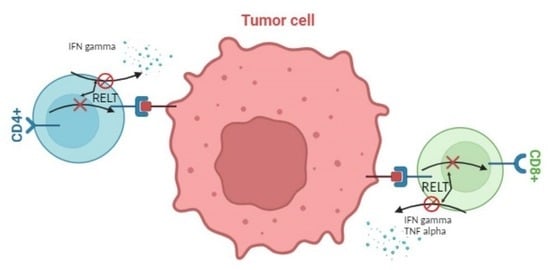
The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development
Abstract
:1. Introduction
2. Discovery, Chromosomal Locations, and Single Nucleotide Polymorphisms (SNPs)
3. Transcription and Transcriptional Regulation
3.1. Expression of RELT mRNA
3.2. Expression of RELL1 mRNA
3.3. Expression of RELL2 mRNA
3.4. Functional RNA Molecules Expressed from RELTfm Genes
4. Protein Structure, Expression, and Localization
4.1. RELT Protein Structure
4.2. RELL1 and RELL2 Protein Structure
4.3. Co-Immunoprecipitations of RELTfms and Localization
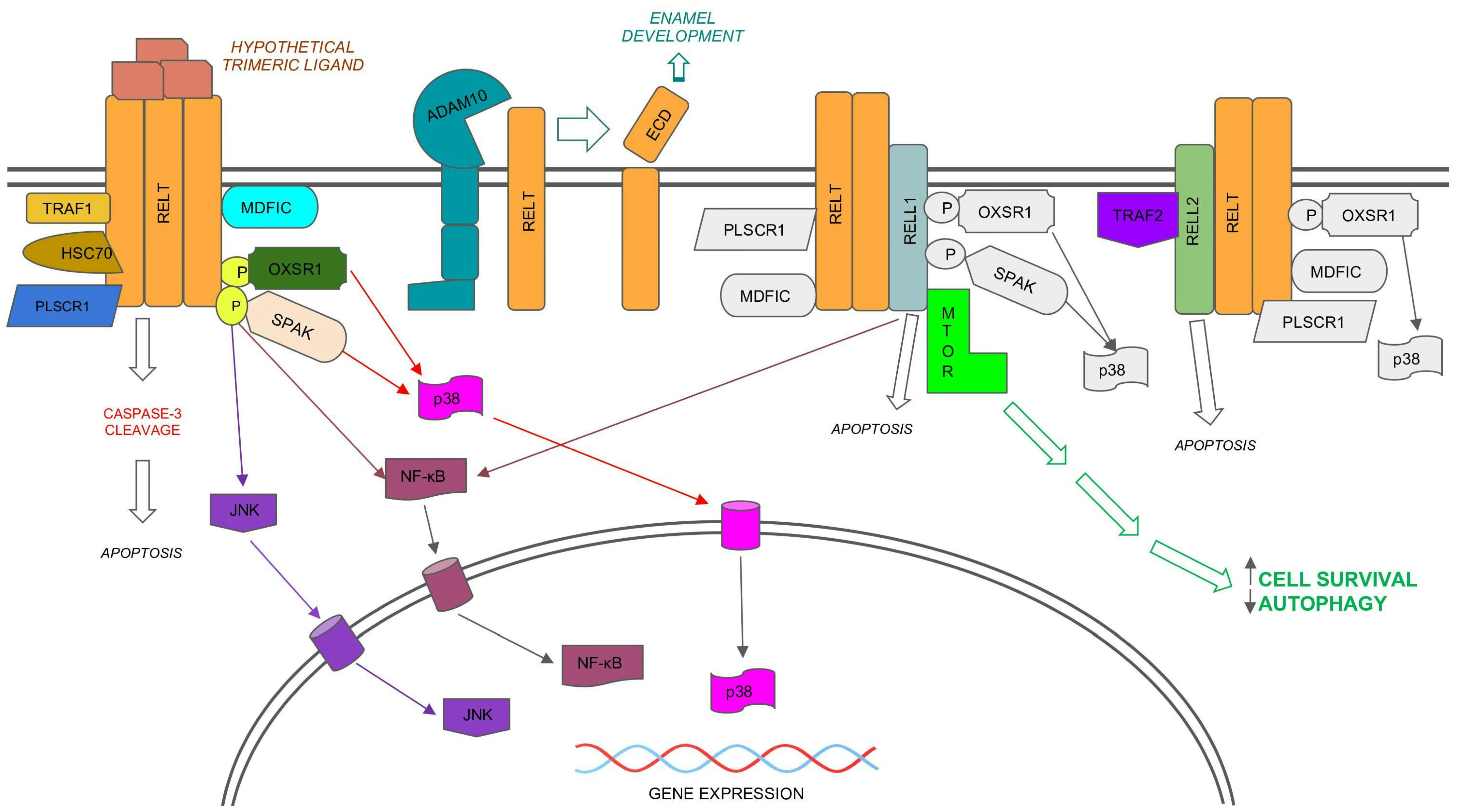
5. Phenotypes of Mice Lacking RELTfms
5.1. Phenotype of RELT Knockout Mouse
5.2. Phenotype of RELL1 Knockout Mouse
6. Activation of Signaling Pathways by RELTfms
6.1. Activation of NF-κB by RELTfms
6.2. Association of RELTfms with Interferon Signaling
7. Activation of p38 and JNK MAPK Pathways by OXSR1 and SPAK Kinases
8. Activation of Cell Death Pathways
8.1. Activation of Apoptosis by RELTfms
8.2. Autophagy Inhibition by RELL1
9. Association of RELTfms with Cancers
9.1. Breast Cancer
9.2. Esophageal Squamous Cell Carcinoma (ESCC)
9.3. Prostate Cancer
9.4. Glioma and Glioblastoma
9.5. Gastric Cancer
9.6. Pancreatic Cancer
9.7. Lung Cancer
9.8. Renal Cancer
9.9. Head and Neck Squamous Cell Carcinoma
9.10. Cancers of Hematopoietic Origin
9.11. Colorectal Cancer
9.12. RELL2 Involvement in Additional Cancers
10. Microbial Infections and Inflammation
10.1. Innate Immune Signaling
10.2. Regulation of Virus Infection
10.3. Regulation of Parasite Infection
10.4. Regulation of Bacterial Infection
11. Other Functions Associated with RELTfms
11.1. Enamel Development
11.2. Reproduction and Development
11.3. Blood Pressure and Myocardial Infarction
11.4. Brain and Behavior
12. Additional Binding Partners of RELTfms
12.1. Hsc70
12.2. PLSCR1
12.3. MDFIC
13. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef]
- Siegel, R.M.; Frederiksen, J.K.; Zacharias, D.A.; Chan, F.K.; Johnson, M.; Lynch, D.; Tsien, R.Y.; Lenardo, M.J. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science 2000, 288, 2354–2357. [Google Scholar] [CrossRef]
- Chan, F.K.; Chun, H.J.; Zheng, L.; Siegel, R.M.; Bui, K.L.; Lenardo, M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 2000, 288, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.A. p75NTR is positively promiscuous: Novel partners and new insights. Neuron 2004, 42, 529–533. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Croft, M.; Benedict, C.A.; Ware, C.F. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 2013, 12, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Sica, G.L.; Zhu, G.; Tamada, K.; Liu, D.; Ni, J.; Chen, L. RELT, a new member of the tumor necrosis factor receptor superfamily, is selectively expressed in hematopoietic tissues and activates transcription factor NF-kappaB. Blood 2001, 97, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Bossen, C.; Ingold, K.; Tardivel, A.; Bodmer, J.L.; Gaide, O.; Hertig, S.; Ambrose, C.; Tschopp, J.; Schneider, P. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J. Biol. Chem. 2006, 281, 13964–13971. [Google Scholar] [CrossRef]
- Cusick, J.K.; Xu, L.G.; Bin, L.H.; Han, K.J.; Shu, H.B. Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem. Biophys. Res. Commun. 2006, 340, 535–543. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Bhat, D.S.; Spies, M.A.; Spies, M. A moving target for drug discovery: Structure activity relationship and many genome (de)stabilizing functions of the RAD52 protein. DNA Repair 2022, 120, 103421. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Kim, S.H.; Kim, Y.H.; Lee, D.G.; Oh, H.S.; Han, C.; Kim, Y.I.; Jeon, Y.; Lee, H.; Kwon, B.S. RELT negatively regulates the early phase of the T-cell response in mice. Eur. J. Immunol. 2018, 48, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Zhang, H.; Seymen, F.; Koruyucu, M.; Hu, Y.; Kang, J.; Kim, Y.J.; Ikeda, A.; Kasimoglu, Y.; Bayram, M.; et al. Mutations in RELT cause autosomal recessive amelogenesis imperfecta. Clin. Genet. 2019, 95, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Bradford, Y.M.; Van Slyke, C.E.; Ruzicka, L.; Singer, A.; Eagle, A.; Fashena, D.; Howe, D.G.; Frazer, K.; Martin, R.; Paddock, H.; et al. Zebrafish information network, the knowledgebase for Danio rerio research. Genetics 2022, 220, iyac016. [Google Scholar] [CrossRef]
- Bowes, J.B.; Snyder, K.A.; Segerdell, E.; Jarabek, C.J.; Azam, K.; Zorn, A.M.; Vize, P.D. Xenbase: Gene expression and improved integration. Nucleic Acids Res. 2010, 38, D607–D612. [Google Scholar] [CrossRef]
- Baldarelli, R.M.; Smith, C.M.; Finger, J.H.; Hayamizu, T.F.; McCright, I.J.; Xu, J.; Shaw, D.R.; Beal, J.S.; Blodgett, O.; Campbell, J.; et al. The mouse Gene Expression Database (GXD): 2021 update. Nucleic Acids Res. 2021, 49, D924–D931. [Google Scholar] [CrossRef]
- Manabe, R.; Tsutsui, K.; Yamada, T.; Kimura, M.; Nakano, I.; Shimono, C.; Sanzen, N.; Furutani, Y.; Fukuda, T.; Oguri, Y.; et al. Transcriptome-based systematic identification of extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 12849–12854. [Google Scholar] [CrossRef]
- Huang, H.S.; Huang, X.Y.; Yu, H.Z.; Xue, Y.; Zhu, P.L. Circular RNA circ-RELL1 regulates inflammatory response by miR-6873-3p/MyD88/NF-kappaB axis in endothelial cells. Biochem. Biophys. Res. Commun. 2020, 525, 512–519. [Google Scholar] [CrossRef]
- Sang, H.; Zhang, W.; Peng, L.; Wei, S.; Zhu, X.; Huang, K.; Yang, J.; Chen, M.; Dang, Y.; Zhang, G. Exosomal circRELL1 serves as a miR-637 sponge to modulate gastric cancer progression via regulating autophagy activation. Cell Death Dis. 2022, 13, 56. [Google Scholar] [CrossRef]
- Angenard, G.; Merdrignac, A.; Louis, C.; Edeline, J.; Coulouarn, C. Expression of long non-coding RNA ANRIL predicts a poor prognosis in intrahepatic cholangiocarcinoma. Dig. Liver Dis. 2019, 51, 1337–1343. [Google Scholar] [CrossRef]
- Moua, P.; Checketts, M.; Xu, L.G.; Shu, H.B.; Reyland, M.E.; Cusick, J.K. RELT family members activate p38 and induce apoptosis by a mechanism distinct from TNFR1. Biochem. Biophys. Res. Commun. 2017, 491, 25–32. [Google Scholar] [CrossRef]
- Ikeda, A.; Shahid, S.; Blumberg, B.R.; Suzuki, M.; Bartlett, J.D. ADAM10 is Expressed by Ameloblasts, Cleaves the RELT TNF Receptor Extracellular Domain and Facilitates Enamel Development. Sci. Rep. 2019, 9, 14086. [Google Scholar] [CrossRef]
- Cusick, J.K.; Alhomsy, Y.; Wong, S.; Talbott, G.; Uversky, V.N.; Hart, C.; Hejazi, N.; Jacobs, A.T.; Shi, Y. RELT stains prominently in B-cell lymphomas and binds the hematopoietic transcription factor MDFIC. Biochem. Biophys. Rep. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Cusick, J.K.; Mustian, A.; Goldberg, K.; Reyland, M.E. RELT induces cellular death in HEK 293 epithelial cells. Cell. Immunol. 2010, 261, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cusick, J.K.; Mustian, A.; Jacobs, A.T.; Reyland, M.E. Identification of PLSCR1 as a protein that interacts with RELT family members. Mol. Cell. Biochem. 2012, 362, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Park, S.; Gunassekaran, G.R.; Jeon, M.; Cho, Y.E.; Baek, M.C.; Park, J.Y.; Shim, G.; Oh, Y.K.; Kim, I.S.; et al. A Peptide Probe Enables Photoacoustic-Guided Imaging and Drug Delivery to Lung Tumors in K-ras(LA2) Mutant Mice. Cancer Res. 2019, 79, 4271–4282. [Google Scholar] [CrossRef]
- Polek, T.C.; Talpaz, M.; Spivak-Kroizman, T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem. Biophys. Res. Commun. 2006, 343, 125–134. [Google Scholar] [CrossRef]
- Feng, L.; Hu, J.; Zhang, W.; Dong, Y.; Xiong, S.; Dong, C. RELL1 inhibits autophagy pathway and regulates Mycobacterium tuberculosis survival in macrophages. Tuberculosis 2020, 120, 101900. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Chen, Q.; Li, S.; Jia, X.; Xu, L.; Wei, L. RELT promotes the growth of esophageal squamous cell carcinoma by activating the NF-kappaB pathway. Cell Cycle 2021, 20, 1231–1241. [Google Scholar] [CrossRef]
- Dickinson, M.E.; Flenniken, A.M.; Ji, X.; Teboul, L.; Wong, M.D.; White, J.K.; Meehan, T.F.; Weninger, W.J.; Westerberg, H.; Adissu, H.; et al. High-throughput discovery of novel developmental phenotypes. Nature 2016, 537, 508–514. [Google Scholar] [CrossRef]
- Rayet, B.; Gelinas, C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 1999, 18, 6938–6947. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Baud’huin, M.; Lamoureux, F.; Duplomb, L.; Redini, F.; Heymann, D. RANKL, RANK, osteoprotegerin: Key partners of osteoimmunology and vascular diseases. Cell. Mol. Life Sci. 2007, 64, 2334–2350. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Nakanishi, C.; Toi, M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef]
- Kumar, D.; Nath, L.; Kamal, M.A.; Varshney, A.; Jain, A.; Singh, S.; Rao, K.V. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 2010, 140, 731–743. [Google Scholar] [CrossRef]
- Gangaplara, A.; Martens, C.; Dahlstrom, E.; Metidji, A.; Gokhale, A.S.; Glass, D.D.; Lopez-Ocasio, M.; Baur, R.; Kanakabandi, K.; Porcella, S.F.; et al. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 2018, 14, e1006985. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, P.; Ma, J.; Xu, J.; Yang, L.; Xu, W.; Que, H.; Chen, M.; Xu, H. Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med. 2019, 8, 1576–1583. [Google Scholar] [CrossRef]
- Delpire, E.; Gagnon, K.B. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol. Genom. 2007, 28, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.B.; Delpire, E. Molecular physiology of SPAK and OSR1: Two Ste20-related protein kinases regulating ion transport. Physiol. Rev. 2012, 92, 1577–1617. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.M.; Naselli, G.; Gonez, L.J.; Martin, R.M.; Harrison, L.C.; DeAizpurua, H.J. SPAK, a STE20/SPS1-related kinase that activates the p38 pathway. Oncogene 2000, 19, 4290–4297. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Ono, K.; Han, J. The p38 signal transduction pathway: Activation and function. Cell. Signal. 2000, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Limon, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Nebreda, A.R. Roles of p38alpha mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015, 282, 1841–1857. [Google Scholar] [CrossRef]
- Yin, N.; Qi, X.; Tsai, S.; Lu, Y.; Basir, Z.; Oshima, K.; Thomas, J.P.; Myers, C.R.; Stoner, G.; Chen, G. p38gamma MAPK is required for inflammation-associated colon tumorigenesis. Oncogene 2016, 35, 1039–1048. [Google Scholar] [CrossRef]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef]
- Fuchs, S.Y.; Adler, V.; Pincus, M.R.; Ronai, Z. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. USA 1998, 95, 10541–10546. [Google Scholar] [CrossRef]
- Tran, T.H.; Andreka, P.; Rodrigues, C.O.; Webster, K.A.; Bishopric, N.H. Jun kinase delays caspase-9 activation by interaction with the apoptosome. J. Biol. Chem. 2007, 282, 20340–20350. [Google Scholar] [CrossRef]
- Yu, C.; Minemoto, Y.; Zhang, J.; Liu, J.; Tang, F.; Bui, T.N.; Xiang, J.; Lin, A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol. Cell 2004, 13, 329–340. [Google Scholar] [CrossRef]
- Staples, C.J.; Owens, D.M.; Maier, J.V.; Cato, A.C.; Keyse, S.M. Cross-talk between the p38alpha and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J. Biol. Chem. 2010, 285, 25928–25940. [Google Scholar] [CrossRef]
- Miura, H.; Kondo, Y.; Matsuda, M.; Aoki, K. Cell-to-Cell Heterogeneity in p38-Mediated Cross-Inhibition of JNK Causes Stochastic Cell Death. Cell Rep. 2018, 24, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Kang, T.B.; Kovalenko, A. The extrinsic cell death pathway and the elan mortel. Cell Death Differ. 2008, 15, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vandevoorde, V.; Haegeman, G.; Fiers, W. Induced expression of trimerized intracellular domains of the human tumor necrosis factor (TNF) p55 receptor elicits TNF effects. J. Cell Biol. 1997, 137, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Krautwald, S.; Ziegler, E.; Rolver, L.; Linkermann, A.; Keyser, K.A.; Steen, P.; Wollert, K.C.; Korf-Klingebiel, M.; Kunzendorf, U. Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J. Biol. Chem. 2010, 285, 19997–20005. [Google Scholar] [CrossRef]
- Zhou, Q.; Snipas, S.; Orth, K.; Muzio, M.; Dixit, V.M.; Salvesen, G.S. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J. Biol. Chem. 1997, 272, 7797–7800. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Ji, H.; Lu, M.; Li, Z.; Qiao, X.; Sun, B.; Zhang, W.; Xue, D. Proteomic analysis of apoptotic and oncotic pancreatic acinar AR42J cells treated with caerulein. Mol. Cell Biochem. 2013, 382, 1–17. [Google Scholar] [CrossRef]
- Johansen, S.; Traynor, S.; Ebstrup, M.L.; Terp, M.G.; Pedersen, C.B.; Ditzel, H.J.; Gjerstorff, M.F. ZBED1 Regulates Genes Important for Multiple Biological Processes of the Placenta. Genes 2022, 13, 133. [Google Scholar] [CrossRef]
- Bi, S.; Ma, X.; Wang, Y.; Chi, X.; Zhang, Y.; Xu, W.; Hu, S. Protective Effect of Ginsenoside Rg1 on Oxidative Damage Induced by Hydrogen Peroxide in Chicken Splenic Lymphocytes. Oxidative Med. Cell. Longev. 2019, 2019, 8465030. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Q.; Du, X.; Chen, Y.; Zhang, T. Targeted regulation of Rell2 by microRNA-18a is implicated in the anti-metastatic effect of polyphyllin VI in breast cancer cells. Eur. J. Pharmacol. 2019, 851, 161–173. [Google Scholar] [CrossRef]
- Leone, R.D.; Amaravadi, R.K. Autophagy: A targetable linchpin of cancer cell metabolism. Trends Endocrinol. Metab. 2013, 24, 209–217. [Google Scholar] [CrossRef]
- Maycotte, P.; Thorburn, A. Targeting autophagy in breast cancer. World J. Clin. Oncol. 2014, 5, 224–240. [Google Scholar] [CrossRef]
- Zhong, L.; Ge, K.; Zu, J.C.; Zhao, L.H.; Shen, W.K.; Wang, J.F.; Zhang, X.G.; Gao, X.; Hu, W.; Yen, Y.; et al. Autoantibodies as potential biomarkers for breast cancer. Breast Cancer Res. 2008, 10, R40. [Google Scholar] [CrossRef]
- Johansson, J.; Tabor, V.; Wikell, A.; Jalkanen, S.; Fuxe, J. TGF-beta1-Induced Epithelial-Mesenchymal Transition Promotes Monocyte/Macrophage Properties in Breast Cancer Cells. Front. Oncol. 2015, 5, 3. [Google Scholar] [CrossRef]
- Tang, S.J.; Shen, H.; An, O.; Hong, H.; Li, J.; Song, Y.; Han, J.; Tay, D.J.T.; Ng, V.H.E.; Bellido Molias, F.; et al. Cis- and trans-regulations of pre-mRNA splicing by RNA editing enzymes influence cancer development. Nat. Commun. 2020, 11, 799. [Google Scholar] [CrossRef]
- Ge, S.; Hua, X.; Chen, J.; Xiao, H.; Zhang, L.; Zhou, J.; Liang, C.; Tai, S. Identification of a Costimulatory Molecule-Related Signature for Predicting Prognostic Risk in Prostate Cancer. Front. Genet. 2021, 12, 666300. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Dong, Y.; Gu, Y.; Kapoor, A.; Lin, X.; Su, Y.; Vega Neira, S.; Tang, D. IQGAP3 is relevant to prostate cancer: A detailed presentation of potential pathomechanisms. J. Adv. Res. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xie, H.; Liu, X.; Shen, Q.; Wang, Z.; Hao, H.; Gu, Y. RELL1, a novel oncogene, accelerates tumor progression and regulates immune infiltrates in glioma. Int. Immunopharmacol. 2020, 87, 106707. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Cardon, T.; Aboulouard, S.; Hajjaji, N.; Kobeissy, F.; Duhamel, M.; Fournier, I.; Salzet, M. Surfaceome Proteomic of Glioblastoma Revealed Potential Targets for Immunotherapy. Front. Immunol. 2021, 12, 746168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qin, C.; Zhao, B.; Wang, Y.; Li, T.; Zhao, Y.; Wang, W. DHX38 restricts chemoresistance by regulating the alternative pre-mRNA splicing of RELL2 in pancreatic ductal adenocarcinoma. PLOS Genet. 2023, 19, e1010847. [Google Scholar] [CrossRef] [PubMed]
- Schrump, D.S.; Fischette, M.R.; Nguyen, D.M.; Zhao, M.; Li, X.; Kunst, T.F.; Hancox, A.; Hong, J.A.; Chen, G.A.; Kruchin, E.; et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin. Cancer Res. 2008, 14, 188–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, P.; Zhang, C.; Luo, Y.; Zhang, G.; Zeng, Q.; Wang, L.; Yang, Z.; Sun, N.; He, J. Tumor Necrosis Factor Family Member Profile Predicts Prognosis and Adjuvant Chemotherapy Benefit for Patients with Small-Cell Lung Cancer. Front. Immunol. 2021, 12, 745769. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, T.; Xu, F.; Zhang, J.; Wang, Y.; Wu, J.; Bu, H.; Fu, D.; Fang, B.; Lv, H.; et al. KCNN4 may weaken anti-tumor immune response via raising Tregs and diminishing resting mast cells in clear cell renal cell carcinoma. Cancer Cell Int. 2022, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.K.; Wang, Z.W.; Su, W.H.; Xu, K.; Jia, H.; Chen, J. Identification of Novel Biomarkers for Predicting Prognosis and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma Based on ceRNA Network and Immune Infiltration Analysis. BioMed Res. Int. 2021, 2021, 4532438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, L.T.; Hu, L.J.; Lv, M. Comprehensive analysis of ECHDC3 as a potential biomarker and therapeutic target for acute myeloid leukemia: Bioinformatic analysis and experimental verification. Front. Oncol. 2022, 12, 947492. [Google Scholar] [CrossRef]
- Kadkhoda, S.; Darbeheshti, F.; Rezaei, N.; Azizi-Tabesh, G.; Zolfaghari, F.; Tavakolibazaz, S.; Taslimi, R.; Tavakkoly-Bazzaz, J. Investigation of circRNA-miRNA-mRNA network in colorectal cancer using an integrative bioinformatics approach. Gastroenterol. Hepatol. Bed Bench 2021, 14, 141–153. [Google Scholar]
- Qiu, J.; Keyser, B.; Lin, Z.T.; Wu, T. Autoantibodies as Potential Biomarkers in Breast Cancer. Biosensors 2018, 8, 67. [Google Scholar] [CrossRef]
- Lacombe, J.; Mange, A.; Solassol, J. Use of autoantibodies to detect the onset of breast cancer. J. Immunol. Res. 2014, 2014, 574981. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, J.; Ouyang, L.; Wang, Y. Polo-like Kinase 1 Inhibitors in Human Cancer Therapy: Development and Therapeutic Potential. J. Med. Chem. 2022, 65, 10133–10160. [Google Scholar] [CrossRef]
- Pommier, Y.; Nussenzweig, A.; Takeda, S.; Austin, C. Human topoisomerases and their roles in genome stability and organization. Nat. Rev. Mol. Cell Biol. 2022, 23, 407–427. [Google Scholar] [CrossRef]
- Musha, K.; Ge, X.; Ablikim, N.; Lu, B.; Chen, C.; Huang, J. Comprehensive Analysis of RELL2 as a Potential Biomarker Associated with Tumor Immune Infiltrating Cells in a Pan-Cancer Analysis. Dis. Markers 2022, 2022, 5009512. [Google Scholar] [CrossRef]
- Oakley, R.H.; Busillo, J.M.; Cidlowski, J.A. Cross-talk between the glucocorticoid receptor and MyoD family inhibitor domain-containing protein provides a new mechanism for generating tissue-specific responses to glucocorticoids. J. Biol. Chem. 2017, 292, 5825–5844. [Google Scholar] [CrossRef]
- Nicolaou, S.A.; Neumeier, L.; Peng, Y.; Devor, D.C.; Conforti, L. The Ca(2+)-activated K(+) channel KCa3.1 compartmentalizes in the immunological synapse of human T lymphocytes. Am. J. Physiol. Physiol. 2007, 292, C1431–C1439. [Google Scholar] [CrossRef]
- Nikolopoulos, G.; Smith, C.E.L.; Brookes, S.J.; El-Asrag, M.E.; Brown, C.J.; Patel, A.; Murillo, G.; O’Connell, M.J.; Inglehearn, C.F.; Mighell, A.J. New missense variants in RELT causing hypomineralised amelogenesis imperfecta. Clin. Genet. 2020, 97, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, S.E.; Mann, S.A.; Bodansky, A.; Kung, A.F.; Quandt, Z.; Ferre, E.M.N.; Landegren, N.; Eriksson, D.; Bastard, P.; Zhang, S.Y.; et al. Autoantibody discovery across monogenic, acquired, and COVID-19-associated autoimmunity with scalable PhIP-seq. Elife 2022, 11, e78550. [Google Scholar] [CrossRef] [PubMed]
- Rakus, J.F.; Kegley, N.R.; Ashley, A.J.; Parsons, M.A.; Takeuchi, M. Lipopolysaccharide stimulation of RAW264.7 cells is a model for identifying novel clients of Hsc70. bioRxiv 2020. [Google Scholar] [CrossRef]
- Vadaq, N.; Zhang, Y.; Vos, W.A.; Groenendijk, A.L.; Blaauw, M.J.; van Eekeren, L.E.; Jacobs-Cleophas, M.; van de Wijer, L.; Dos Santos, J.C.; Gasem, M.H.; et al. High-throughput proteomic analysis reveals systemic dysregulation in virally suppressed people living with HIV. JCI Insight 2023, 8, e166166. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Basmaciogullari, S.; Bouchet, J.; Zarka, M.; Moura, I.C.; Benhamou, M.; Monteiro, R.C.; Hocini, H.; Madrid, R.; Benichou, S. The phospholipid scramblases 1 and 4 are cellular receptors for the secretory leukocyte protease inhibitor and interact with CD4 at the plasma membrane. PLoS ONE 2009, 4, e5006. [Google Scholar] [CrossRef]
- Li, Y.; Shang, W.; Xiao, G.; Zhang, L.K.; Zheng, C. A Comparative Quantitative Proteomic Analysis of HCMV-Infected Cells Highlights pUL138 as a Multifunctional Protein. Molecules 2020, 25, 2520. [Google Scholar] [CrossRef]
- DiNardo, A.R.; Nishiguchi, T.; Mace, E.M.; Rajapakshe, K.; Mtetwa, G.; Kay, A.; Maphalala, G.; Secor, W.E.; Mejia, R.; Orange, J.S.; et al. Schistosomiasis Induces Persistent DNA Methylation and Tuberculosis-Specific Immune Changes. J. Immunol. 2018, 201, 124–133. [Google Scholar] [CrossRef]
- Brown, E.L.; Below, J.E.; Fischer, R.S.; Essigmann, H.T.; Hu, H.; Huff, C.; Robinson, D.A.; Petty, L.E.; Aguilar, D.; Bell, G.I.; et al. Genome-Wide Association Study of Staphylococcus aureus Carriage in a Community-Based Sample of Mexican-Americans in Starr County, Texas. PLoS ONE 2015, 10, e0142130. [Google Scholar] [CrossRef]
- Simonsen, J.R.; Karajamaki, A.; Antikainen, A.A.; Toppila, I.; Ahlqvist, E.; Prasad, R.; Mansour-Aly, D.; Harjutsalo, V.; Jarvinen, A.; Tuomi, T.; et al. Genetic factors affect the susceptibility to bacterial infections in diabetes. Sci. Rep. 2021, 11, 9464. [Google Scholar] [CrossRef] [PubMed]
- Onteru, S.K.; Fan, B.; Du, Z.Q.; Garrick, D.J.; Stalder, K.J.; Rothschild, M.F. A whole-genome association study for pig reproductive traits. Anim. Genet. 2012, 43, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Shim, J.; Oh, K.B.; Lee, H.; Park, M.R. cd26 Knockdown Negatively Affects Porcine Parthenogenetic Preimplantation Embryo Development. Animals 2022, 12, 1662. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Matos, M.C.; Seno, L.O.; Romero, A.R.; Garcia, J.F.; Grisolia, A.B. Genome wide association study on early puberty in Bos indicus. Genet. Mol. Res. 2016, 15, gmr.15017548. [Google Scholar] [CrossRef]
- Wan, X.; Jing, J.N.; Wang, D.F.; Lv, F.H. Whole-genome selective scans detect genes associated with important phenotypic traits in goat (Capra hircus). Front. Genet. 2023, 14, 1173017. [Google Scholar] [CrossRef]
- Chai, T.; Tian, M.; Yang, X.; Qiu, Z.; Lin, X.; Chen, L. Association of Circulating Cathepsin B Levels with Blood Pressure and Aortic Dilation. Front. Cardiovasc. Med. 2022, 9, 762468. [Google Scholar] [CrossRef]
- Ngo, D.; Sinha, S.; Shen, D.; Kuhn, E.W.; Keyes, M.J.; Shi, X.; Benson, M.D.; O’Sullivan, J.F.; Keshishian, H.; Farrell, L.A.; et al. Aptamer-Based Proteomic Profiling Reveals Novel Candidate Biomarkers and Pathways in Cardiovascular Disease. Circulation 2016, 134, 270–285. [Google Scholar] [CrossRef]
- Lind, L.; Zanetti, D.; Ingelsson, M.; Gustafsson, S.; Arnlov, J.; Assimes, T.L. Large-Scale Plasma Protein Profiling of Incident Myocardial Infarction, Ischemic Stroke, and Heart Failure. J. Am. Heart Assoc. 2021, 10, e023330. [Google Scholar] [CrossRef]
- Brown, A.; Meor Azlan, N.F.; Wu, Z.; Zhang, J. WNK-SPAK/OSR1-NCC kinase signaling pathway as a novel target for the treatment of salt-sensitive hypertension. Acta Pharmacol. Sin. 2021, 42, 508–517. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Y.; Wang, X.; Wang, C.; Li, M.; Liu, H.; Han, W.; Wang, H.; Zhang, W.; Wei, H.; et al. The Vulnerability to Methamphetamine Dependence and Genetics: A Case-Control Study Focusing on Genetic Polymorphisms at Chromosomal Region 5q31.3. Front. Psychiatry 2022, 13, 870322. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, J.; Stout, J.G.; Luhm, R.A.; Wiedmer, T.; Sims, P.J. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 1997, 272, 18240–18244. [Google Scholar] [CrossRef]
- Fadok, V.A.; de Cathelineau, A.; Daleke, D.L.; Henson, P.M.; Bratton, D.L. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 2001, 276, 1071–1077. [Google Scholar] [CrossRef]
- Zhou, Q.; Ben-Efraim, I.; Bigcas, J.L.; Junqueira, D.; Wiedmer, T.; Sims, P.J. Phospholipid scramblase 1 binds to the promoter region of the inositol 1,4,5-triphosphate receptor type 1 gene to enhance its expression. J. Biol. Chem. 2005, 280, 35062–35068. [Google Scholar] [CrossRef]
- Bailey, K.; Cook, H.W.; McMaster, C.R. The phospholipid scramblase PLSCR1 increases UV induced apoptosis primarily through the augmentation of the intrinsic apoptotic pathway and independent of direct phosphorylation by protein kinase C delta. Biochim. Biophys. Acta 2005, 1733, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rogulski, K.; Zhou, Q.; Sims, P.J.; Prochownik, E.V. The negative c-Myc target onzin affects proliferation and apoptosis via its obligate interaction with phospholipid scramblase 1. Mol. Cell Biol. 2006, 26, 3401–3413. [Google Scholar] [CrossRef]
- Yu, A.; McMaster, C.R.; Byers, D.M.; Ridgway, N.D.; Cook, H.W. Stimulation of phosphatidylserine biosynthesis and facilitation of UV-induced apoptosis in Chinese hamster ovary cells overexpressing phospholipid scramblase 1. J. Biol. Chem. 2003, 278, 9706–9714. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zhou, Q.; Zhao, J.; Zhou, A.; Harty, R.N.; Bose, S.; Banerjee, A.; Slee, R.; Guenther, J.; Williams, B.R.; et al. Phospholipid scramblase 1 potentiates the antiviral activity of interferon. J. Virol. 2004, 78, 8983–8993. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yamashita, T.; Shiozawa, E.; Nagasawa, A.; Okabe-Kado, J.; Nakamaki, T.; Tomoyasu, S.; Kimura, F.; Motoyoshi, K.; Honma, Y.; et al. MmTRA1b/phospholipid scramblase 1 gene expression is a new prognostic factor for acute myelogenous leukemia. Leuk. Res. 2004, 28, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.W.; Li, X.; Zhao, Q.; Huang, Y.; Li, D.; Peng, Z.G.; Shen, W.Z.; Zhao, J.; Zhou, Q.; Chen, Z.; et al. Protein kinase Cdelta mediates retinoic acid and phorbol myristate acetate-induced phospholipid scramblase 1 gene expression: Its role in leukemic cell differentiation. Blood 2004, 104, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Q.; Zhou, C.X.; Gu, Z.M.; Li, D.; Xu, H.Z.; Wiedmer, T.; Sims, P.J.; Zhao, K.W.; Chen, G.Q. Antileukemic roles of human phospholipid scramblase 1 gene, evidence from inducible PLSCR1-expressing leukemic cells. Oncogene 2006, 25, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Liao, R.; Chen, X.; Wu, X.; Li, X.; Wang, Y.; Cao, Q.; Dong, C. Nuclear translocation of PLSCR1 activates STAT1 signaling in basal-like breast cancer. Theranostics 2020, 10, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.W.; Chen, C.Y.; Chen, K.T.; Shen, C.R.; Kuo, Y.B.; Chen, Y.S.; Chou, Y.P.; Wei, W.S.; Chan, E.C. Blockade of phospholipid scramblase 1 with its N-terminal domain antibody reduces tumorigenesis of colorectal carcinomas in vitro and in vivo. J. Transl. Med. 2012, 10, 254. [Google Scholar] [CrossRef]
- Mastorci, K.; Montico, B.; Fae, D.A.; Sigalotti, L.; Ponzoni, M.; Inghirami, G.; Dolcetti, R.; Dal Col, J. Phospholipid scramblase 1 as a critical node at the crossroad between autophagy and apoptosis in mantle cell lymphoma. Oncotarget 2016, 7, 41913–41928. [Google Scholar] [CrossRef]
- Shi, K.; An, J.; Qian, K.; Zhao, X.; Li, F.; Ma, X.; Wang, Y.; Zhang, Y. p53 controls the switch between autophagy and apoptosis through regulation of PLSCR1 in sodium selenite-treated leukemia cells. Exp. Cell Res. 2020, 389, 111879. [Google Scholar] [CrossRef]
- Thebault, S.; Gachon, F.; Lemasson, I.; Devaux, C.; Mesnard, J.M. Molecular cloning of a novel human I-mfa domain-containing protein that differently regulates human T-cell leukemia virus type I and HIV-1 expression. J. Biol. Chem. 2000, 275, 4848–4857. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Dean, J.; Oliveira, A.L.; Sheehy, N.; Hall, W.W.; Gautier, V.W. Expression profile and differential regulation of the Human I-mfa domain-Containing protein (HIC) gene in immune cells. Immunol. Lett. 2009, 123, 179–184. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, C.J.; Yang, S.F.; Huang, M.S.; Liu, Y.P. The MyoD family inhibitor domain-containing protein enhances the chemoresistance of cancer stem cells in the epithelial state by increasing beta-catenin activity. Oncogene 2020, 39, 2377–2390. [Google Scholar] [CrossRef]
- Sui, Y.; Li, X.; Oh, S.; Zhang, B.; Freeman, W.M.; Shin, S.; Janknecht, R. Opposite Roles of the JMJD1A Interaction Partners MDFI and MDFIC in Colorectal Cancer. Sci. Rep. 2020, 10, 8710. [Google Scholar] [CrossRef]
- Tripputi, P.; Bianchi, P.; Fermo, E.; Bignotto, M.; Zanella, A. Chromosome 7q31.1 deletion in myeloid neoplasms. Hum. Pathol. 2014, 45, 368–371. [Google Scholar] [CrossRef]
- Kojima, T.; Morikawa, Y.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Senba, E.; Kitamura, T. TROY, a newly identified member of the tumor necrosis factor receptor superfamily, exhibits a homology with Edar and is expressed in embryonic skin and hair follicles. J. Biol. Chem. 2000, 275, 20742–20747. [Google Scholar] [CrossRef] [PubMed]
- Loftus, J.C.; Dhruv, H.; Tuncali, S.; Kloss, J.; Yang, Z.; Schumacher, C.A.; Cao, B.; Williams, B.O.; Eschbacher, J.M.; Ross, J.T.; et al. TROY (TNFRSF19) promotes glioblastoma survival signaling and therapeutic resistance. Mol. Cancer Res. 2013, 11, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Lin, Y.X.; Qi, X.K.; He, G.P.; Zhang, Y.; Zhang, H.J.; Xu, M.; Feng, Q.S.; Bei, J.X.; Zeng, Y.X.; et al. TNFRSF19 Inhibits TGFbeta Signaling through Interaction with TGFbeta Receptor Type I to Promote Tumorigenesis. Cancer Res. 2018, 78, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; O’Rourke, K.; Yu, G.L.; Lyons, R.H.; Garg, M.; Duan, D.R.; Xing, L.; Gentz, R.; Ni, J.; Dixit, V.M. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science 1996, 274, 990–992. [Google Scholar] [CrossRef]
- Migone, T.S.; Zhang, J.; Luo, X.; Zhuang, L.; Chen, C.; Hu, B.; Hong, J.S.; Perry, J.W.; Chen, S.F.; Zhou, J.X.; et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 2002, 16, 479–492. [Google Scholar] [CrossRef]
- Wang, E.C.; Thern, A.; Denzel, A.; Kitson, J.; Farrow, S.N.; Owen, M.J. DR3 regulates negative selection during thymocyte development. Mol. Cell Biol. 2001, 21, 3451–3461. [Google Scholar] [CrossRef]
- Soroosh, P.; Ine, S.; Sugamura, K.; Ishii, N. Differential requirements for OX40 signals on generation of effector and central memory CD4+ T cells. J. Immunol. 2007, 179, 5014–5023. [Google Scholar] [CrossRef]
- Byun, M.; Ma, C.S.; Akcay, A.; Pedergnana, V.; Palendira, U.; Myoung, J.; Avery, D.T.; Liu, Y.; Abhyankar, A.; Lorenzo, L.; et al. Inherited human OX40 deficiency underlying classic Kaposi sarcoma of childhood. J. Exp. Med. 2013, 210, 1743–1759. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, S.; Hori, T.; Imura, A.; Takaori-Kondo, A.; Uchiyama, T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J. Biol. Chem. 1998, 273, 5808–5814. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.R.; Song, J.; Gramaglia, I.; Killeen, N.; Croft, M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 2001, 15, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.F. Network communications: Lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005, 23, 787–819. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.P.; Naniche, D.; Crowley, M.T.; Koni, P.A.; Flavell, R.A.; Oldstone, M.B. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology 1999, 260, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Seleznik, G.M.; Zeller, N.; Heikenwalder, M. The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: From lymphoid tissue formation to liver and prostate cancer development. Oncogene 2010, 29, 5006–5018. [Google Scholar] [CrossRef]
| Cancer or Cancer Cell Line | Gene or Protein | Predicted Effect, Prognostic Indicator, or Expression Level | Details of Study Implicating RELTfm with Cancer | Reference |
|---|---|---|---|---|
| Breast cancer | RELT | Autoantibodies against RELT potential biomarker for cancer | RELT likely upregulated or altered in breast cancer, based on the identification of RELT autoantibodies in serum of women with breast cancer, that are absent in healthy controls | [65]: Zhong L. et al., 2008 |
| Breast cancer | RELT | Upregulated in cancer | Upregulated RELT RNA in mouse mammary EpRas tumor cells treated with TGF-β for two weeks to induce EMT. RELT associated with cluster of genes upregulated in human triple-negative breast cancer, based on microarray analysis of 38 breast cancer cell lines and 107 human breast cancer tissues. | [66]: Johansson J. et al., 2015 |
| Breast cancer | RELL2 | Protective against cancer | Expression of RELL2 inhibits migration and invasion capabilities of breast cancer cell lines 4T1 and MDA-MB-231 (231) ex vivo. The saponin PP-VI indirectly upregulates RELL2 expression, and PP-VI treatment inhibited growth of 4T1 in mice in vivo and induced cell cycle arrest and apoptosis of 4T1 and 231 cells ex vivo. | [62]: Wang P. et al., 2019 |
| ESCC | RELT | Pro-tumorigenic, Poor prognostic indicator, Upregulated in cancer | RELT functions as an oncogene that upregulates NF-κB and inhibits pathways based on both ex-vivo and in-vivo studies. RELT expression upregulated ESCC based on bioinformatic and laboratory studies. High levels of RELT expression serves as a poor prognostic indicator for survival of ESCC patients. | [29]: Yao W. et al., 2021 |
| ESCC | RELL2 | Protective against cancer, expression decreased in ESCC cell line. | RELL2 expression protective against ESCC both in vivo and ex vivo. Endogenous expression of RELL2 is low in tumorigenic ESCC cell line. Report also highlighted alternative splicing of RELL2 hnRNA that can lead to nonsense mediated decay message, and less RELL2 protein expression, in ESCC cell line. | [67]:Tang S.J. et al., 2020 |
| Prostate cancer | RELT | Poor prognostic indicator, Upregulated expression | Upregulated RNA in cancer compared with non-malignant prostate tissue, expression increases with advanced staging. Bioinformatic analysis indicates higher RELT expression is poor prognostic indicator for prostate cancer patients. | [68]: Ge S. et al., 2021 |
| Prostate cancer | RELT | Poor prognostic indicator | RELT a member of a gene signature, SigIQGAP3NW, that predicts poor outcomes in prostate cancer. Bioinformatic analysis indicates RELT expression associated with an immunosuppressive environment and poor outcomes in prostate cancer. | [69]: Mei W. et al., 2023 |
| Glioma | RELL1 | Poor prognostic indicator, Upregulated expression | Bioinformatic analysis indicates RELL1 RNA is upregulated in glioma and correlates with more severe cases of glioma and an immunosuppressive environment. Expression of RELL1 inversely correlated with NK cell activity. | [70]: Jin X. et al., 2020 |
| Glioblastoma | RELL1 | Potentially pro-tumorigenic | RELL1 containing a N255D mutation in intracellular domain only expressed in glioblastoma cell lines, not in normal astrocyte cell lines. | [71]: Rose M. et al., 2021 |
| Gastric cancer | RELL1 circRNA | Protective against cancer, Downregulated expression in cancer | Demonstrated that circRELL1 expression inhibits gastric cancer both in vivo and ex vivo. Propose that circRELL1 protects against cancer by sponging pro-tumorigenic miR637. Exosomal circRELL1 downregulated in gastric cancer. | [19]: Sang H. et al., 2022 |
| PDAC | RELL2 | Anti-tumorigenic, Good prognostic indicator | RELL2 induces apoptosis of AsPC-1 cell line. Splicing factor DHX38 required to remove intron 4 of RELL2 transcript to avoid nonsense mediated decay of RELL2 transcript. Higher levels of RELL2 expression a good prognostic indicator. | [72]: Li Z. et al., 2023 |
| Lung (NSCLC) | RELT | Upregulated | RELT RNA upregulated 2.1-fold in lung cancer in tumors of 4 human patients, most patients had NSCLC. RELT expression downregulated 1.9-fold in response to romidepsin treatment in at least one patient with lung cancer. | [73]: Schrump D. et al., 2008 |
| Lung | RELT | Upregulated | RELT protein upregulated in mouse lung tumors 2-fold. Tumorigenesis in mouse lungs induced by K-ras G12D mutation. | [26]: Jung H. et al., 2019 |
| Lung (SCLC) | RELT | Good prognostic indicator | RELT expression expressed at higher amounts in low-risk group for SCLC. | [74]: Zhang Z. et al., 2021 |
| Renal cancer | RELT | Poor prognostic indicator | Bioinformatical data indicates RELT expression associated with poor survival, RELT expression may be correlated with an immunosuppressive environment. | [75]: Cui Y. et al., 2022 |
| Head and neck cancer | RELT | Poor prognostic indicator | RELT expression inversely correlated with overall patient survival. Bioinformatic data indicates RELT expression associated with immunosuppression markers, and that RELT expression is downregulated by several chemotherapeutic agents. | [76]: Guo Y. et al., 2021 |
| B cell lymphoma | RELT | Upregulated | RELT protein expression upregulated in B cell lymphoma biopsies versus healthy lymph nodes. | [23]: Cusick J. et al., 2020 |
| Non-acute promyelocytic leukemia | RELL2 | Poor prognostic indicator | RELL2 is one of 9 genes whose expression was correlated with poor survival of patients with non-acute promyelocytic leukemia. | [77]: Zhao Y. et al., 2022 |
| Colorectal cancer | RELL1 | Predicted to be pro-tumorigenic | Bioinformatic analysis indicates RELL1 may contribute to dysregulation of gene expression in colorectal cancer by competing for miRNA molecules. | [78]: Kadkhoda S. et al., 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusick, J.K.; Alcaide, J.; Shi, Y. The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development. Biomedicines 2023, 11, 2695. https://doi.org/10.3390/biomedicines11102695
Cusick JK, Alcaide J, Shi Y. The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development. Biomedicines. 2023; 11(10):2695. https://doi.org/10.3390/biomedicines11102695
Chicago/Turabian StyleCusick, John K., Jessa Alcaide, and Yihui Shi. 2023. "The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development" Biomedicines 11, no. 10: 2695. https://doi.org/10.3390/biomedicines11102695