Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Administration
2.3. Behavioral Test
2.3.1. Catalepsy Test
2.3.2. Open-Field Spontaneous Locomotor Activity
2.3.3. Rota-Rod Test
2.4. Tissue Preparation
Immunohistochemistry
2.5. Quantification of Immunohistochemistry
2.6. Statistical Analyses
3. Results
3.1. Antipsychotic Treatment-Induced Extrapyramidal Side Effects (EPS)
3.1.1. Catalepsy Test
3.1.2. Open-Field Exploration
3.1.3. Rota-Rod Test
3.2. Effects of Chronic Drug Treatment on FosB and DARPP-32 Immunoreactivity in Neostriatum
3.2.1. FosB Labeling in the Neostriatum and NAc
3.2.2. DARPP32-Thr34 and DARPP32-Thr75 Labeling in the Neostriatum and NAc
3.3. Correlations
4. Discussion
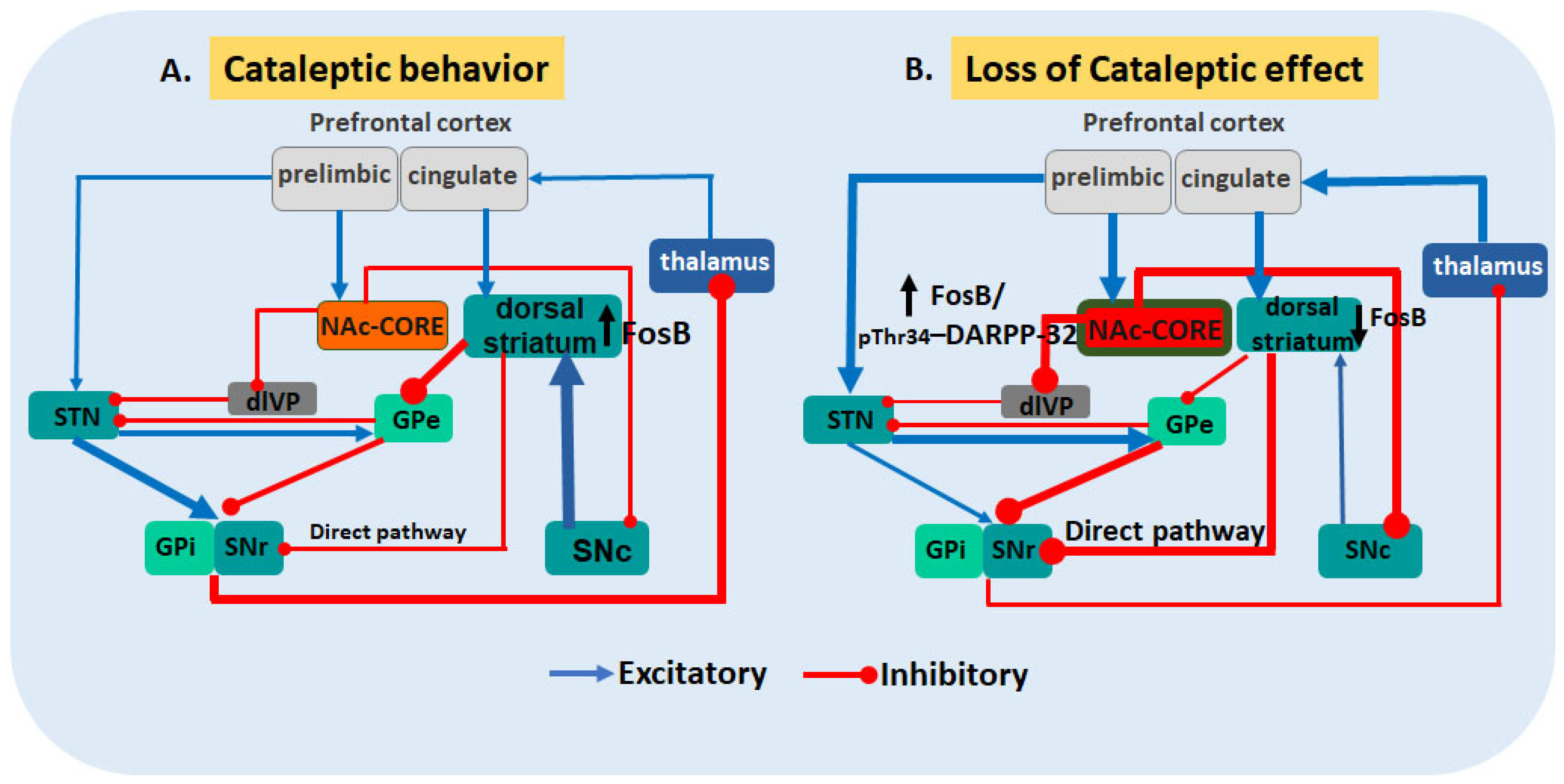
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peacock, L.; Solgaard, T.; Lublin, H.; Gerlach, J. Clozapine versus Typical Antipsychotics: A Retro- and Prospective Study of Extrapyramidal Side Effects. Psychopharmacology 1996, 124, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Gareri, P.; De Fazio, P.; Stilo, M.; Ferreri, G.; De Sarro, G. Conventional and Atypical Antipsychotics in the Elderly: A Review. Clin. Drug Investig. 2003, 23, 287–322. [Google Scholar] [CrossRef] [PubMed]
- Breekveldt-Postma, N.S.; Schillevoort, I.; Nolen, W.A.; Veraart, C.P.W.M.; Herings, R.M.C. Selective Prescribing of Atypical Antipsychotics. Pharmacoepidemiol. Drug Saf. 2005, 14, 25–30. [Google Scholar] [CrossRef]
- Miller, L.; Mohr, F.; Umbricht, D.; Woerner, M.; Fleischhacker, W.; Lieberman, J. Metoclopramide-Induced Movement Disorders: Clinical Findings with a Review of the Literature. Arch. Intern. Med. 1998, 149, 2486–2492. [Google Scholar] [CrossRef]
- Bishnoi, M.; Chopra, K.; Kulkarni, S.K. Activation of Striatal Inflammatory Mediators and Caspase-3 Is Central to Haloperidol-Induced Orofacial Dyskinesia. Eur. J. Pharmacol. 2008, 590, 241–245. [Google Scholar] [CrossRef]
- Ng, Q.X.; Chong, J.W.X.; Chee, K.T. Add-on Pharmacotherapy for Patients with First-Episode Schizophrenia: A Clinical Perspective. Eur. J. Clin. Pharmacol. 2021, 77, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Coppens, H.J.; Sebens, J.B.; Korf, J. Catalepsy, Fos Protein, and Dopamine Receptor Occupancy after Long-Term Haloperidol Treatment. Pharmacol. Biochem. Behav. 1995, 51, 175–182. [Google Scholar] [CrossRef]
- Glazer, W.M. Extrapyramidal Side Effects, Tardive Dyskinesia, and the Concept of Atypicality. J. Clin. Psychiatry 2000, 61, 16–21. [Google Scholar]
- Klawans, H.L.; Goetz, C.G.; Perlik, S. Tardive Dyskinesia: Review and Update. Am. J. Psychiatry 1980, 137, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Lévesque, D.; Blanchet, P.J. Upregulation of Dopamine D3, Not D2, Receptors Correlates with Tardive Dyskinesia in a Primate Model. Mov. Disord. 2014, 29, 1125–1133. [Google Scholar] [CrossRef]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for Schizophrenia: A Critical Review of Pharmacology and Mechanisms of Action of Antipsychotic Drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Ushijima, I.; Mizuki, Y.; Yamada, M. Alteration of Cataleptic Responses Induced by Dopamine Receptor Antagonists after Chronic Cocaine Administration in Mice. Eur. J. Pharmacol. 1995, 285, 55–59. [Google Scholar] [CrossRef]
- Kapur, S.; Barsoum, S.C.; Seeman, P. Dopamine D2 Receptor Blockade by Haloperidol: 3H-Raclopride Reveals Much Higher Occupancy than EEDQ. Neuropsychopharmacology 2000, 23, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Zipursky, R.; Remington, G. Clinical and Theoretical Implications of 5-HT2 and D2 Receptor Occupancy of Clozapine, Risperidone, and Olanzapine in Schizophrenia. Am. J. Psychiatry 1999, 156, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, H.H.M.; Bunzoe, J.R.; Guan, H.-C.; Sunahara, R.K.; Seeman, P.; Niznik, H.B.; Civelli, O. Cloning of the Gene for a Human Dopamine D4 Receptor with High Affinity for the Antipsychotic Clozapine. Lett. Nat. 1991, 350, 610–614. [Google Scholar] [CrossRef]
- Egan, C.T.; Herrick-Davis, K.; Teitler, M. Creation of a Constitutively Activated State of the 5-Hydroxytryptamine2A Receptor by Site-Directed Mutagenesis: Inverse Agonist Activity of Antipsychotic Drugs. J. Pharmacol. Exp. Ther. 1998, 286, 85–90. [Google Scholar]
- Matsubara, S.; Matsubara, R.; Kusumi, I.; Koyama, T.; Yamashita, I. Dopamine D1, D2 and Serotonin2 Receptor Occupation by Typical and Atypical Antipsychotic Drugs in Vivo. J. Pharmacol. Exp. Ther. 1993, 265, 498–508. [Google Scholar] [PubMed]
- Millan, M.J.; Schreiber, R.; Dekeyne, A.; Rivet, J.; Bervoets, K.; Mavridis, M.; Sebban, C.; Maurel-remy, S.; Newman-tancredi, A.; Spedding, M.; et al. Antipsychotic with Marked Serotonin (5-HT) 1A Agonist Properties: II. Functional Profile in Comparison to Clozapine and Haloperidol. Pharmacology 1998, 286, 1356–1373. [Google Scholar]
- Cussac, D.; Duqueyroix, D.; Newman-Tancredi, A.; Millan, M.J. Stimulation by Antipsychotic Agents of Mitogen-Activated Protein Kinase (MAPK) Coupled to Cloned, Human (h)Serotonin (5-HT)1A Receptors. Psychopharmacology 2002, 162, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Beasley, C.M.; Dellva, M.A.; Tamura, R.N.; Morgenstern, H.; Glazer, W.M.; Ferguson, K.; Tollefson, G.D. Randomised Double-Blind Comparison of the Incidence of Tardive Dyskinesia in Patients with Schizophrenia during Long-Term Treatment with Olanzapine or Haloperidol. Br. J. Psychiatry 1999, 174, 23–30. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.M.; Gommeren, W.; Luyten, W.H.M.L.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone Compared with New and Reference Antipsychotic Drugs: In Vitro and in Vivo Receptor Binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Devoto, P.; Flore, G. On the Origin of Cortical Dopamine: Is It a Co-Transmitter in Noradrenergic Neurons? Curr. Neuropharmacol. 2006, 4, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.; Craig, T.K.J.; McConnell, B.; Barnes, T.R.E. Side-Effect Monitoring of Continuing LAI Antipsychotic Medication in UK Adult Mental Health Services. Ther. Adv. Psychopharmacol. 2021, 11, 2045125321991278. [Google Scholar] [CrossRef] [PubMed]
- Bonito-Oliva, A.; Pallottino, S.; Bertran-Gonzalez, J.; Girault, J.A.; Valjent, E.; Fisone, G. Haloperidol Promotes MTORC1-Dependent Phosphorylation of Ribosomal Protein S6 via Dopamine- and CAMP-Regulated Phosphoprotein of 32 KDa and Inhibition of Protein Phosphatase-1. Neuropharmacology 2013, 72, 197–203. [Google Scholar] [CrossRef]
- Robertson, G.S.; Matsumura, H.; Fibiger, H.C. Induction Patterns of Fos-like Immunoreactivity in the Forebrain as Predictors of Atypical Antipsychotic Activity. J. Pharmacol. Exp. Ther. 1994, 271, 1058–1066. [Google Scholar] [PubMed]
- Merchant, K.M.; Dorsa, D.M. Differential Induction of Neurotensin and C-Fos Gene Expression by Typical versus Atypical Antipsychotics. Proc. Natl. Acad. Sci. USA 1993, 90, 3447–3451. [Google Scholar] [CrossRef]
- Hervé, D.; Le Moine, C.; Corvol, J.C.; Belluscio, L.; Ledent, C.; Fienberg, A.A.; Jaber, M.; Studler, J.M.; Girault, J.A. Galpha(Olf) Levels Are Regulated by Receptor Usage and Control Dopamine and Adenosine Action in the Striatum. J. Neurosci. 2001, 21, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Bonito-Oliva, A.; Feyder, M.; Fisone, G. Deciphering the Actions of Antiparkinsonian and Antipsychotic Drugs on CAMP/DARPP-32 Signaling. Front. Neuroanat. 2011, 5, 38. [Google Scholar] [CrossRef]
- Konradi, C.; Heckers, S. Haloperidol-Induced Fos Expression in Striatum Is Dependent upon Transcription Factor Cyclic AMP Response Element Binding Protein. Neuroscience 1995, 65, 1051–1061. [Google Scholar] [CrossRef]
- Håkansson, K.; Galdi, S.; Hendrick, J.; Snyder, G.; Greengard, P.; Fisone, G. Regulation of Phosphorylation of the GluR1 AMPA Receptor by Dopamine D2 Receptors. J. Neurochem. 2006, 96, 482–488. [Google Scholar] [CrossRef]
- Bertran-gonzalez, J.; Håkansson, K.; Borgkvist, A.; Brami-cherrier, K.; Usiello, A.; Greengard, P.; Hervé, D.; Girault, J.; Valjent, E. Histone H3 Phosphorylation Is Under the Opposite Tonic Control of Dopamine D2 and Adenosine A2A Receptors in Striatopallidal Neurons. Neuropsychopharmacology 2009, 34, 1710–1720. [Google Scholar] [CrossRef]
- Nishi, A.; Watanabe, Y.; Higashi, H.; Tanaka, M.; Nairn, A.C.; Greengard, P. Glutamate Regulation of DARPP-32 Phosphorylation in Neostriatal Neurons Involves Activation of Multiple Signaling Cascades. Proc. Natl. Acad. Sci. USA 2005, 102, 3–8. [Google Scholar] [CrossRef]
- Svenningsson, P.; Nishi, A.; Fisone, G.; Girault, J.-A.; Nairn, A.C.; Greengard, P. DARPP-32: An Integrator of Neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 269–296. [Google Scholar] [CrossRef]
- Greengard, P.; Allen, P.B.; Nairn, A.C. Beyond the Dopamine Receptor: The DARPP-32/Protein Phosphatase-1 Cascade. Neuron 1999, 23, 435–447. [Google Scholar] [CrossRef]
- Yuste, J.E.; Echeverry, M.B.; Ros-Bernal, F.; Gomez, A.; Ros, C.M.; Campuzano, C.M.; Fernandez-Villalba, E.; Herrero, M.T. 7-Nitroindazole down-Regulates Dopamine/DARPP-32 Signaling in Neostriatal Neurons in a Rat Model of Parkinson’s Disease. Neuropharmacology 2012, 63, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Bateup, H.S.; Santini, E.; Shen, W.; Birnbaum, S.; Valjent, E.; Surmeier, D.J.; Fisone, G.; Nestler, E.J.; Greengard, P. Distinct Subclasses of Medium Spiny Neurons Differentially Regulate Striatal Motor Behaviors. Proc. Natl. Acad. Sci. USA 2010, 107, 14845–14850. [Google Scholar] [CrossRef]
- Cohen, B.M.; Keck, P.E.; Satlin, A.; Cole, J.O. Prevalence and Severity of Akathisia in Patients on Clozapine. Biol. Psychiatry 1991, 29, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, A.A.; Ghaemi, S.N. Side Effects of Atypical Antipsychotics: Extrapyramidal Symptoms and the Metabolic Syndrome. Harv. Rev. Psychiatry 2006, 14, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. Biomed. Res. Int. 2014, 2014, 656370. [Google Scholar] [CrossRef] [PubMed]
- Josiassen, R.C.; Joseph, A.; Kohegyi, E.; Stokes, S.; Dadvand, M.; Paing, W.W.; Shaughnessy, R.A. Clozapine Augmented with Risperidone in the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Psychiatry 2005, 162, 130–136. [Google Scholar] [CrossRef]
- Chertkow, Y.; Weinreb, O.; Youdim, M.B.H.; Silver, H. Molecular Mechanisms Underlying Synergistic Effects of SSRI-Antipsychotic Augmentation in Treatment of Negative Symptoms in Schizophrenia. J. Neural. Transm. 2009, 116, 1529–1541. [Google Scholar] [CrossRef]
- Turrone, P.; Remington, G.; Kapur, S.; Nobrega, J.N. Continuous but Not Intermittent Olanzapine Infusion Induces Vacuous Chewing Movements in Rats. Biol. Psychiatry 2005, 57, 406–411. [Google Scholar] [CrossRef]
- Prieto, S.C.; Silva, J.C.S.; De Lima, M.O.; Almeida, M.C.; Echeverry, M.B. Cross-Tolerance between Nitric Oxide Synthase Inhibition and Atypical Antipsychotics Modify Nicotinamide-Adenine-Dinucleotide Phosphate-Diaphorase Activity in Mouse Lateral Striatum. Behav. Pharmacol. 2019, 30, 67–78. [Google Scholar] [CrossRef]
- Sebens, J.B.; Koch, T.; Horst, G.J.T.; Korf, J. Olanzapine-Induced Fos Expression in the Rat Forebrain; Cross-Tolerance with Haloperidol and Clozapine. Eur. J. Pharmacol. 1998, 353, 13–21. [Google Scholar] [CrossRef]
- Bardin, L.; Kleven, M.S.; Barret-Grévoz, C.; Depoortère, R.; Newman-Tancredi, A. Antipsychotic-like vs Cataleptogenic Actions in Mice of Novel Antipsychotics Having D2 Antagonist and 5-HT1A Agonist Properties. Neuropsychopharmacology 2006, 31, 1869–1879. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Yovtcheva, S.P.; Stanley-Tilt, C.; Moles, J.K. Reemergence of Tardive Dyskinesia after Discontinuation of Clozapine Treatment. Schizophr. Res. 2000, 46, 107–109. [Google Scholar] [CrossRef]
- Ahmed, S.; Chengappa, K.N.R.; Naidu, V.R.; Baker, R.W.; Parepally, H.; Schooler, N.R. Clozapine Withdrawal-Emergent Dystonias and Dyskinesias: A Case Series. J. Clin. Psychiatry 1998, 59, 472–477. [Google Scholar] [CrossRef]
- Alblowi, M.A.; Alosaimi, F.D. Tardive Dyskinesia Occurring in a Young Woman after Withdrawal of an Atypical Antipsychotic Drug. Neurosciences 2015, 20, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Bunsey, M.D.; Giordano, M.; Norman, A.B. The Catalepsy Test: Its Ups and Downs. Behav. Neurosci. 1988, 102, 748–759. [Google Scholar] [CrossRef]
- Echeverry, M.B.; Salgado, M.L.; Ferreira, F.R.; da-Silva, C.A.; Del Bel, E.A. Intracerebroventricular Administration of Nitric Oxide-Sensitive Guanylyl Cyclase Inhibitors Induces Catalepsy in Mice. Psychopharmacology 2007, 194, 271–278. [Google Scholar] [CrossRef]
- Del Bel, E.; Souza, A.; Guimarães, F.; Da-Silva, C.; Nucci-da-Silva, L. Motor Effects of Acute and Chronic Inhibition of Nitric Oxide Synthesis in Mice. Psychopharmacology 2002, 161, 32–37. [Google Scholar] [CrossRef]
- Dekundy, A.; Lundblad, M.; Danysz, W.; Cenci, M.A. Modulation of L-DOPA-Induced Abnormal Involuntary Movements by Clinically Tested Compounds: Further Validation of the Rat Dyskinesia Model. Behav. Brain Res. 2007, 179, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, L.I.; Hadeishi, Y.; Ulery, P.G.; Barrot, M.; Monteggia, L.; Duman, R.S.; Nestler, E.J. Induction of ΔFosB in Reward-Related Brain Structures after Chronic Stress. J. Neurosci. 2004, 24, 10594–10602. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, D.; Laorden, M.L.; Milanés, M.V. Regulation of Pleiotrophin, Midkine, Receptor Protein Tyrosine Phosphatase β/ζ, and Their Intracellular Signaling Cascades in the Nucleus Accumbens during Opiate Administration. Int. J. Neuropsychopharmacol. 2016, 19, pyv077. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, R.; Vallejo, M.; Elibol, B.; Graybiel, A.M. D1-Class Dopamine Receptors Influence Cocaine-Induced Persistent Expression of Fos-Related Proteins in Striatum. Neuroreport 1996, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.; Zhu, H.; Martin, A.B.; Lee, M.; Ortiz, O.; Hiroi, N.; Moratalla, R. Chronic Treatment with Atypical Neuroleptics Induces Striosomal FosB/DeltaFosB Expression in Rats. Biol. Psychiatry 2004, 55, 457–463. [Google Scholar] [CrossRef]
- McClung, C.A.; Ulery, P.G.; Perrotti, L.I.; Zachariou, V.; Berton, O.; Nestler, E.J. ΔfosB: A Molecular Switch for Long-Term Adaptation in the Brain. Mol. Brain Res. 2004, 132, 146–154. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K. The Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Burton, A.C.; Nakamura, K.; Roesch, M.R. From Ventral-Medial to Dorsal-Lateral Striatum: Neural Correlates of Reward-Guided Decision-Making. Neurobiol. Learn Mem. 2015, 117, 51–59. [Google Scholar] [CrossRef]
- Shilliam, C.S.; Dawson, L.A. The Effect of Clozapine on Extracellular Dopamine Levels in the Shell Subregion of the Rat Nucleus Accumbens Is Reversed Following Chronic Administration: Comparison with a Selective 5-HT(2C) Receptor Antagonist. Neuropsychopharmacology 2005, 30, 372–380. [Google Scholar] [CrossRef]
- dos-Santos-Pereira, M.; Acuña, L.; Hamadat, S.; Rocca, J.; González-Lizárraga, F.; Chehín, R.; Sepulveda-Diaz, J.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Microglial Glutamate Release Evoked by α-Synuclein Aggregates Is Prevented by Dopamine. Glia 2018, 66, 2353–2365. [Google Scholar] [CrossRef]
- Casey, D.E. Implications of the CATIE Trial on Treatment: Extrapyramidal Symptoms. CNS Spectr. 2006, 11, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.; Barnes, T.R.E.; Davies, L.; Dunn, G.; Lloyd, H.; Hayhurst, K.P.; Murray, R.M.; Markwick, A.; Lewis, S.W. Randomized Controlled Trial of the Effect on Quality of Life of Second- vs. First-Generation Antipsychotic Drugs in Schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch. Gen. Psychiatry 2006, 63, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Li, Z.; Kaneda, Y.; Ichikawa, J. Serotonin Receptors: Their Key Role in Drugs to Treat Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- White, F.; Wang, R. Differential Effects of Classical and Atypical Antipsychotic Drugs on A9 and A10 Dopamine Neurons. Science 1983, 221, 1054–1056. [Google Scholar] [CrossRef]
- Farde, L.; Nordstrom, A.-L.; Wiesel, F.-A.; Pauli, S.; Halldin, C.; Sedvall, C. Positron Emission Tomographic Analysis of Central D1 and D2 Dopamine Receptor Occupancy in Patients Treated with Classical Neuroleptics and Clozapine Relation to extrapyramidal side effects. Arch. Gen. Psychiatry 1992, 49, 538–544. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Gavaudan, S.; Conte, C.; Chaput, C.; Touzard, M.; Verrièle, L.; Audinot, V.; Millan, M.J. Agonist and Antagonist Actions of Antipsychotic Agents at 5-HT(1A) Receptors: A [35S] GTPγS Binding Study. Eur. J. Pharmacol. 1998, 355, 245–256. [Google Scholar] [CrossRef]
- Haleem, D.J. 5-HT1A Receptor-Dependent Control of Nigrostriatal Dopamine Neurotransmission in the Pharmacotherapy of Parkinson’s Disease and Schizophrenia. Behav. Pharmacol. 2015, 26, 45–58. [Google Scholar] [CrossRef]
- Ohno, Y.; Shimizu, S.; Imaki, J.; Ishihara, S.; Sofue, N.; Sasa, M.; Kawai, Y. Anticataleptic 8-OH-DPAT Preferentially Counteracts with Haloperidol-Induced Fos Expression in the Dorsolateral Striatum and the Core Region of the Nucleus Accumbens. Neuropharmacology 2008, 55, 717–723. [Google Scholar] [CrossRef]
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Robert Lane, J.; Charlton, S.J. Extrapyramidal Side Effects of Antipsychotics Are Linked to Their Association Kinetics at Dopamine D2 Receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef]
- Nye, H.E.; Nestler, E.J. Induction of Chronic Fos-Related Antigens in Rat Brain by Chronic Morphine Administration—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8609891/ (accessed on 24 August 2023).
- Sonego, A.B.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Cannabidiol Attenuates Haloperidol-Induced Catalepsy and c-Fos Protein Expression in the Dorsolateral Striatum via 5-HT1A Receptors in Mice. Behav. Brain Res. 2016, 309, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Orozco, P.E.; Robbe, D. The Striatum Multiplexes Contextual and Kinematic Information to Constrain Motor Habits Execution. Nat. Neurosci. 2015, 18, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.A.; Viana, T.G.; Silveira, V.T.; Aguiar, D.C.; Moreira, F.A. Effects of Aripiprazole on Caffeine-Induced Hyperlocomotion and Neural Activation in the Striatum. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2016, 389, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, N.; Graybiel, A.M. Atypical and Typical Neuroleptic Treatments Induce Distinct Programs of Transcription Factor Expression in the Striatum. J. Comp. Neurol. 1996, 374, 70–83. [Google Scholar] [CrossRef]
- Lobo, M.K.; Zaman, S.; Damez-Werno, D.M.; Koo, J.W.; Bagot, R.C.; DiNieri, J.A.; Nugent, A.; Finkel, E.; Chaudhury, D.; Chandra, R.; et al. FosB Induction in Striatal Medium Spiny Neuron Subtypes in Response to Chronic Pharmacological, Emotional, and Optogenetic Stimuli. J. Neurosci. 2013, 33, 18381–18395. [Google Scholar] [CrossRef] [PubMed]
- Kontkanen, O.; Lakso, M.; Wong, G.; Castrén, E. Chronic Antipsychotic Drug Treatment Induces Long-Lasting Expression of Fos and Jun Family Genes and Activator Protein 1 Complex in the Rat Prefrontal Cortex. Neuropsychopharmacology 2002, 27, 152–162. [Google Scholar] [CrossRef]
- Majercikova, Z.; Horvathova, L.; Osacka, J.; Pecenak, J.; Kiss, A. Impact of Repeated Asenapine Treatment on FosB/ΔFosB Expression in the Forebrain Structures under Normal Conditions and Mild Stress Preconditioning in the Rat. Brain Res. Bull. 2016, 127, 29–37. [Google Scholar] [CrossRef]
- Maldonado-Irizarry, C.S.; Kelleyt, A.E. Excitotoxic Lesions of the Core and Shell Subregions of the Nucleus Accumbens Differentially Disrupt Body Weight Regulation and Motor Activity in Rat. Brain Res. Bull. 1995, 38, 551–559. [Google Scholar] [CrossRef]
- Atkins, J.B.; Chlan-Fourney, J.; Nye, H.E.; Hiroi, N.; Carlezon, W.A.; Nestler, E.J. Region-Specific Induction of FosB by Repeated Administration of Typical versus Atypical Antipsychotic Drugs. Synapse 1999, 33, 118–128. [Google Scholar] [CrossRef]
- Guesdon, B.; Denis, R.G.P.; Richard, D. Additive Effects of Olanzapine and Melanin-Concentrating Hormone Agonism on Energy Balance. Behav. Brain Res. 2010, 207, 14–20. [Google Scholar] [CrossRef]
- Radke, J.M.; Owens, M.J.; Ritchie, J.C.; Nemeroff, C.B. Atypical Antipsychotic Drugs Selectively Increase Neurotensin Efflux in Dopamine Terminal Regions. Proc. Natl. Acad. Sci. USA 1998, 95, 11462–11464. [Google Scholar] [CrossRef] [PubMed]
- Prinssen, E.P.M.; Bemelmans, F.J. Evidence for a Role of the Shell of the Nucleus Behavior of Freely Moving Rats in Oral. J. Neurosci. 1994, 74, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Volavka, J.; Czobor, P.; Sheitman, B.; Lindenmayer, J.P.; Citrome, L.; McEvoy, J.P.; Cooper, T.B.; Chakos, M.; Lieberman, J.A. Clozapine, Olanzapine, Risperidone, and Haloperidol in the Treatment of Patients with Chronic Schizophrenia and Schizoaffective Disorder. Am. J. Psychiatry 2002, 159, 255–262. [Google Scholar] [CrossRef]
- Wang, H.; Farhan, M.; Xu, J.; Lazarovici, P.; Zheng, W. The Involvement of DARPP-32 in the Pathophysiology of Schizophrenia. Oncotarget 2017, 8, 53791–53803. [Google Scholar] [CrossRef]
- Petryszyn, S.; Sánchez, M.G.; Gagnon, D.; Beaulieu, J.M.; Parent, A.; Parent, M. A Dense Cluster of D1+ Cells in the Mouse Nucleus Accumbens. Synapse 2017, 71, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, S. Dopamine Reward Circuitry: Two Projection Systems from the Ventral Midbrain to the Nucleus Accumbens-Olfactory Tubercle Complex. Brain Res. 2007, 56, 27–78. [Google Scholar] [CrossRef]
- Rushlow, W.J.; Seah, Y.H.; Belliveau, D.J.; Rajakumar, N. Changes in Calcineurin Expression Induced in the Rat Brain by the Administration of Antipsychotics. J. Neurochem. 2005, 94, 587–596. [Google Scholar] [CrossRef]
- Nishi, A.; Bibb, J.A.; Matsuyama, S.; Hamada, M.; Higashi, H.; Nairn, A.C.; Greengard, P. Regulation of DARPP-32 Dephosphorylation at PKA- and Cdk5-Sites by NMDA and AMPA Receptors: Distinct Roles of Calcineurin and Protein Phosphatase-2A. J. Neurochem. 2002, 81, 832–841. [Google Scholar] [CrossRef]
- Agid, O.; Kapur, S.; Arenovich, R.; Zipursky, B. Delayed Onset Hypoyhesis of Antipsychotic Action—A Hipothesis Tested and Rejected. Arch. Gen. Psychiatry 2003, 60, 1228–1235. [Google Scholar] [CrossRef]
- Sesack, S.R.; Grace, A.A. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology 2010, 35, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Lobo, M.K.; Spencer, S.; Kalivas, P.W. Cocaine-Induced Adaptations in D1 and D2 Accumbens Projection Neurons (a Dichotomy Not Necessarily Synonymous with Direct and Indirect Pathways). Curr. Opin. Neurobiol. 2013, 23, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Stefanik, M.T.; Kupchik, Y.M.; Brown, R.M.; Kalivas, P.W. Optogenetic Evidence That Pallidal Projections, Not Nigral Projections, from the Nucleus Accumbens Core Are Necessary for Reinstating Cocaine Seeking. J. Neurosci. 2013, 33, 13654–13662. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto, S.G.; Almeida, M.C.; Silva, J.C.S.; Del-Bel, E.; Echeverry, M.B. Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens. Biomedicines 2023, 11, 2677. https://doi.org/10.3390/biomedicines11102677
Prieto SG, Almeida MC, Silva JCS, Del-Bel E, Echeverry MB. Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens. Biomedicines. 2023; 11(10):2677. https://doi.org/10.3390/biomedicines11102677
Chicago/Turabian StylePrieto, Sonia G., Maria Camila Almeida, João C. S. Silva, Elaine Del-Bel, and Marcela B. Echeverry. 2023. "Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens" Biomedicines 11, no. 10: 2677. https://doi.org/10.3390/biomedicines11102677
APA StylePrieto, S. G., Almeida, M. C., Silva, J. C. S., Del-Bel, E., & Echeverry, M. B. (2023). Extrapyramidal Side Effects with Chronic Atypical Antipsychotic Can Be Predicted by Labeling Pattern of FosB and phosphoThr34-DARPP-32 in Nucleus Accumbens. Biomedicines, 11(10), 2677. https://doi.org/10.3390/biomedicines11102677





