The Importance of Optimal Hydration in Patients with Heart Failure—Not Always Too Much Fluid
Abstract
:1. Introduction
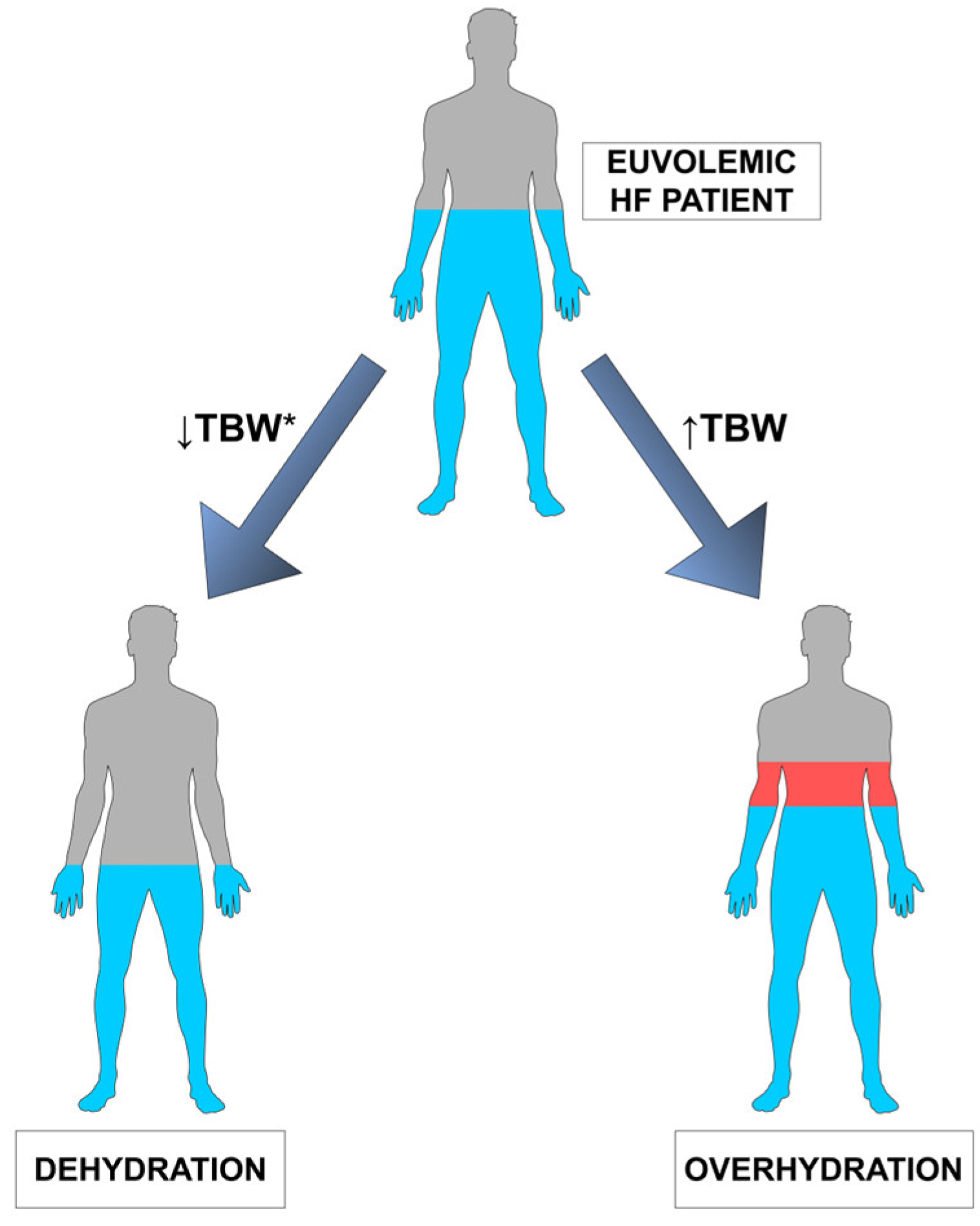
2. Human Body Water—Brief Overview
3. Congestion/Overhydration in Heart Failure
3.1. Congestion in Heart Failure—Overview and Pathogenesis
3.1.1. Role of Sympathetic Nervous System
3.1.2. Role of Renin–Angiotensin–Aldosterone System
3.1.3. Role of Natriuretic Peptide System
3.1.4. Development of Tissue Edema
3.2. Diagnostics
3.2.1. Gold Standard
3.2.2. Symptoms
3.2.3. Signs
3.2.4. Laboratory Tests
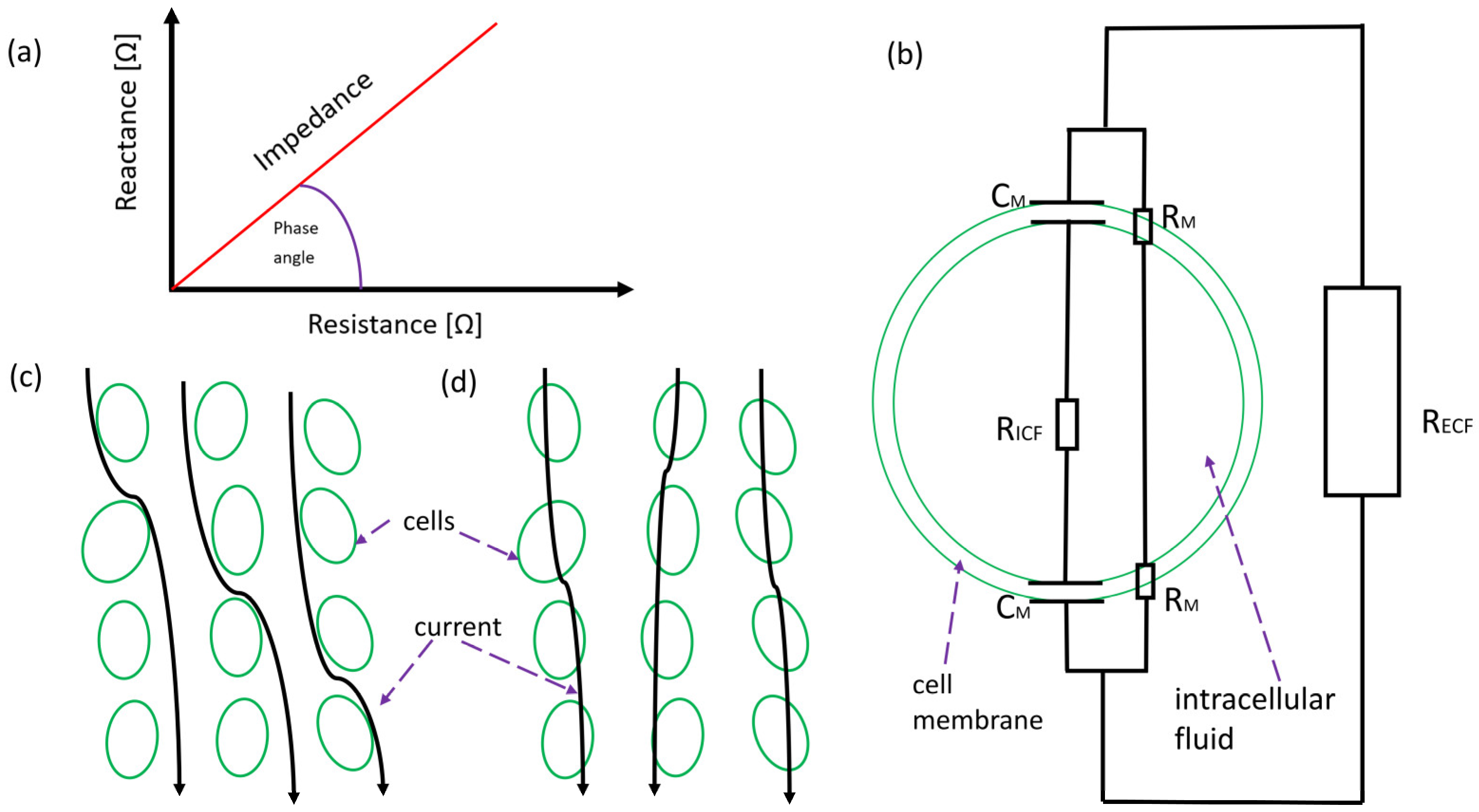
3.2.5. Bioelectrical Impedance Analysis
3.2.6. Imaging
3.3. Treatment Overview
3.3.1. Diuretics
3.3.2. Other Drugs
3.3.3. Sodium-Glucose Co-Transporter 2 Inhibitors (SGLT2 Inhibitors)
3.3.4. Angiotensin-Converting Enzyme Inhibitors (ACE-Is)
3.3.5. Mineralocorticoid Receptor Antagonists (MRAs)
3.3.6. Sacubitril/Valsartan (ARNI)
3.3.7. Beta Blockers
3.4. Congestion-Self-Care and Ambulatory Care of Heart Failure Patients
3.5. Congestion/Overhydration in Heart Failure—Summary
4. Dehydration—Overview
4.1. Objective Methods for Diagnosing Dehydration
4.2. Clinical Diagnosis of Dehydration—Signs and Symptoms
4.3. Treatment Overview
4.4. Dehydration—Summary
5. Dehydration and Heart Failure
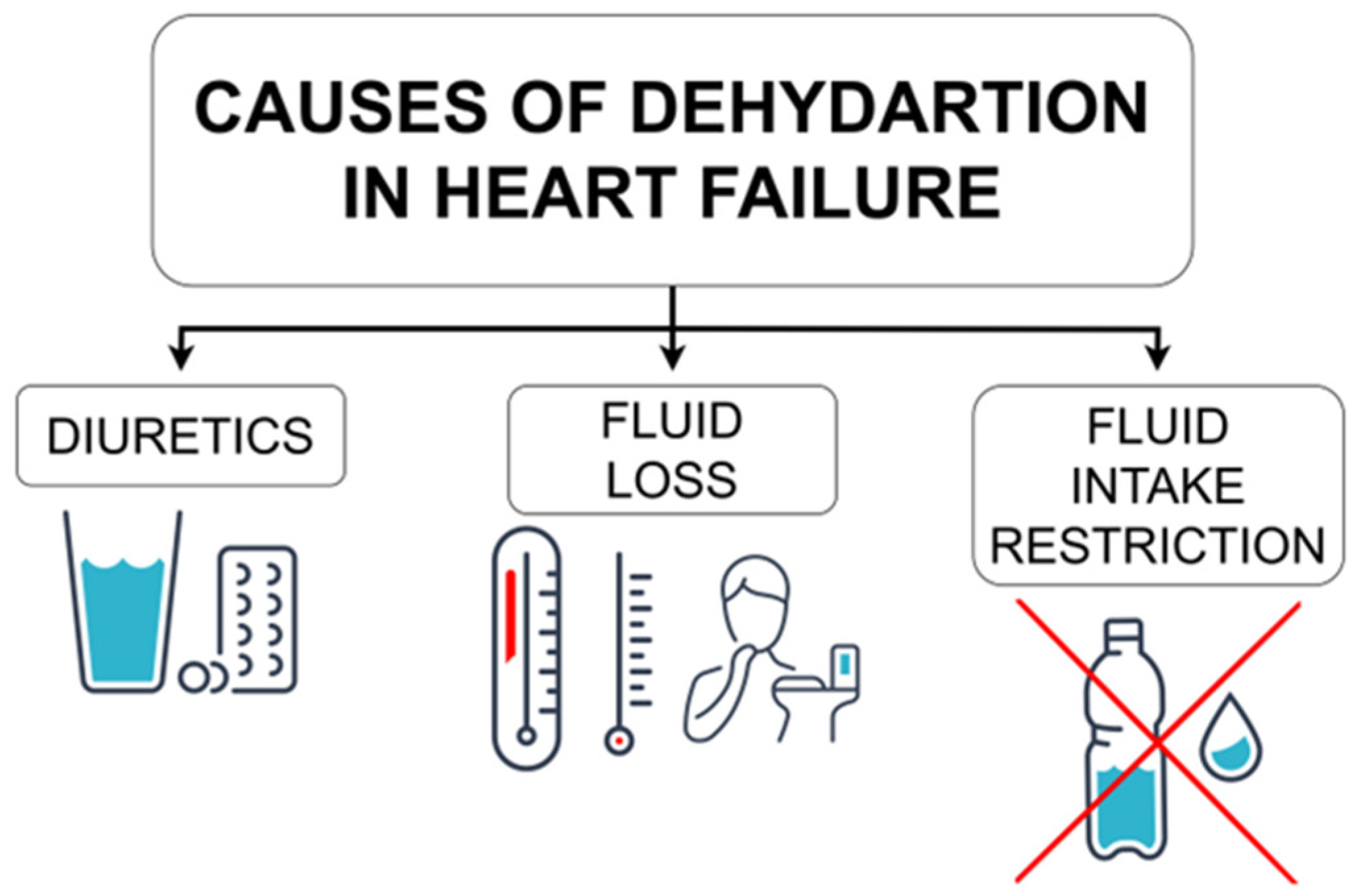
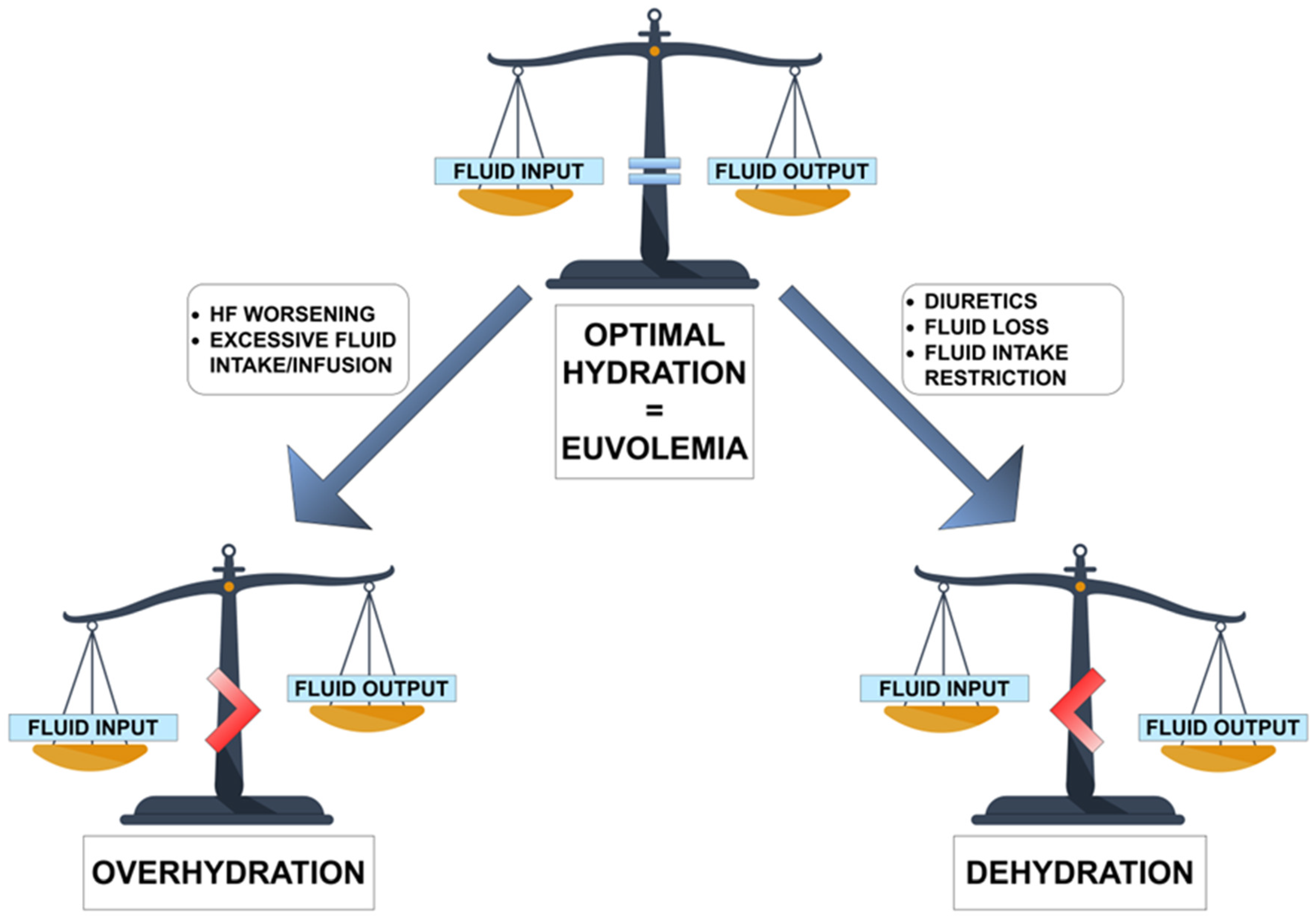
5.1. Dehydration in Heart Failure—Possible Causes
5.1.1. Diuretics
5.1.2. Fluid Loss
5.1.3. Fluid Intake Restriction
5.1.4. Summary
5.2. Heart Failure and Dehydration in the Literature
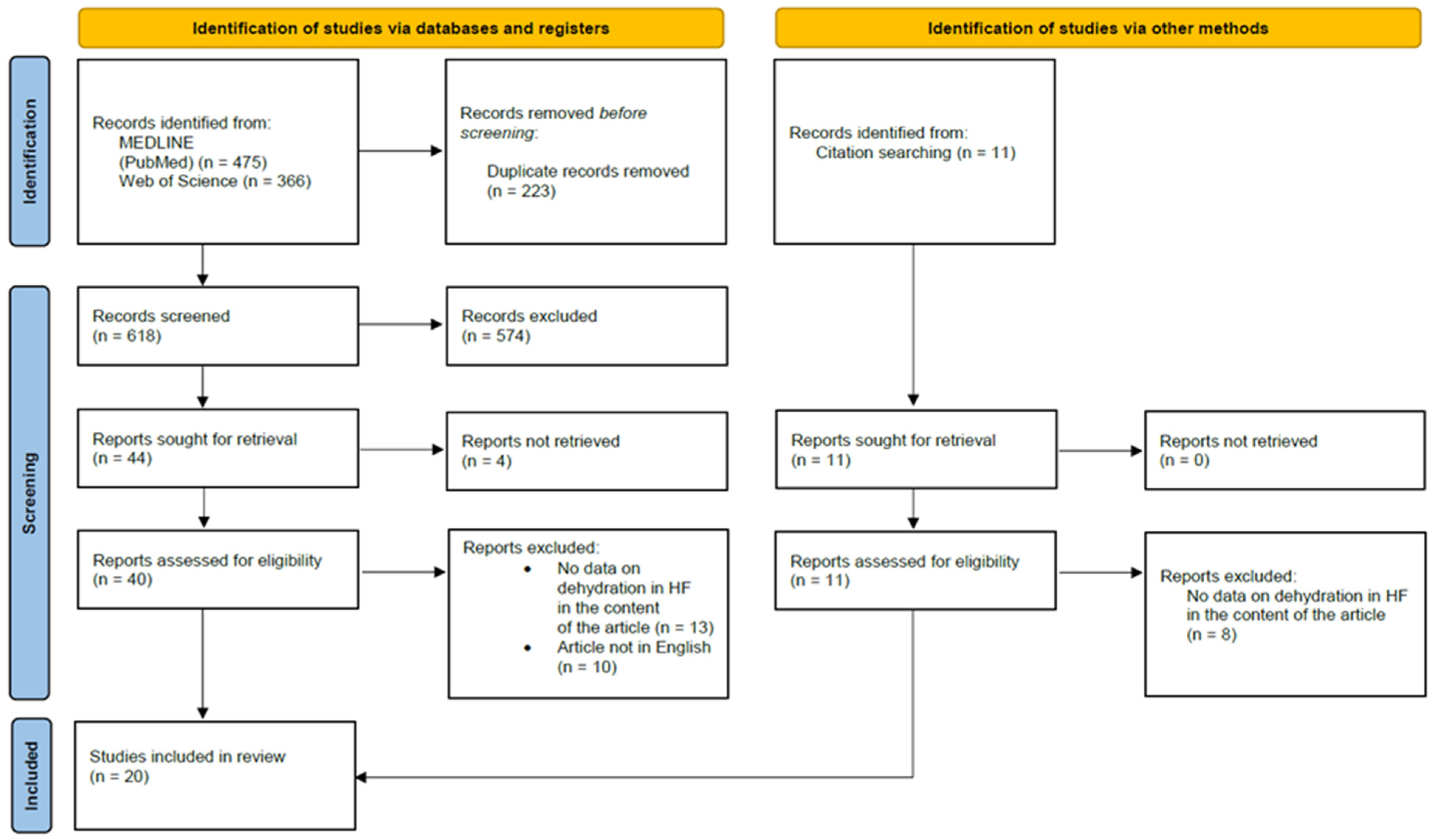
5.2.1. Methodology
5.2.2. Case Reports
5.2.3. Review of Included Articles
5.3. Heart Failure and Hypohydration
5.4. Dehydration-Self-Care and Ambulatory Care of Heart Failure Patients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Riet, E.E.S.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.J.; Rutten, F.H. Epidemiology of Heart Failure: The Prevalence of Heart Failure and Ventricular Dysfunction in Older Adults over Time. A Systematic Review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Baron, S.; Courbebaisse, M.; Lepicard, E.M.; Friedlander, G. Assessment of Hydration Status in a Large Population. Br. J. Nutr. 2015, 113, 147–158. [Google Scholar] [CrossRef]
- Kavouras, S.A. Hydration, Dehydration, Underhydration, Optimal Hydration: Are We Barking up the Wrong Tree? Eur. J. Nutr. 2019, 58, 471–473. [Google Scholar] [CrossRef]
- Rush, E.C. Water: Neglected, Unappreciated and under Researched. Eur. J. Clin. Nutr. 2013, 67, 492–495. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.P.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-Care of Heart Failure Patients: Practical Management Recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Jéquier, E.; Constant, F. Water as an Essential Nutrient: The Physiological Basis of Hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Austin, P. Physiology of Human Fluid Balance. Anaesth. Intensive Care Med. 2021, 22, 644–651. [Google Scholar] [CrossRef]
- Katz, S.D. In Search of Euvolemia in Heart Failure. JACC Heart Fail. 2014, 2, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.; Ballard, B.D.; Mohiuddin, S.S. Physiology, Water Balance—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541059/ (accessed on 19 August 2023).
- O’Connor, M.E.; Prowle, J.R. Fluid Overload. Crit. Care Clin. 2015, 31, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, E.M.; ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in Heart Failure: A Contemporary Look at Physiology, Diagnosis and Treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Chen, H.H.; Schrier, R.W. Pathophysiology of Volume Overload in Acute Heart Failure Syndromes. Am. J. Med. 2006, 119, S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Nijst, P.; Mullens, W. Current Approach to Decongestive Therapy in Acute Heart Failure. Curr. Heart Fail. Rep. 2015, 12, 367–378. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abraham, W.T. Hormones and Hemodynamics in Heart Failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef]
- Zucker, I.H.; Schultz, H.D.; Li, Y.F.; Wang, Y.; Wang, W.; Patel, K.P. The Origin of Sympathetic Outflow in Heart Failure: The Roles of Angiotensin II and Nitric Oxide. Prog. Biophys. Mol. Biol. 2004, 84, 217–232. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal Activation in Heart Failure with Reduced Ejection Fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef]
- Borovac, J.A.; D’Amario, D.; Bozic, J.; Glavas, D. Sympathetic Nervous System Activation and Heart Failure: Current State of Evidence and the Pathophysiology in the Light of Novel Biomarkers. World J. Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically Mediated Changes in Capacitance: Redistribution of the Venous Reservoir as a Cause of Decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Abassi, Z.; Khoury, E.E.; Karram, T.; Aronson, D. Edema Formation in Congestive Heart Failure and the Underlying Mechanisms. Front. Cardiovasc. Med. 2022, 9, 933215. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Venous Function and Central Venous Pressure: A Physiologic Story. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Jones, W.S.; Boortz-Marx, R.L.; Ganesh, A.; Green, C.L.; Hernandez, A.F.; Patel, M.R. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018, 138, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Orsborne, C.; Chaggar, P.S.; Shaw, S.M.; Williams, S.G. The Renin-Angiotensin-Aldosterone System in Heart Failure for the Non-Specialist: The Past, the Present and the Future. Postgrad. Med. J. 2017, 93, 29–37. [Google Scholar] [CrossRef]
- Sayer, G.; Bhat, G. The Renin-Angiotensin-Aldosterone System and Heart Failure. Cardiol. Clin. 2014, 32, 21–32. [Google Scholar] [CrossRef]
- Hackenthal, E.; Paul, M.; Ganten, D.; Taugner, R. Morphology, Physiology, and Molecular Biology of Renin Secretion. Physiol. Rev. 1990, 70, 1067–1116. [Google Scholar] [CrossRef]
- Kurtz, A. Renin Release: Sites, Mechanisms, and Control. Annu. Rev. Physiol. 2011, 73, 377–399. [Google Scholar] [CrossRef]
- Sullivan, R.D.; Mehta, R.M.; Tripathi, R.; Reed, G.L.; Gladysheva, I.P. Renin Activity in Heart Failure with Reduced Systolic Function—New Insights. Int. J. Mol. Sci. 2019, 20, 3182. [Google Scholar] [CrossRef]
- Chen, L.; Kim, S.M.; Eisner, C.; Oppermann, M.; Huang, Y.; Mizel, D.; Li, L.; Chen, M.; Sequeira Lopez, M.L.; Weinstein, L.S.; et al. Stimulation of Renin Secretion by Angiotensin II Blockade Is Gsα-Dependent. J. Am. Soc. Nephrol. 2010, 21, 986–992. [Google Scholar] [CrossRef]
- Bauersachs, J.; López-Andrés, N. Mineralocorticoid Receptor in Cardiovascular Diseases-Clinical Trials and Mechanistic Insights. Br. J. Pharmacol. 2022, 179, 3119–3134. [Google Scholar] [CrossRef]
- Rossier, B.C.; Baker, M.E.; Studer, R.A. Epithelial Sodium Transport and Its Control by Aldosterone: The Story of Our Internal Environment Revisited. Physiol. Rev. 2015, 95, 297–340. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T. Aldosterone in Congestive Heart Failure. N. Engl. J. Med. 2001, 345, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Charloux, A.; Piquard, F.; Doutreleau, S.; Brandenberger, G.; Geny, B. Mechanisms of Renal Hyporesponsiveness to ANP in Heart Failure. Eur. J. Clin. Investig. 2003, 33, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Egom, E.E.; Feridooni, T.; Hotchkiss, A.; Kruzliak, P.; Pasumarthi, K.B.S. Mechanisms of Renal Hyporesponsiveness to BNP in Heart Failure. Can. J. Physiol. Pharmacol. 2015, 93, 399–403. [Google Scholar] [CrossRef]
- Ibebuogu, U.N.; Gladysheva, I.P.; Houng, A.K.; Reed, G.L. Decompensated Heart Failure Is Associated with Reduced Corin Levels and Decreased Cleavage of Pro-Atrial Natriuretic Peptide. Circ. Heart Fail. 2011, 4, 114–120. [Google Scholar] [CrossRef]
- Zaidi, S.S.; Ward, R.D.; Ramanathan, K.; Yu, X.; Gladysheva, I.P.; Reed, G.L. Possible Enzymatic Downregulation of the Natriuretic Peptide System in Patients with Reduced Systolic Function and Heart Failure: A Pilot Study. Biomed Res. Int. 2018, 2018, 7279036. [Google Scholar] [CrossRef]
- Gladysheva, I.P.; Sullivan, R.D.; Reed, G.L. Falling Corin and ANP Activity Levels Accelerate Development of Heart Failure and Cardiac Fibrosis. Front. Cardiovasc. Med. 2023, 10, 1120487. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Lapp, H.; Boerrigter, G.; Lerman, A.; Bufe, A.; Macheret, F.; Heublein, D.M.; Larue, C.; Burnett, J.C. Secretion of Prohormone of B-Type Natriuretic Peptide, proBNP1-108, Is Increased in Heart Failure. JACC Heart Fail. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Niederkofler, E.E.; Kiernan, U.A.; O’Rear, J.; Menon, S.; Saghir, S.; Protter, A.A.; Nelson, R.W.; Schellenberger, U. Detection of Endogenous B-Type Natriuretic Peptide at Very Low Concentrations in Patients with Heart Failure. Circ. Heart Fail. 2008, 1, 258–264. [Google Scholar] [CrossRef]
- Volpe, M.; Carnovali, M.; Mastromarino, V. The Natriuretic Peptides System in the Pathophysiology of Heart Failure: From Molecular Basis to Treatment. Clin. Sci. 2016, 130, 57–77. [Google Scholar] [CrossRef]
- McKie, P.M.; Burnett, J.C. Rationale and Therapeutic Opportunities for Natriuretic Peptide System Augmentation in Heart Failure. Curr. Heart Fail. Rep. 2015, 12, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Cleland, J.G.F. Causes and Treatment of Oedema in Patients with Heart Failure. Nat. Rev. Cardiol. 2013, 10, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Dini, F.L.; Pugliese, N.R.; Ameri, P.; Attanasio, U.; Badagliacca, R.; Correale, M.; Mercurio, V.; Tocchetti, C.G.; Agostoni, P.; Palazzuoli, A. Right Ventricular Failure in Left Heart Disease: From Pathophysiology to Clinical Manifestations and Prognosis. Heart Fail. Rev. 2022, 10, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.F. Pulmonary Edema: Pathophysiology and Diagnosis [Review Article]. Int. J. Tuberc. Lung Dis. 2011, 15, 155–160. [Google Scholar] [PubMed]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.A.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and Grading Congestion in Acute Heart Failure: A Scientific Statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and Endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [CrossRef]
- van’t Laar, A. Why Is the Measurement of Jugular Venous Pressure Discredited? Neth. J. Med. 2003, 61, 268–272. [Google Scholar]
- Breidthardt, T.; Moreno-Weidmann, Z.; Uthoff, H.; Sabti, Z.; Aeppli, S.; Puelacher, C.; Stallone, F.; Twerenbold, R.; Wildi, K.; Kozhuharov, N.; et al. How Accurate Is Clinical Assessment of Neck Veins in the Estimation of Central Venous Pressure in Acute Heart Failure? Insights from a Prospective Study. Eur. J. Heart Fail. 2018, 20, 1160–1162. [Google Scholar] [CrossRef]
- Wynne, J. The Clinical Meaning of the Third Heart Sound. Am. J. Med. 2001, 111, 157–158. [Google Scholar] [CrossRef]
- Kelder, J.C.; Cramer, M.J.; Van Wijngaarden, J.; Van Tooren, R.; Mosterd, A.; Moons, K.G.M.; Lammers, J.W.; Cowie, M.R.; Grobbee, D.E.; Hoes, A.W. The Diagnostic Value of Physical Examination and Additional Testing in Primary Care Patients with Suspected Heart Failure. Circulation 2011, 124, 2865–2873. [Google Scholar] [CrossRef]
- Daniels, L.B.; Maisel, A.S. Natriuretic Peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Thanapholsart, J.; Khan, E.; Lee, G.A. A Current Review of the Uses of Bioelectrical Impedance Analysis and Bioelectrical Impedance Vector Analysis in Acute and Chronic Heart Failure Patients: An Under-Valued Resource? Biol. Res. Nurs. 2022, 25, 109980042211328. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Gryglewska-Wawrzak, K.; Sakowicz, A.; Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction. Biomedicines 2022, 10, 3257. [Google Scholar] [CrossRef]
- Showkat, I.; Khanday, F.A.; Beigh, M.R. A Review of Bio-Impedance Devices. Med. Biol. Eng. Comput. 2023, 61, 927–950. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance and The Frequency Dependent Current Conduction Through Biological Tissues: A Short Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 331, 12005. [Google Scholar] [CrossRef]
- Dovancescu, S.; Saporito, S.; Herold, I.H.F.; Korsten, H.H.M.; Aarts, R.M.; Mischi, M. Monitoring Thoracic Fluid Content Using Bioelectrical Impedance Spectroscopy and Cole Modeling. J. Electr. Bioimpedance 2017, 8, 107–115. [Google Scholar] [CrossRef]
- Bera, T.K.; Nagaraju, J. Electrical Impedance Spectroscopic Studies on Broiler Chicken Tissue Suitable for the Development of Practical Phantoms in Multifrequency EIT. J. Electr. Bioimpedance 2011, 2, 48–63. [Google Scholar] [CrossRef]
- Ruiz-Vargas, A.; Ivorra, A.; Arkwright, J.W. Design, Construction and Validation of an Electrical Impedance Probe with Contact Force and Temperature Sensors Suitable for in-Vivo Measurements. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Chabin, X.; Taghli-Lamallem, O.; Mulliez, A.; Bordachar, P.; Jean, F.; Futier, E.; Massoullié, G.; Andonache, M.; Souteyrand, G.; Ploux, S.; et al. Bioimpedance Analysis Is Safe in Patients with Implanted Cardiac Electronic Devices. Clin. Nutr. 2019, 38, 806–811. [Google Scholar] [CrossRef]
- Roehrich, L.; Knierim, J.; Hajduczenia, M.; Mulzer, J.; Mueller, M.; Pergantis, P.; Hummel, M.; Falk, V.; Potapov, E.; Suendermann, S.; et al. Early- and Late-Onset Arrhythmias after Bioelectrical Impedance Analysis in End-Stage Heart Failure Patients under Inotropic Support. J. Heart Lung Transplant. 2019, 38, S377. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Gryglewska, K.; Sakowicz, A.; Rybak, M.; Janikowski, K.; Banach, M. Obesity and Body Mass Components Influence Exercise Tolerance and the Course of Hypertension in Perimenopausal Women. J. Cardiovasc. Dev. Dis. 2022, 9, 238. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Gryglewska, K.; Sakowicz, A.; von Haehling, S.; Janikowski, K.; Maciejewski, M.; Banach, M. Factors and Prognostic Significance of Impaired Exercise Tolerance in Women over 40 with Arterial Hypertension. J. Pers. Med. 2021, 11, 759. [Google Scholar] [CrossRef]
- Prasad, A.; Roy, M. Bioimpedance Analysis of Vascular Tissue and Fluid Flow in Human and Plant Body: A Review. Biosyst. Eng. 2020, 197, 170–187. [Google Scholar] [CrossRef]
- Di Somma, S.; De Berardinis, B.; Bongiovanni, C.; Marino, R.; Ferri, E.; Alfei, B. Use of BNP and Bioimpedance to Drive Therapy in Heart Failure Patients. Congest. Heart Fail. 2010, 16 (Suppl. 1), S56–S61. [Google Scholar] [CrossRef] [PubMed]
- di Somma, S.; Lalle, I.; Magrini, L.; Russo, V.; Navarin, S.; Castello, L.; Avanzi, G.C.; Di Stasio, E.; Maisel, A. Additive Diagnostic and Prognostic Value of Bioelectrical Impedance Vector Analysis (BIVA) to Brain Natriuretic Peptide “grey-Zone” in Patients with Acute Heart Failure in the Emergency Department. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, S.; Russo, V.; Lalle, I.; De Berardinis, B.; Navarin, S.; Magrini, L.; Piccoli, A.; Codognotto, M.; Castello, L.M.; Avanzi, G.C.; et al. Usefulness of Combining Admission Brain Natriuretic Peptide (BNP) plus Hospital Discharge Bioelectrical Impedance Vector Analysis (BIVA) in Predicting 90 Days Cardiovascular Mortality in Patients with Acute Heart Failure. Intern. Emerg. Med. 2017, 12, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Valle, R.; Aspromonte, N.; Milani, L.; Peacock, F.W.; Maisel, A.S.; Santini, M.; Ronco, C. Optimizing Fluid Management in Patients with Acute Decompensated Heart Failure (ADHF): The Emerging Role of Combined Measurement of Body Hydration Status and Brain Natriuretic Peptide (BNP) Levels. Heart Fail. Rev. 2011, 16, 519–529. [Google Scholar] [CrossRef]
- Platz, E.; Merz, A.A.; Jhund, P.S.; Vazir, A.; Campbell, R.; McMurray, J.J. Dynamic Changes and Prognostic Value of Pulmonary Congestion by Lung Ultrasound in Acute and Chronic Heart Failure: A Systematic Review. Eur. J. Heart Fail. 2017, 19, 1154–1163. [Google Scholar] [CrossRef]
- Ceriani, E.; Casazza, G.; Peta, J.; Torzillo, D.; Furlotti, S.; Cogliati, C. Residual Congestion and Long-Term Prognosis in Acutely Decompensated Heart Failure Patients. Intern. Emerg. Med. 2020, 15, 719–724. [Google Scholar] [CrossRef]
- Speets, A.M.; van der Graaf, Y.; Hoes, A.W.; Kalmijn, S.; Sachs, A.P.E.; Rutten, M.J.C.M.; Gratama, J.W.C.; Montauban van Swijndregt, A.D.; Mali, W.P.T.M. Chest Radiography in General Practice: Indications, Diagnostic Yield and Consequences for Patient Management. Br. J. Gen. Pract. 2006, 56, 574. [Google Scholar] [PubMed]
- Mant, J.; Doust, J.; Roalfe, A.; Barton, P.; Cowie, M.R.; Glasziou, P.; Mant, D.; McManus, R.J.; Holder, R.; Deeks, J.; et al. Systematic Review and Individual Patient Data Meta-Analysis of Diagnosis of Heart Failure, with Modelling of Implications of Different Diagnostic Strategies in Primary Care. Health Technol. Assess. 2009, 13, 1–207. [Google Scholar] [CrossRef] [PubMed]
- Mahdyoon, H.; Klein, R.; Eyler, W.; Lakier, J.B.; Chakko, S.C.; Gheorghiade, M. Radiographic Pulmonary Congestion in End-Stage Congestive Heart Failure. Am. J. Cardiol. 1989, 63, 625–627. [Google Scholar] [CrossRef]
- Collins, S.P.; Lindsell, C.J.; Storrow, A.B.; Abraham, W.T. Prevalence of Negative Chest Radiography Results in the Emergency Department Patient With Decompensated Heart Failure. Ann. Emerg. Med. 2006, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Barile, M.; Hida, T.; Hammer, M.; Hatabu, H. Simple Quantitative Chest CT for Pulmonary Edema. Eur. J. Radiol. Open 2020, 7, 100273. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.; Apanasiewicz, W.; Zukowska, M.; Jaczewski, G.; Dcabrowska, M. Diagnosing Lung Abnormalities Related to Heart Failure in Chest Radiogram, Lung Ultrasound and Thoracic Computed Tomography. Adv. Respir. Med. 2023, 91, 103–122. [Google Scholar] [CrossRef]
- Butler, J.; Usman, M.S.; Khan, M.S.; Greene, S.J.; Friede, T.; Vaduganathan, M.; Filippatos, G.; Coats, A.J.S.; Anker, S.D. Efficacy and Safety of SGLT2 Inhibitors in Heart Failure: Systematic Review and Meta-Analysis. ESC Heart Fail. 2020, 7, 3298–3309. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, ehad195. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Dewan, P.; Anand, I.S.; Bělohlávek, J.; Bengtsson, O.; De Boer, R.A.; Böhm, M.; Boulton, D.W.; Chopra, V.K.; Demets, D.L.; et al. Dapagliflozin and Diuretic Use in Patients With Heart Failure and Reduced Ejection Fraction in DAPA-HF. Circulation 2020, 142, 1040–1054. [Google Scholar] [CrossRef]
- Sullivan, R.D.; McCune, M.E.; Hernandez, M.; Reed, G.L.; Gladysheva, I.P. Suppression of Cardiogenic Edema with Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure with Reduced Ejection Fraction: Mechanisms and Insights from Pre-Clinical Studies. Biomedicines 2022, 10, 2016. [Google Scholar] [CrossRef]
- Hernandez, M.; Sullivan, R.D.; McCune, M.E.; Reed, G.L.; Gladysheva, I.P. Sodium-Glucose Cotransporter-2 Inhibitors Improve Heart Failure with Reduced Ejection Fraction Outcomes by Reducing Edema and Congestion. Diagnostics 2022, 12, 989. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why Do SGLT2 Inhibitors Reduce Heart Failure Hospitalization? A Differential Volume Regulation Hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Biegus, J.; Fudim, M.; Salah, H.M.; Heerspink, H.J.L.; Voors, A.A.; Ponikowski, P. Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure: Potential Decongestive Mechanisms and Current Clinical Studies. Eur. J. Heart Fail. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, A.; Ortiz, F.; Radhakrishnan, J.; Schrier, R.W.; Colombo, P.C. Mineralocorticoid Receptor Antagonists as Diuretics: Can Congestive Heart Failure Learn from Liver Failure? Heart Fail. Rev. 2015, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, P.; Ménard, J.; Fay, R.; Gustafsson, F.; Pitt, B.; Zannad, F. Eplerenone Survival Benefits in Heart Failure Patients Post-Myocardial Infarction Are Independent from Its Diuretic and Potassium-Sparing Effects. Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) Substudy. J. Am. Coll. Cardiol. 2011, 58, 1958–1966. [Google Scholar] [CrossRef]
- Hensen, J.; Abraham, W.T.; Dürr, J.A.; Schrier, R.W. Aldosterone in Congestive Heart Failure: Analysis of Determinants and Role in Sodium Retention. Am. J. Nephrol. 1991, 11, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Bonou, Μ.; Malliaras, K.; Athanasiadi, E.; Vakrou, S.; Skouloudi, M.; Masoura, C.; Barbetseas, J. Association of Loop Diuretics Use and Dose with Outcomes in Outpatients with Heart Failure: A Systematic Review and Meta-Analysis of Observational Studies Involving 96,959 Patients. Heart Fail. Rev. 2022, 27, 147–161. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Claggett, B.; Kachadourian, J.; Desai, A.S.; Packer, M.; Rouleau, J.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. Reduced Loop Diuretic Use in Patients Taking Sacubitril/Valsartan Compared with Enalapril: The PARADIGM-HF Trial. Eur. J. Heart Fail. 2019, 21, 337–341. [Google Scholar] [CrossRef]
- Wand, A.L.; Russell, S.D.; Gilotra, N.A. Ambulatory Management of Worsening Heart Failure: Current Strategies and Future Directions. Heart Int. 2021, 15, 49–53. [Google Scholar] [CrossRef]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A Multidisciplinary Consensus on Dehydration: Definitions, Diagnostic Methods and Clinical Implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Watson, P.; Neal, K.R.; Ljungqvist, O.; Maughan, R.J.; Sahota, O.; Lobo, D.N. Hydration and Outcome in Older Patients Admitted to Hospital (The HOOP Prospective Cohort Study). Age Ageing 2015, 44, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. Preventable Hospitalizations of Dehydration: Implications of Inadequate Primary Health Care in the United States. Ann. Epidemiol. 2007, 17, 736. [Google Scholar] [CrossRef]
- Thomas, D.R.; Cote, T.R.; Lawhorne, L.; Levenson, S.A.; Rubenstein, L.Z.; Smith, D.A.; Stefanacci, R.G.; Tangalos, E.G.; Morley, J.E. Understanding Clinical Dehydration and Its Treatment. J. Am. Med. Dir. Assoc. 2008, 9, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN Guideline on Clinical Nutrition and Hydration in Geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Ely, B.R.; Kenefick, R.W.; Sawka, M.N. Biological Variation and Diagnostic Accuracy of Dehydration Assessment Markers. Am. J. Clin. Nutr. 2010, 92, 565–573. [Google Scholar] [CrossRef]
- Khajuria, A.; Krahn, J. Osmolality Revisited–Deriving and Validating the Best Formula for Calculated Osmolality. Clin. Biochem. 2005, 38, 514–519. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical Symptoms, Signs and Tests for Identification of Impending and Current Water-Loss Dehydration in Older People. Cochrane Database Syst. Rev. 2015, 2015, CD009647. [Google Scholar] [CrossRef]
- Crecelius, C. Dehydration: Myth and Reality. J. Am. Med. Dir. Assoc. 2008, 9, 287–288. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence Overview | Intravenous Fluid Therapy in Adults in Hospital | Guidance | NICE. Available online: https://www.nice.org.uk/guidance/cg174 (accessed on 19 August 2023).
- Williams, B. The National Early Warning Score: From Concept to NHS Implementation. Clin. Med. 2022, 22, 499–505. [Google Scholar]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on Dietary Reference Values for Water. EFSA J. 2010, 8, 1459. [Google Scholar] [CrossRef]
- Hoste, E.A.; Maitland, K.; Brudney, C.S.; Mehta, R.; Vincent, J.L.; Yates, D.; Kellum, J.A.; Mythen, M.G.; Shaw, A.D. Four Phases of Intravenous Fluid Therapy: A Conceptual Model. Br. J. Anaesth. 2014, 113, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Girerd, N.; Boffa, J.J.; Courivaud, C.; Isnard, R.; Juillard, L.; Lamblin, N.; Legrand, M.; Logeart, D.; Mariat, C.; et al. Practical Management of Worsening Renal Function in Outpatients with Heart Failure and Reduced Ejection Fraction: Statement from a Panel of Multidisciplinary Experts and the Heart Failure Working Group of the French Society of Cardiology. Arch. Cardiovasc. Dis. 2020, 113, 660–670. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The Use of Diuretics in Heart Failure with Congestion—A Position Statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Huxel, C.; Raja, A.; Ollivierre-Lawrence, M.D. Loop Diuretics. Available online: http://www.ncbi.nlm.nih.gov/books/NBK546656/ (accessed on 20 August 2023).
- Balmain, B.N.; Sabapathy, S.; Jay, O.; Adsett, J.; Stewart, G.M.; Jayasinghe, R.; Morris, N.R. Heart Failure and Thermoregulatory Control: Can Patients With Heart Failure Handle the Heat? J. Card. Fail. 2017, 23, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.J.; Beckers-Wesche, F.; Baltussen, L.E.H.J.M.; Verdijk, M.H.I.; Bellersen, L.; Brunner-la Rocca, H.P.; Jaarsma, T.; Pisters, R.; Sanders-van Wijk, S.; Rodwell, L.; et al. Fluid REStriction in Heart Failure vs Liberal Fluid UPtake: Rationale and Design of the Randomized FRESH-UP Study. J. Card. Fail. 2022, 28, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Van Der Wal, M.H.L.; Strömberg, A.; Waldréus, N.; Jaarsma, T. Fluid Restriction in Patients with Heart Failure: How Should We Think? Eur. J. Cardiovasc. Nurs. 2016, 15, 301–304. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, V.; Rada, G. Is Fluid Restriction Needed in Heart Failure? Medwave 2017, 17, e6817. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Supakul, S.; Nishikawa, Y.; Teramura, M.; Takase, T. Short-Term Treatment with Empagliflozin Resulted in Dehydration and Cardiac Arrest in an Elderly Patient with Specific Complications: A Case Report and Literature Review. Medicina 2022, 58, 815. [Google Scholar] [CrossRef]
- Todani, S.; Takahashi, M. Recurrent Takotsubo Syndrome Complicated with Ischemic Enteritis Successfully Treated by Hydration: A Case Report. J. Med. Case Rep. 2021, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Mun, J.B.; Kim, E.Y.; Moon, J. Paradoxical Heart Failure Precipitated by Profound Dehydration: Intraventricular Dynamic Obstruction and Significant Mitral Regurgitation in a Volume-Depleted Heart. Yonsei Med. J. 2013, 54, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Alzand, B.S.N.; Mihl, C.; Brunner La Rocca, H.P. Dehydration with High Natriuretic Peptide Levels! A Word of Caution. Cardiology 2011, 118, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Galas, A.; Krzesinski, P.; Gielerak, G. Diuretics in Heart Failure—Rationale for Use in Heart Failure. Pediatr. I Med. Rodz. Paediatr. Fam. Med. 2017, 13, 450–459. [Google Scholar] [CrossRef]
- De Vecchis, R.; Ciccarelli, A.; Pucciarelli, A. Unloading Therapy by Intravenous Diuretic in Chronic Heart Failure: A Double-Edged Weapon? J. Cardiovasc. Med. 2010, 11, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kubota, Y.; Nishino, T.; Tara, S.; Kato, K.; Hayashi, D.; Mozawa, K.; Matsuda, J.; Tokita, Y.; Yasutake, M.; et al. Utility of Fractional Excretion of Urea Nitrogen in Heart Failure Patients with Chronic Kidney Disease. ESC Heart Fail. 2023, 10, 1706–1716. [Google Scholar] [CrossRef]
- Akhtar, A.M.; Ghouri, N.; Chahal, C.A.A.; Patel, R.; Ricci, F.; Sattar, N.; Waqar, S.; Khanji, M.Y. Ramadan Fasting: Recommendations for Patients with Cardiovascular Disease. Heart 2022, 108, 258. [Google Scholar] [CrossRef]
- Chuda, A.; Kaszkowiak, M.; Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. The Relationship of Dehydration and Body Mass Index With the Occurrence of Atrial Fibrillation in Heart Failure Patients. Front. Cardiovasc. Med. 2021, 8, 668653. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Adams, K.F.; Abraham, W.T.; Yancy, C.W.; Boscardin, W.J. Risk Stratification for In-Hospital Mortality in Acutely Decompensated Heart Failure: Classification and Regression Tree Analysis. JAMA 2005, 293, 572–580. [Google Scholar] [CrossRef]
- Gotsman, I.; Zwas, D.; Planer, D.; Admon, D.; Lotan, C.; Keren, A. The Significance of Serum Urea and Renal Function in Patients with Heart Failure. Medicine 2010, 89, 197–203. [Google Scholar] [CrossRef]
- Nunez, J.; Mascarell, B.; Stubbe, H.; Ventura, S.; Bonanad, C.; Bodi, V.; Nunez, E.; Minana, G.; Facila, L.; Bayes-Genis, A.; et al. Bioelectrical Impedance Vector Analysis and Clinical Outcomes in Patients with Acute Heart Failure. J. Cardiovasc. Med. 2016, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, A.; Takabayashi, K.; Okazaki, Y.; Ogami, M.; Ichinohe, T.; Yamamoto, T.; Hujita, R.; Takenaka, H.; Haruna, Y.; Kitaguchi, S.; et al. P605Lower Body Mass Index in Patients with Acute Heart Failure Is Independently Associated with Higher Mortality and Hospitalization by Dehydration in Community-Based Registry; KICKOFF Registry. Eur. Heart J. 2017, 38, 605. [Google Scholar] [CrossRef]
- Asada, K.; Fujiu, K.; Imai, Y.; Kojima, T.; Sugiyama, H.; Suzuki, T.; Kinugawa, K.; Hirata, Y.; Nagai, R. Intrathoracic Impedance Monitoring in Patients with Heart Failure: Correlation with Dehydration and Bleeding Events. Circ. J. Off. J. Jpn. Circ. Soc. 2012, 76, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Waldreus, N.; Sjostrand, F.; Hahn, R.G. Thirst in the Elderly with and without Heart Failure. Arch. Gerontol. Geriatr. 2011, 53, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.E.; Weber, H. Dehydration despite Drinking: Beyond the BUN/Creatinine Ratio. J. Am. Med. Dir. Assoc. 2004, 5, S67–S71. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Liu, D.; Wu, C.O.; Boehm, M. Middle Age Serum Sodium Levels in the Upper Part of Normal Range and Risk of Heart Failure. Eur. Heart J. 2022, 43, 3335–3348. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Springer, D.A.; Burg, M.B.; Boehm, M.; Dmitrieva, N.I. Suboptimal Hydration Remodels Metabolism, Promotes Degenerative Diseases, and Shortens Life. JCI Insight 2019, 4, e130949. [Google Scholar] [CrossRef]
- Lang, F.; Guelinckx, I.; Lemetais, G.; Melander, O. Two Liters a Day Keep the Doctor Away? Considerations on the Pathophysiology of Suboptimal Fluid Intake in the Common Population. Kidney Blood Press. Res. 2017, 42, 483–494. [Google Scholar] [CrossRef]
- Hassanein, M.; Bashier, A.; Randeree, H.; Abouelmagd, M.; AlBaker, W.; Afandi, B.; Abu Hijleh, O.; Shaltout, I.; EI-Sharkawy, M.; Dagdelen, S.; et al. Use of SGLT2 Inhibitors during Ramadan: An Expert Panel Statement. Diabetes Res. Clin. Pract. 2020, 169, 108465. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Herrera Soto, J.A.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary Indices during Dehydration, Exercise, and Rehydration. Int. J. Sport. Nutr. 1998, 8, 345–355. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Burg, M.B. Elevated Sodium and Dehydration Stimulate Inflammatory Signaling in Endothelial Cells and Promote Atherosclerosis. PLoS ONE 2015, 10, e0128870. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, N.I.; Burg, M.B. Secretion of von Willebrand Factor by Endothelial Cells Links Sodium to Hypercoagulability and Thrombosis. Proc. Natl. Acad. Sci. USA 2014, 111, 6485–6490. [Google Scholar] [CrossRef] [PubMed]
- Enhörning, S.; Hedblad, B.; Nilsson, P.M.; Engström, G.; Melander, O. Copeptin Is an Independent Predictor of Diabetic Heart Disease and Death. Am. Heart J. 2015, 169, 549. [Google Scholar] [CrossRef] [PubMed]
- The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. Am. J. Epidemiol. 1989, 129, 687–702. [Google Scholar] [CrossRef]
- Chuda, A.; Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. Role of Confirmed and Potential Predictors of an Unfavorable Outcome in Heart Failure in Everyday Clinical Practice. Ir. J. Med. Sci. 2022, 191, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Chuda-Wietczak, A.; Sakowicz, A.; Tycinska, A.; Bytyci, I.; Bielecka-Dabrowa, A. The GLVC Scoring System: A Single-Center Model for Predicting Survival and Hospitalization in Patients with Heart Failure. Ir. J. Med. Sci. 2023. online ahead of print. [Google Scholar] [CrossRef]
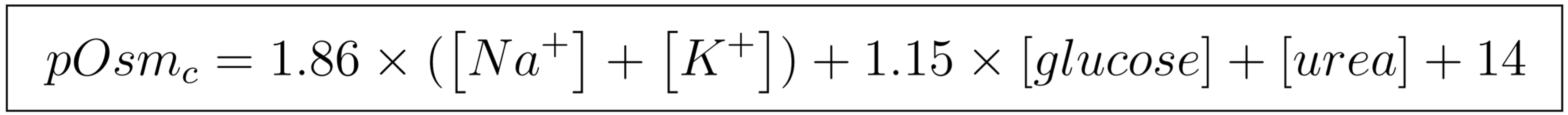
| Clinical Finding | Overhydration (Congestion) | Dehydration |
|---|---|---|
| Symptoms | Dyspnea (also paroxysmal nocturnal dyspnea), orthopnea, bendopnea, peripheral edema (ankle swelling) | Confusion, fatigue |
| Signs | Jugular venous distention, the third heart sound, pitting edema | Tongue dryness and furrows, dry mucous membranes, low urine output, speech difficulty, sunken eyes, low blood pressure, increased pulse rate |
| Laboratory findings | Elevated circulating levels of natriuretic peptides | Elevated plasma osmolality (direct laboratory measurement or calculated); creatinine, urinary sodium, BUN (blood urea nitrogen)/urea |
| Non-invasive methods | Bioelectrical impedance analysis (BIA) and bioelectrical impedance vector analysis (BIVA); lung ultrasound (LUS); chest X-ray; chest computed tomography (CT) | Tracer dilution techniques for total body water (TBW) measurement (gold standard); bioelectrical impedance analysis (BIA), especially resistance at 50 kHz |
| Invasive methods | Cardiac catheterization—measurement of the right atrial pressure and pulmonary capillary wedge pressure (PCWP) | - |
| - | Case 1 [113] | Case 2 [114] | Case 3 [115] | Case 4 [116] |
|---|---|---|---|---|
| Date of publication | 2022 | 2021 | 2013 | 2011 |
| Title | Short-Term Treatment with Empagliflozin Resulted in Dehydration and Cardiac Arrest in an Elderly Patient with Specific Complications: A Case Report and Literature Review | Recurrent Takotsubo syndrome complicated with ischemic enteritis successfully treated by hydration: a case report | Paradoxical Heart Failure Precipitated by Profound Dehydration: Intraventricular Dynamic Obstruction and Significant Mitral Regurgitation in a Volume-Depleted Heart | Dehydration with High Natriuretic Peptide Levels! A Word of Caution |
| Authors; country | Supakul et al.; Japan | Shunsuke Todani and Mao Takahashi; Japan | Kim et al.; South Korea | Alzand et al.; The Netherlands |
| Age and sex of patient | 68 years; man | 80 years; woman | 59 years; woman | 81 years; woman |
| Clinical presentation | The patient was hospitalized for a cerebral infarction. During hospitalization, he was diagnosed with type 2 diabetes mellitus and HF (ejection fraction of 39% with global hypokinesia). Empagliflozin 10 mg/day was started to treat hyperglycemia and HF. Coronary angiography was performed on day 18 and showed 90% stenosis of the left anterior descending artery. A drug-eluting stent was deployed on day 31 to treat the lesion. During hospitalization, the patient experienced decreased appetite. On day 33, intravenous hydration was discontinued. On day 40, the patient had a cardiac arrest. The ECG showed asystole, and the patient did not respond to cardiopulmonary resuscitation. The cause of death was suspected to be related to dehydration due to low food and fluid intake associated with empagliflozin treatment, which may have led to acute kidney injury, hyperkalemia, and subsequent cardiac arrest. | The patient was admitted to the hospital with upper abdominal pain and bloody stools. She had a history of Takotsubo syndrome (TTS) complicated by ischemic enteritis 4 months earlier. Blood work showed that the patient’s brain natriuretic peptide (BNP) level had increased to 1578 pg/mL. Echocardiography showed wall motion abnormality centered on the left central ventricle with apical ballooning. A recurrence of TTS was diagnosed. The authors suspected that the abdominal pain and dehydration due to ischemic enteritis may have contributed to the development of TTS. A coronary angiography and an acetylcholine provocation test were conducted. No significant coronary artery stenosis was found, but the acetylcholine provocation test revealed significant multivessel coronary spasm in the left coronary artery, suggesting coronary vasospastic angina pectoris associated with TTS. | The patient presented with dyspnea (NYHA III/IV), chest radiography showed pulmonary congestion. Transthoracic echocardiography revealed systolic anterior motion (SAM) of the mitral valve (MV), resulting in dynamic left ventricular outflow tract (LVOT) obstruction and significant mitral regurgitation. The hemodynamic abnormalities were exacerbated by dehydration, which resulted in an acute decrease in cardiac output and hemodynamic compromise. Despite pulmonary edema, the authors decided to restore the patient’s systemic volume because the cause of pulmonary congestion was not due to absolute systemic volume overload, but to intracardiac volume maldistribution resulting from SAM of the MV in the volume depleted heart. | The patient presented to an outpatient clinic with complaints of palpitations. She was taking chlorthalidone 50 mg and atenolol 100 mg. Physical examination revealed sinus tachycardia of 120/min. Cardiac echocardiography showed systolic anterior motion of the mitral valve (MV) with a maximal gradient of 82 mmHg over the left ventricular outflow tract and a grade 3+ MV regurgitation (MR). Laboratory findings revealed a high NT-proBNP level of 1357 pmol/L, which was significantly elevated compared to the routine chemistry check-up 4 weeks prior to presentation. Despite the MR, there was no evidence of increased filling pressures. The patient was diagnosed with dehydration and admitted to the hospital, where diuretics were discontinued and she received an intravenous infusion of 1.5 L/day. |
| Cause of dehydration | Decreased appetite, inadequate fluid intake, discontinuation of IV fluids | Ischemic enteritis, hematochezia | The patient had not taken any food or water for a week due to longstanding anorexia and vomiting associated with her brain disease (medical history of old cerebrovascular accident and epilepsy). | Use of diuretics (chlorthalidone) and presumably inadequate fluid intake |
| Treatment | See above | Oxygen (2 L/min); 10,000 units/day of continuous intravenous heparin for 2 days; fluid replacement of 1500 mL/day to treat TTS | Fluid therapy with crystalloid and other nutritional support for five days. | Diuretics were ceased and the patient received 1.5 L/day intravenous infusion of 0.9% normal saline. |
| Follow-up | - | The BNP level and myocardial wall motion were normalized on the fourth day after admission. Subsequent follow-up showed no recurrence of takotsubo syndrome over 3 years. | After receiving fluid therapy, the patient’s condition stabilized and laboratory findings gradually returned to normal. | The NT-proBNP level normalized and the clinical as well as the echocardiographic parameters stabilized 3 days after treatment. |
| Author’s conclusions | “Although there are many advantages to using SGLT-2 inhibitors, it is important to note that there are individual risks that potentially lead to serious adverse effects, as shown in this case. Careful monitoring of elderly patients with neurological deficits who receive this medication is strongly recommended”. | “Our treatment of TTS in the acute phase centered on replenishment of fluid to increase coronary blood flow, improve heart load without exacerbating heart failure, and stabilize circulation dynamics. We recommend that clinicians consider treating TTS using hydration rather than diuretics in patients with dehydration”. | “The case provides helpful insights into intracardiac hemodynamics in a volume-depleted heart”. | “The present case report may have significant clinical implication, illustrating that elevated BNP or NT-proBNP levels should not robotically be assumed to be related to high filling pressures and reflexively resulting in intensifying diuretic therapy”. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittczak, A.; Ślot, M.; Bielecka-Dabrowa, A. The Importance of Optimal Hydration in Patients with Heart Failure—Not Always Too Much Fluid. Biomedicines 2023, 11, 2684. https://doi.org/10.3390/biomedicines11102684
Wittczak A, Ślot M, Bielecka-Dabrowa A. The Importance of Optimal Hydration in Patients with Heart Failure—Not Always Too Much Fluid. Biomedicines. 2023; 11(10):2684. https://doi.org/10.3390/biomedicines11102684
Chicago/Turabian StyleWittczak, Andrzej, Maciej Ślot, and Agata Bielecka-Dabrowa. 2023. "The Importance of Optimal Hydration in Patients with Heart Failure—Not Always Too Much Fluid" Biomedicines 11, no. 10: 2684. https://doi.org/10.3390/biomedicines11102684
APA StyleWittczak, A., Ślot, M., & Bielecka-Dabrowa, A. (2023). The Importance of Optimal Hydration in Patients with Heart Failure—Not Always Too Much Fluid. Biomedicines, 11(10), 2684. https://doi.org/10.3390/biomedicines11102684







