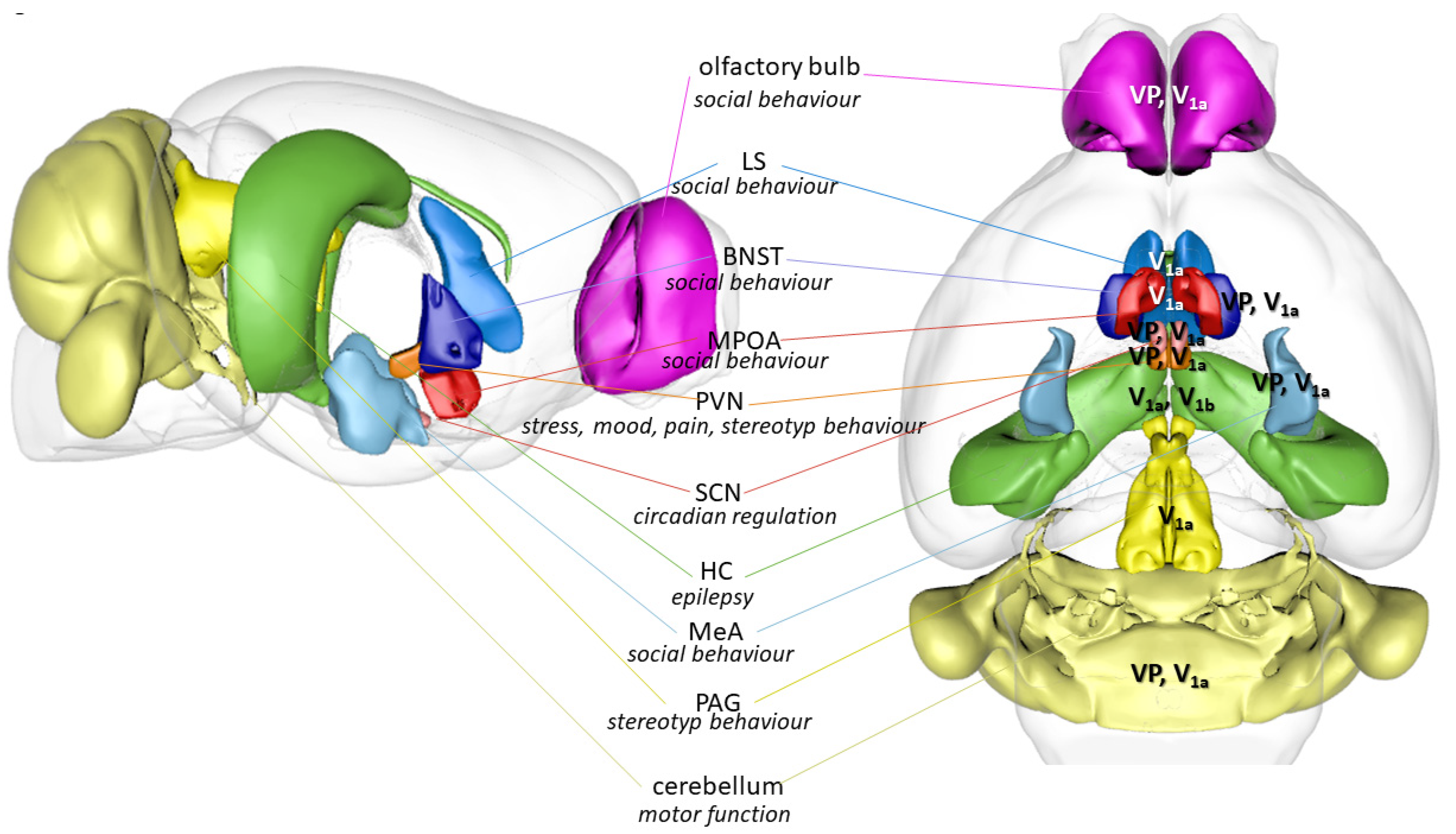
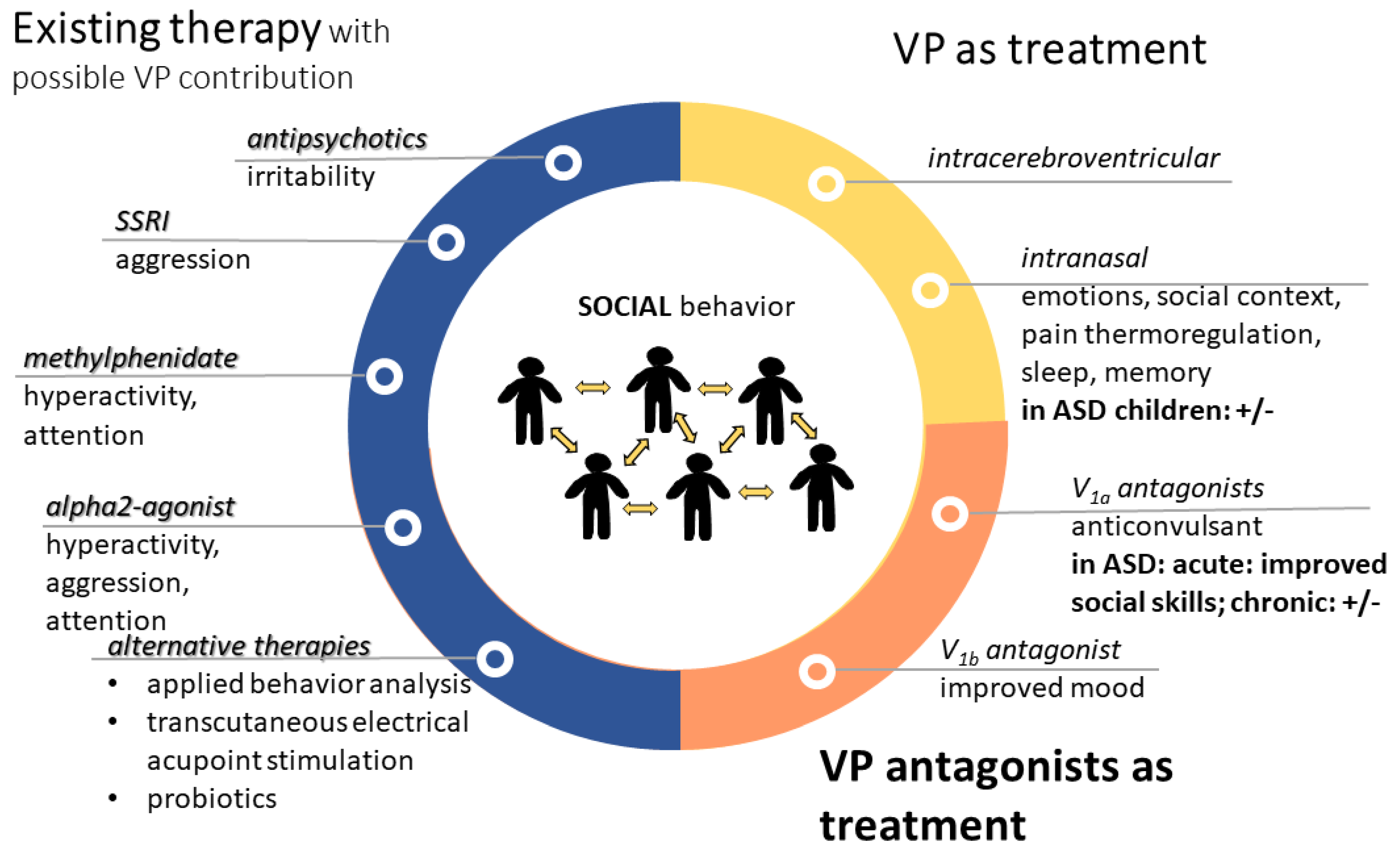
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
László, K.; Vörös, D.; Correia, P.; Fazekas, C.L.; Török, B.; Plangár, I.; Zelena, D. Vasopressin as Possible Treatment Option in Autism Spectrum Disorder. Biomedicines 2023, 11, 2603. https://doi.org/10.3390/biomedicines11102603
László K, Vörös D, Correia P, Fazekas CL, Török B, Plangár I, Zelena D. Vasopressin as Possible Treatment Option in Autism Spectrum Disorder. Biomedicines. 2023; 11(10):2603. https://doi.org/10.3390/biomedicines11102603
Chicago/Turabian StyleLászló, Kristóf, Dávid Vörös, Pedro Correia, Csilla Lea Fazekas, Bibiána Török, Imola Plangár, and Dóra Zelena. 2023. "Vasopressin as Possible Treatment Option in Autism Spectrum Disorder" Biomedicines 11, no. 10: 2603. https://doi.org/10.3390/biomedicines11102603
APA StyleLászló, K., Vörös, D., Correia, P., Fazekas, C. L., Török, B., Plangár, I., & Zelena, D. (2023). Vasopressin as Possible Treatment Option in Autism Spectrum Disorder. Biomedicines, 11(10), 2603. https://doi.org/10.3390/biomedicines11102603






