Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection
Abstract
:1. Introduction
Statement of Significance
2. Materials and Methods
2.1. Materials
2.2. Biological Evaluation of VD3 Solutions and BG+VD3 Thin Films
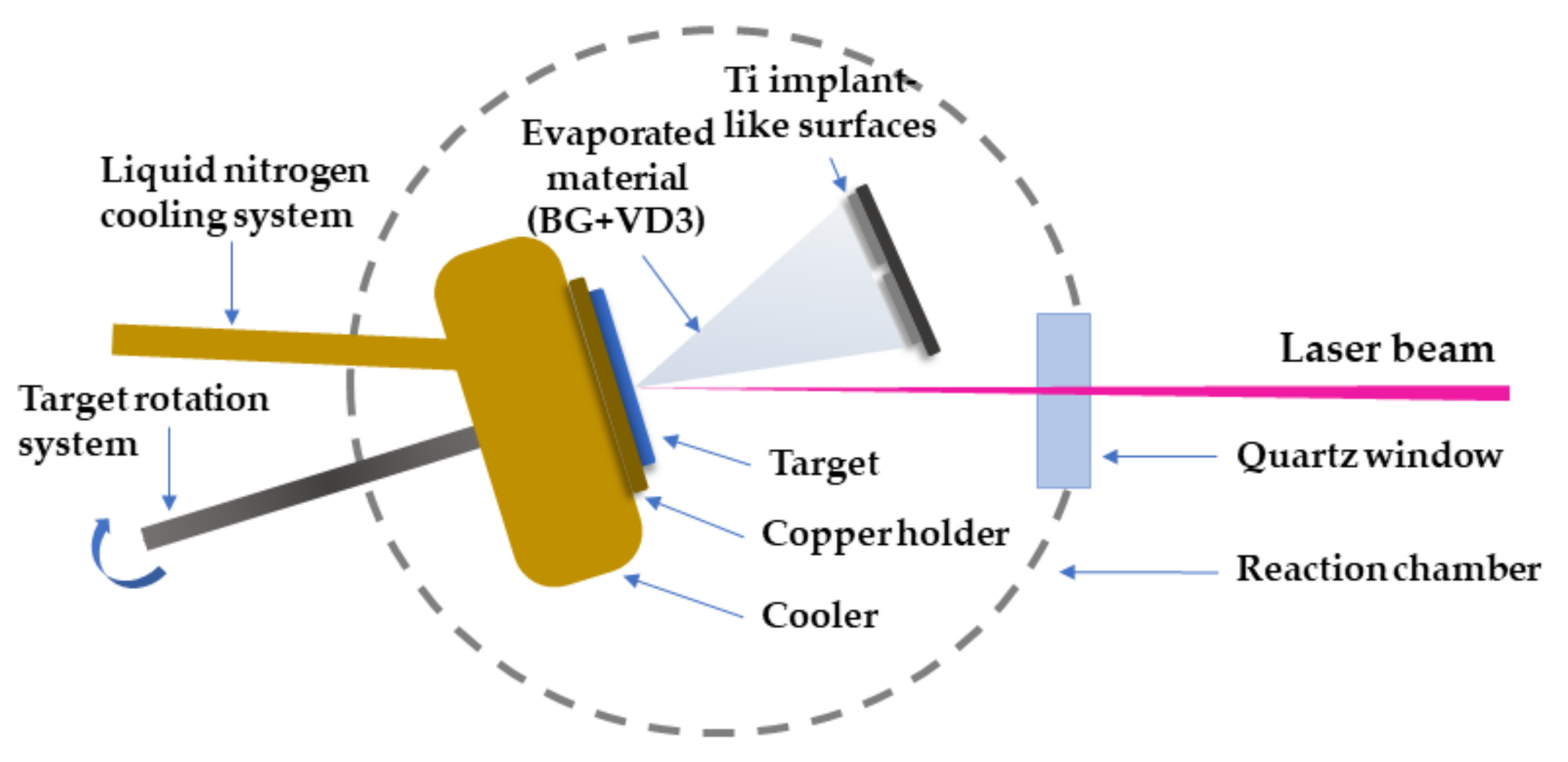
2.3. Experimental Conditions for MAPLE Transfer
- (a)
- BG57—Using a magnetic stirrer, 0.08 g of BG powder and 20 mL of DMSO were combined, and the mixture was subsequently homogenized.
- (b)
- Considering the biological assays performed on VD3 before incorporating it into thin films, 0.5, 0.25, 0.125 mg/mL VD3 in DMSO were mixed with 0.08 g of BG57. The obtained mixtures were further denoted as BG57+VD3_05, BG57+VD3_025 and BG57+VD3_0125. A magnetic stirrer was used to homogenize the mixtures.
2.4. Thin Films Characterization Methods
2.5. Electrochemical Investigation
3. Results and Discussion
3.1. Biological Evaluation of the VD3 Solution Cytotoxicity before Incorporation into Thin Films
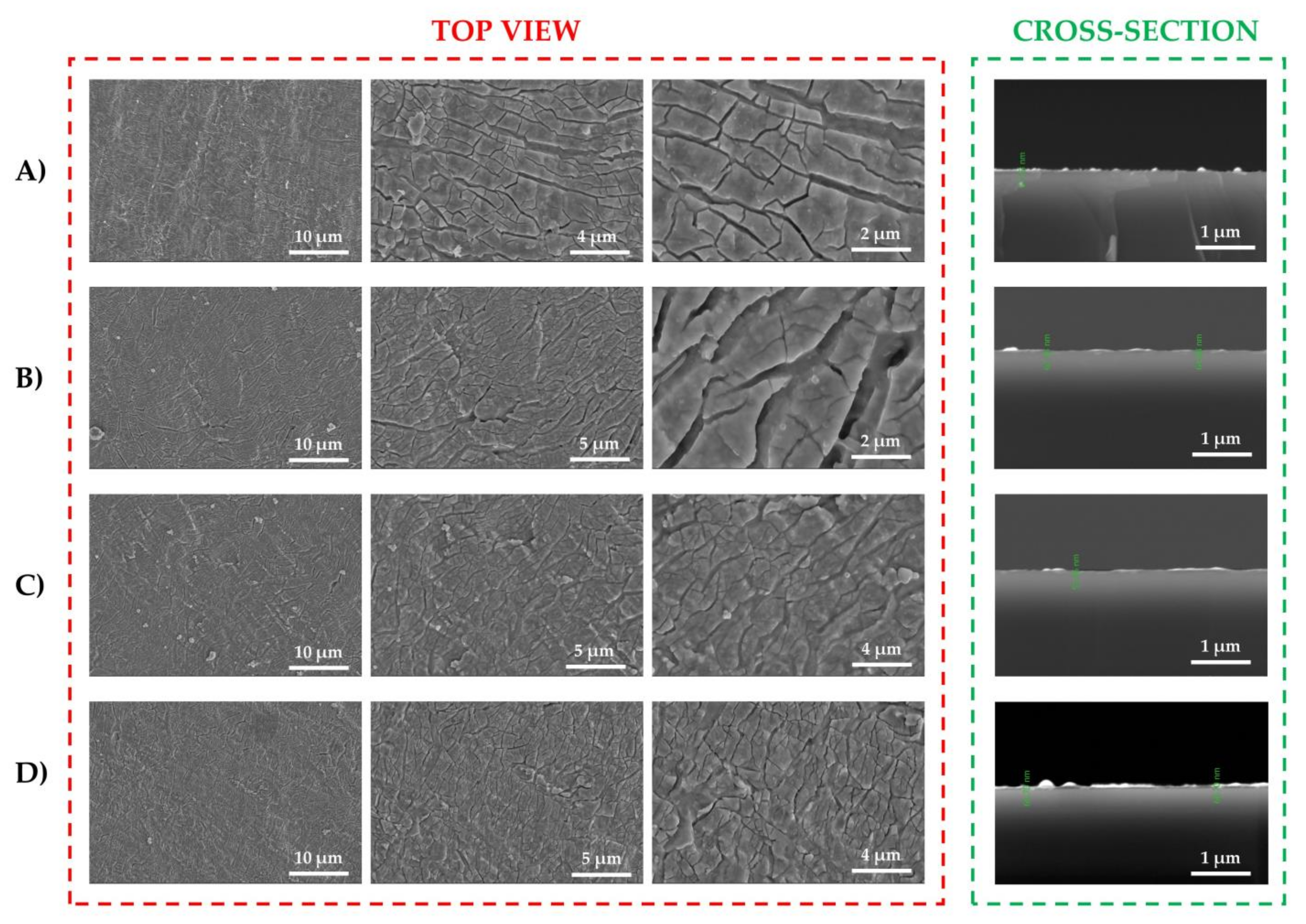
3.2. Surface Investigation of As-Deposited Thin Films
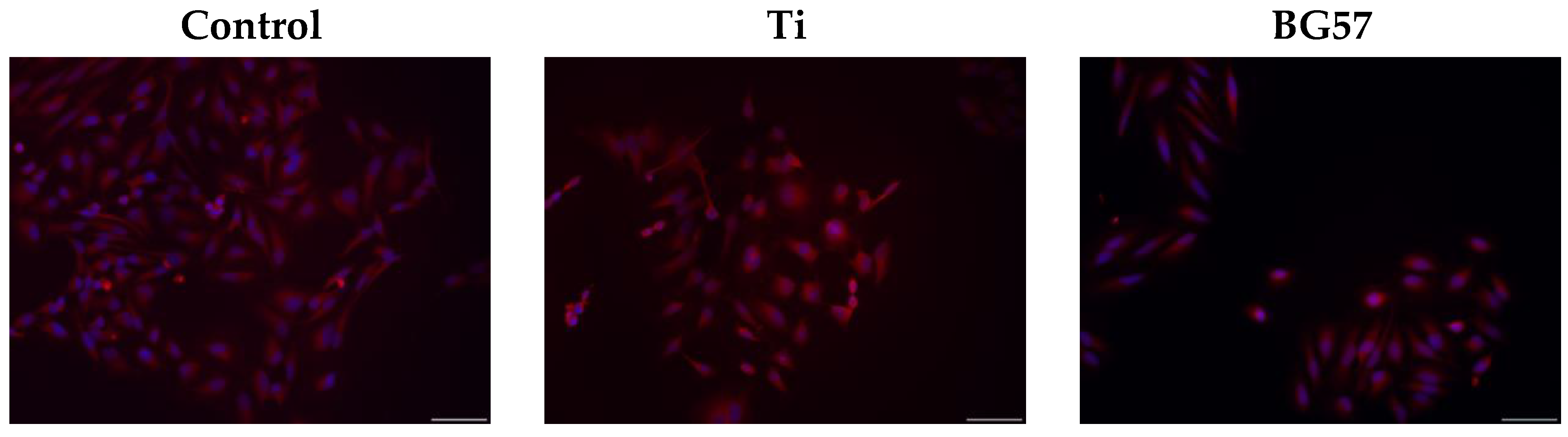
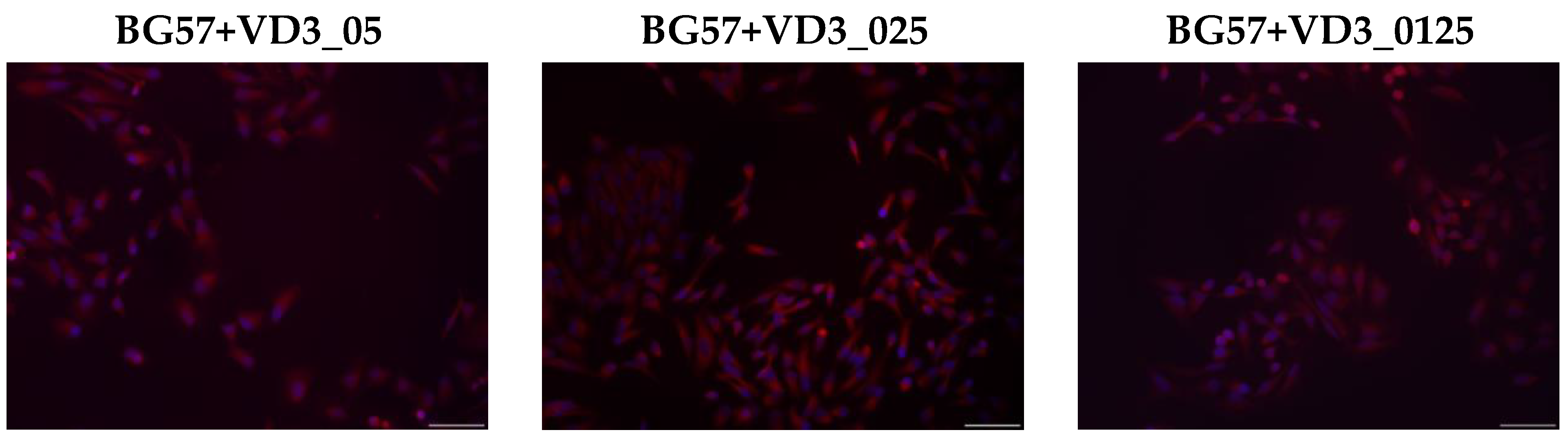
3.3. Biological Evaluation of the MAPLE-Deposited Thin Films
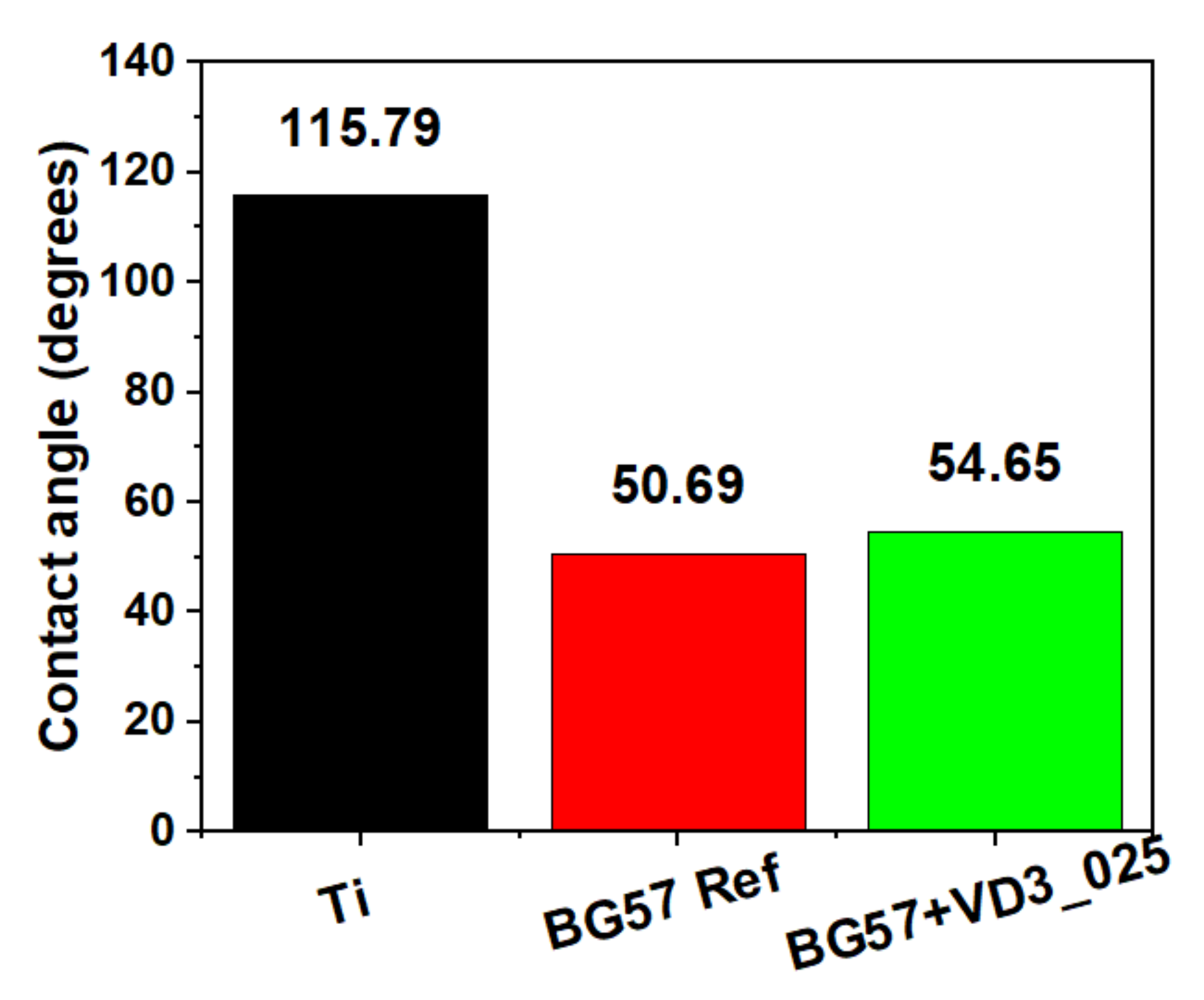
3.4. AFM and Contact Angle Measurement
3.5. Electrochemical Performance of the Tested Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Yan, H.; Afroz, S.; Dalanon, J.; Goto, N.; Hosoki, M.; Matsuka, Y. Metal allergy patient treated by titanium implant denture: A case report with at least 4-year follow-up. Clin. Case Rep. 2018, 6, 1972–1977. [Google Scholar] [CrossRef]
- Ayele, S.; Sharo, N.; Chrcanovic, B.R. Marginal bone loss around dental implants: Comparison between diabetic and non-diabetic patients—A retrospective clinical study. Clin. Oral. Investig. 2023, 27, 2833–2841. [Google Scholar] [CrossRef]
- Albrektsson, T.; Becker, W.; Coli, P.; Jemt, T.; Mölne, J.; Sennerby, L. Bone loss around oral and orthopedic implants: An immunologically based condition. Clin. Implant. Dent. Relat. Res. 2019, 21, 786–795. [Google Scholar] [CrossRef]
- Seo, D.-I.; Lee, J.-B. Localized Corrosion Resistance on Additively Manufactured Ti Alloys by Means of Electrochemical Critical Localized Corrosion Potential in Biomedical Solution Environments. Materials 2021, 14, 7481. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Pedeferri, M.; Ormellese, M. Enhancement of pure titanium localized corrosion resistance by anodic oxidation. Mater. Corros. 2018, 69, 503–509. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Zhang, P. Effect of Glucose Concentration on Electrochemical Corrosion Behavior of Pure Titanium TA2 in Hanks’ Simulated Body Fluid. Materials 2016, 9, 874. [Google Scholar] [CrossRef]
- Chevalley, T.; Brandi, M.L.; Cavalier, E.; Harvey, N.C.; Iolascon, G.; Cooper, C.; Hannouche, D.; Kaux, J.-F.; Kurth, A.; Maggi, S.; et al. How can the orthopedic surgeon ensure optimal vitamin D status in patients operated for an osteoporotic fracture? Osteoporos. Int. 2021, 32, 1921–1935. [Google Scholar] [CrossRef]
- Knechtle, B.; Jastrzębski, Z.; Hill, L.; Nikolaidis, P.T. Vitamin D and Stress Fractures in Sport: Preventive and Therapeutic Measures—A Narrative Review. Medicina 2021, 57, 223. [Google Scholar] [CrossRef]
- Laky, M.; Bertl, K.; Haririan, H.; Andrukhov, O.; Seemann, R.; Volf, I.; Assinger, A.; Gruber, R.; Moritz, A.; Rausch-Fan, X. Serum levels of 25-hydroxyvitamin D are associated with periodontal disease. Clin. Oral. Investig. 2017, 21, 1553–1558. [Google Scholar] [CrossRef]
- Mangano, F.; Mortellaro, C.; Mangano, N.; Mangano, C. Is Low Serum Vitamin D Associated with Early Dental Implant Failure? A Retrospective Evaluation on 1625 Implants Placed in 822 Patients. Mediat. Inflamm. 2016, 2016, e5319718. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Xiong, A.; Sun, R.; Qiu, L.; Xie, Z. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: An updated meta-analysis. Clin. Nutr. 2021, 40, 5531–5537. [Google Scholar] [CrossRef]
- Sim, M.; Zhu, K.; Lewis, J.R.; Hodgson, J.M.; Prince, R.L. Association between vitamin D status and long-term falls-related hospitalization risk in older women. J. Am. Geriatr. Soc. 2021, 69, 3114–3123. [Google Scholar] [CrossRef]
- Slobogean, G.P.; Bzovsky, S.; O’Hara, N.N.; Marchand, L.S.; Hannan, Z.D.; Demyanovich, H.K.; Connelly, D.W.; Adachi, J.D.; Thabane, L.; Sprague, S.; et al. Effect of Vitamin D3 Supplementation on Acute Fracture Healing: A Phase II Screening Randomized Double-Blind Controlled Trial. JBMR Plus 2023, 7, e10705. [Google Scholar] [CrossRef] [PubMed]
- Unune, D.R.; Brown, G.R.; Reilly, G.C. Thermal based surface modification techniques for enhancing the corrosion and wear resistance of metallic implants: A review. Vacuum 2022, 203, 111298. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C.; Tozar, T.; Dinu, M.; Parau, A.C.; Grumezescu, V.; Hapenciuc, C.; Popa, M.; Stan, M.S.; Marutescu, L.; et al. Implant Surfaces Containing Bioglasses and Ciprofloxacin as Platforms for Bone Repair and Improved Resistance to Microbial Colonization. Pharmaceutics 2022, 14, 1175. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef]
- Mojarad Shafiee, B.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220. [Google Scholar] [CrossRef]
- Przykaza, K.; Jurak, M.; Kalisz, G.; Mroczka, R.; Wiącek, A.E. Characteristics of Hybrid Bioglass-Chitosan Coatings on the Plasma Activated PEEK Polymer. Molecules 2023, 28, 1729. [Google Scholar] [CrossRef] [PubMed]
- Kreller, T.; Sahm, F.; Bader, R.; Boccaccini, A.R.; Jonitz-Heincke, A.; Detsch, R. Biomimetic Calcium Phosphate Coatings for Bioactivation of Titanium Implant Surfaces: Methodological Approach and In Vitro Evaluation of Biocompatibility. Materials 2021, 14, 3516. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Ristoscu, C. Bioactive Glasses for Soft and Hard Tissue Healing Applications—A Short Review. Appl. Sci. 2023, 13, 6151. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Vafa, E.; Tayebi, L.; Abbasi, M.; Azizli, M.J.; Bazargan-Lari, R.; Talaiekhozani, A.; Zareshahrabadi, Z.; Vaez, A.; Amani, A.M.; Kamyab, H.; et al. A better roadmap for designing novel bioactive glasses: Effective approaches for the development of innovative revolutionary bioglasses for future biomedical applications. Environ. Sci. Pollut. Res. 2022, 36456674. [Google Scholar] [CrossRef]
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Mirza Rosca, J.C.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional Bioglass—Biopolymer Double Nanostructure for Natural Antimicrobial Drug Extracts Delivery. Nanomaterials 2020, 10, 385. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Mihailescu, N.; Negut, I.; Badea, M.; Ursutiu, D.; Chifiriuc, M.C.; Urzica, I.; Dyia, H.M.; Bleotu, C.; et al. Functionalized Antimicrobial Composite Thin Films Printing for Stainless Steel Implant Coatings. Molecules 2016, 21, 740. [Google Scholar] [CrossRef]
- Floroian, L.; Ristoscu, C.; Candiani, G.; Pastori, N.; Moscatelli, M.; Mihailescu, N.; Negut, I.; Badea, M.; Gilca, M.; Chiesa, R.; et al. Antimicrobial thin films based on ayurvedic plants extracts embedded in a bioactive glass matrix. Appl. Surf. Sci. 2017, 417, 224–233. [Google Scholar] [CrossRef]
- Anene, F.; Aiza Jaafar, C.; Zainol, I.; Azmah Hanim, M.; Suraya, M. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Gyorgy, E.; Grigorescu, S.; Socol, G.; Mihailescu, I.N.; Janackovic, D.; Dindune, A.; Kanepe, Z.; Palcevskis, E.; Zdrentu, E.L.; Petrescu, S.M. Bioactive glass and hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 7981–7986. [Google Scholar] [CrossRef]
- Tanaskovic, D.; Jokic, B.; Socol, G.; Popescu, A.; Mihailescu, I.N.; Petrovic, R.; Janackovic, D.J. Synthesis of functionally graded bioactive glass-apatite multistructures on Ti substrates by pulsed laser deposition. Appl. Surf. Sci. 2007, 254, 1279–1282. [Google Scholar] [CrossRef]
- Negut, I.; Visan, A.I.; Popescu, C.; Cristescu, R.; Ficai, A.; Grumezescu, A.M.; Chifiriuc, M.C.; Boehm, R.D.; Yamaleyeva, D.; Taylor, M.; et al. Successful Release of Voriconazole and Flavonoids from MAPLE Deposited Bioactive Surfaces. Appl. Sci. 2019, 9, 786. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Costa, L.C.; Graça, M.P.F. Biocompatibility, Bioactivity, and Antibacterial Behaviour of Cerium-Containing Bioglass®. Nanomaterials 2022, 12, 4479. [Google Scholar] [CrossRef]
- Youness, R.A.; Dawoud, A.; ElTahtawy, O.; Farag, M.A. Fat-soluble vitamins: Updated review of their role and orchestration in human nutrition throughout life cycle with sex differences. Nutr. Metab. 2022, 19, 60. [Google Scholar] [CrossRef]
- Borojević, A.; Jauković, A.; Kukolj, T.; Mojsilović, S.; Obradović, H.; Trivanović, D.; Živanović, M.; Zečević, Ž.; Simić, M.; Gobeljić, B.; et al. Vitamin D3 Stimulates Proliferation Capacity, Expression of Pluripotency Markers, and Osteogenesis of Human Bone Marrow Mesenchymal Stromal/Stem Cells, Partly through SIRT1 Signaling. Biomolecules 2022, 12, 323. [Google Scholar] [CrossRef]
- Werny, J.G.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does vitamin D have an effect on osseointegration of dental implants? A systematic review. Int. J. Implant. Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Halfon, M.; Phan, O.; Teta, D. Vitamin D: A Review on Its Effects on Muscle Strength, the Risk of Fall, and Frailty. Biomed. Res. Int. 2015, 2015, 953241. [Google Scholar] [CrossRef]
- Abad-Javier, M.E.; Cajero-Juárez, M.; Nuñez-Anita, R.E.; Contreras-García, M.E. Effect of collagen type I and vitamin D3 functionalization of biomimetic bioglass scaffolds on hydroxyapatite condensation. J. Eur. Ceram. Soc. 2019, 39, 3505–3512. [Google Scholar] [CrossRef]
- Ignjatović, N.; Uskoković, V.; Ajduković, Z.; Uskoković, D. Multifunctional hydroxyapatite and poly(d,l-lactide-co-glycolide) nanoparticles for the local delivery of cholecalciferol. Mater. Sci. Eng. C 2013, 33, 943–950. [Google Scholar] [CrossRef]
- Zia, R.; Nazir, A.; Khan, M.K.I.; Maan, A.A.; Rashid, A. Preparation of Ascorbic Acid and Cholecalciferol Microsponges for Topical Application. Int. J. Pharm. Pharm. Sci. 2017, 9, 280–287. [Google Scholar] [CrossRef]
- Behary, N.; Eap, S.; Cayla, A.; Chai, F.; Benkirane-Jessel, N.; Campagne, C. Nano-Structured Ridged Micro-Filaments (≥100 µm Diameter) Produced Using a Single Step Strategy for Improved Bone Cell Adhesion and Proliferation in Textile Scaffolds. Molecules 2022, 27, 3790. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.; Trossmann, V.T.; Scheibel, T. Selective Topography Directed Cell Adhesion on Spider Silk Surfaces. Adv. Mater. Interfaces 2023, 10, 2201936. [Google Scholar] [CrossRef]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in vitro. Med. Devices Evid. Res. 2022, 15, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Pierfelice, T.V.; D’Amico, E.; Iezzi, G.; Piattelli, A.; Di Pietro, N.; D’Arcangelo, C.; Comuzzi, L.; Petrini, M. Nanoporous Titanium Enriched with Calcium and Phosphorus Promotes Human Oral Osteoblast Bioactivity. Int. J. Environ. Res. Public Health 2022, 19, 6212. [Google Scholar] [CrossRef]
- Mahbub, M.R.; Kovach, L.; Wolfe, A.; Lalvani, S.; James, P.F.; Jahan, M.P. Enhancing cell adhesion and corrosion performance of titanium alloy by surface and sub-surface engineering using WEDM. Surf. Coat. Technol. 2022, 429, 127929. [Google Scholar] [CrossRef]
- Hou, C.; An, J.; Zhao, D.; Ma, X.; Zhang, W.; Zhao, W.; Wu, M.; Zhang, Z.; Yuan, F. Surface Modification Techniques to Produce Micro/Nano-scale Topographies on Ti-Based Implant Surfaces for Improved Osseointegration. Front. Bioeng. Biotechnol. 2022, 10, 835008. [Google Scholar] [CrossRef]
- Ramezani, M.; Ripin, Z.M. An Overview of Enhancing the Performance of Medical Implants with Nanocomposites. J. Compos. Sci. 2023, 7, 199. [Google Scholar] [CrossRef]
- Bin Anwar Fadzil, A.F.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of surface quality on biocompatibility of implants—A review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Cao, X.; Lu, M.; Liu, X. Tailoring micro/nano-materials with special wettability for biomedical devices. Biomed. Technol. 2023, 2, 15–30. [Google Scholar] [CrossRef]
- Zhang, B.; Leng, J.; Ouyang, Z.; Yang, Z.; Zhang, Q.; Li, Q.; Li, D.; Zhao, H. Superhydrophilic and topography-regulatable surface grafting on PEEK to improve cellular affinity. Biomater. Adv. 2023, 146, 213310. [Google Scholar] [CrossRef]
- Sun, Y.; Bu, X.; Ulusoy, U.; Guven, O.; Vaziri Hassas, B.; Dong, X. Effect of surface roughness on particle-bubble interaction: A critical review. Miner. Eng. 2023, 201, 108223. [Google Scholar] [CrossRef]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass® composite coatings on stainless steel deposited by direct current and alternating current electrophoretic deposition. Surf. Coat. Technol. 2013, 233, 49–56. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.; Cheon, K.-H.; Jung, H.-D.; Song, J.; Kim, H.-E.; Jang, T.-S. Antibacterial and bioactive properties of stabilized silver on titanium with a nanostructured surface for dental applications. Appl. Surf. Sci. 2018, 451, 232–240. [Google Scholar] [CrossRef]
- Mostafavi, P.; Naeimi, M. Investigation of vitamin D-loaded silk fibroin electrospun scaffolds for bone tissue engineering applications. Mater. Technol. 2022, 37, 1329–1337. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Strehblow, H.-H. Passive film on orthopaedic TiAlV alloy formed in physiological solution investigated by X-ray photoelectron spectroscopy. Biomaterials 2000, 21, 2103–2113. [Google Scholar] [CrossRef]
- Gugelmin, B.S.; Santos, L.S.; Ponte, H.D.A.; Marino, C.E.B. Electrochemical Stability and Bioactivity Evaluation of Ti6Al4V Surface Coated with Thin Oxide by EIS for Biomedical Applications. Mat. Res. 2015, 18, 602–607. [Google Scholar] [CrossRef]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical Impedance Spectroscopy. In Nanostructures and Thin Films for Multifunctional Applications: Technology, Properties and Devices; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; NanoScience and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–42. ISBN 978-3-319-30198-3. [Google Scholar]
- Córdoba-Torres, P. Relationship between constant-phase element (CPE) parameters and physical properties of films with a distributed resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Shoar Abouzari, M.R.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid. State Ion. 2009, 180, 922–927. [Google Scholar] [CrossRef]






| Oxides (wt%) | ||||||
|---|---|---|---|---|---|---|
| BG | SiO2 | Na2O | K2O | CaO | MgO | P2O5 |
| 56.5 | 11 | 3 | 15 | 8.5 | 6 | |
| Sample | 2D Image | 3D Image | Roughness Parameters (nm) | |
|---|---|---|---|---|
| Ra | Rrms | |||
| BG57 |  |  | 80.3 | 103.4 |
| BG57+VD3 _025 | 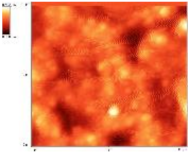 |  | 62.1 | 79.4 |
| Electrochemical Parameters | Time | Samples | ||
|---|---|---|---|---|
| Ti | BG57 Ref | BG57+VD3_025 | ||
| Rs (Ω cm2) | 1 h | 27 | 22 | 56 |
| 12 h | 23 | 19 | 52 | |
| 24 h | 19 | 15 | 42 | |
| Qcoat (μF s(α−1) cm−2) | 1 h | 19.89 | 294.34 | 147.95 |
| 12 h | 19.95 | 301.59 | 175.39 | |
| 24 h | 20.25 | 486.12 | 226.97 | |
| αcoat | 1 h | 0.96 | 0.61 | 0.48 |
| 12 h | 0.96 | 0.60 | 0.45 | |
| 24 h | 0.96 | 0.54 | 0.43 | |
| Rpore (Ω cm2) | 1 h | 87 | 132 | 3047 |
| 12 h | 75 | 123 | 3057 | |
| 24 h | 70 | 130 | 2163 | |
| Qdl (μF s(α−1) cm−2) | 1 h | 9.90 | 1193.00 | 806.34 |
| 12 h | 9.68 | 1501.90 | 634.33 | |
| 24 h | 9.13 | 1427.60 | 577.22 | |
| αdl | 1 h | 0.93 | 0.68 | 0.90 |
| 12 h | 0.93 | 0.63 | 0.90 | |
| 24 h | 0.93 | 0.66 | 0.90 | |
| Rct (Ω cm2) | 1 h | - | - | 5076 |
| 12 h | - | - | 6014 | |
| 24 h | - | - | 4304 | |
| χ2 | 1 h | 2 × 10−4 | 8 × 10−4 | 8 × 10−4 |
| 12 h | 2 × 10−4 | 7 × 10−4 | 1 × 10−3 | |
| 24 h | 2 × 10−4 | 8 × 10−4 | 9 × 10−4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Gradisteanu-Pircalabioru, G.; Dinu, M.; Bita, B.; Parau, A.C.; Grumezescu, V.; Ristoscu, C.; Chifiriuc, M.C. Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection. Biomedicines 2023, 11, 2772. https://doi.org/10.3390/biomedicines11102772
Negut I, Gradisteanu-Pircalabioru G, Dinu M, Bita B, Parau AC, Grumezescu V, Ristoscu C, Chifiriuc MC. Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection. Biomedicines. 2023; 11(10):2772. https://doi.org/10.3390/biomedicines11102772
Chicago/Turabian StyleNegut, Irina, Gratiela Gradisteanu-Pircalabioru, Mihaela Dinu, Bogdan Bita, Anca Constantina Parau, Valentina Grumezescu, Carmen Ristoscu, and Mariana Carmen Chifiriuc. 2023. "Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection" Biomedicines 11, no. 10: 2772. https://doi.org/10.3390/biomedicines11102772
APA StyleNegut, I., Gradisteanu-Pircalabioru, G., Dinu, M., Bita, B., Parau, A. C., Grumezescu, V., Ristoscu, C., & Chifiriuc, M. C. (2023). Bioglass and Vitamin D3 Coatings for Titanium Implants: Osseointegration and Corrosion Protection. Biomedicines, 11(10), 2772. https://doi.org/10.3390/biomedicines11102772








