Pediatric Neuroendocrine Neoplasia of the Parathyroid Glands: Delving into Primary Hyperparathyroidism
Abstract
:1. Introduction
Aim
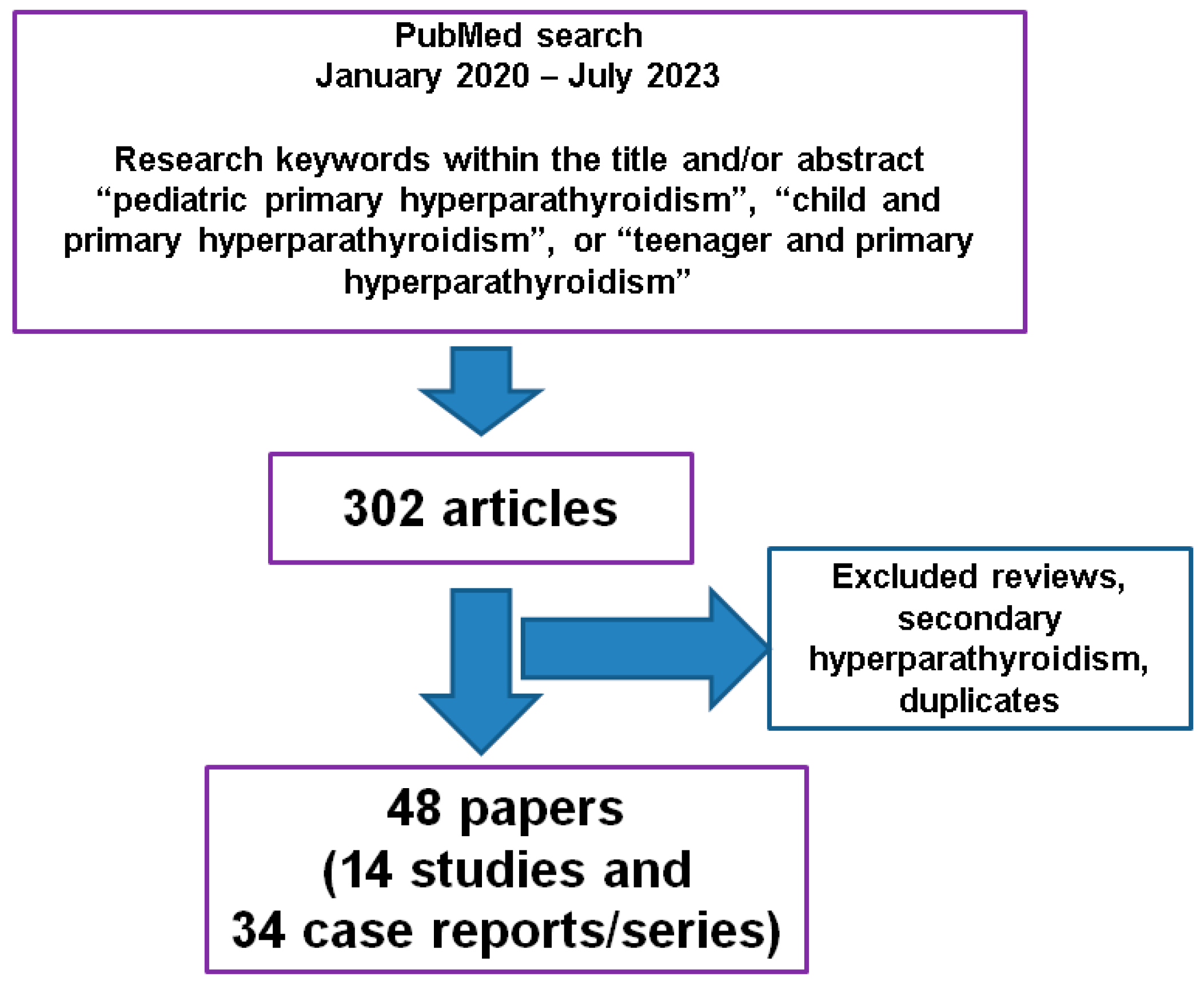
2. Materials and Methods
3. An Update of Pediatric Primary Hyperparathyroidism: From Admission to Outcome
3.1. Clinical Presentation
3.2. Genetic Considerations
3.3. Lab Findings
3.4. Imaging Tools
3.5. Surgical Procedures
3.6. Outcome
4. Discussion
4.1. Changes in Terminology over the Years concerning Parathyroid Tumours
4.2. Neonatal Primary Hyperparathyroidism
4.3. Calcium-Lowering Drugs in Children with PHP
4.4. Paediatric PHP: Osteoporosis and Fracture Issues
4.5. Trans-Pandemic Insights into Paediatric PHP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP2S1 | adaptor protein complex 2 subunit sigma |
| CASR | Calcium-sensing receptor |
| BMD | bone mineral density |
| CT | computed tomography |
| CDC73 | cell division cycle |
| DXA | Dual-Energy X-ray Absorptiometry |
| GHRH | Growth-Hormone-Releasing Hormone |
| GNA11 | guanine nucleotide-binding protein subunit alpha-11 |
| HRpQCT | high-resolution peripheral quantitative computed tomography |
| MEN | multiple endocrine neoplasia |
| PHP | primary hyperparathyroidism |
| PTH | parathyroid hormone |
| PHP | primary hyperparathyroidism |
| PET/CT | positron emission tomography/computed tomography |
| Tc | Technetium |
| TBS | trabecular bone score |
| SPECT/CT | single-photon emission computerized tomography/computed tomography |
| VHL | von Hipple–Lindau |
| VATS | Video-Assisted Thoracoscopic Surgery |
References
- Motlaghzadeh, Y.; Bilezikian, J.P.; Sellmeyer, D.E. Rare Causes of Hypercalcemia: 2021 Update. J. Clin. Endocrinol. Metab. 2021, 106, 3113–3128. [Google Scholar] [CrossRef] [PubMed]
- Zanocco, K.A.; Yeh, M.W. Primary Hyperparathyroidism: Effects on Bone Health. Endocrinol. Metab. Clin. N. Am. 2017, 46, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari, H.; Cheeney, S.H.E.; Manning, S.C.; Parisi, M.T. Pediatric hyperparathyroidism: Review and imaging update. Pediatr. Radiol. 2021, 51, 1106–1120. [Google Scholar] [CrossRef]
- Stanciu, M.; Boicean, L.C.; Popa, F.L. The role of combined techniques of scintigraphy and SPECT/CT in the diagnosis of primary hyperparathyroidism: A case report. Medicine 2019, 98, e14154. [Google Scholar] [CrossRef] [PubMed]
- Rampp, R.D.; Mancilla, E.E.; Adzick, N.S.; Levine, M.A.; Kelz, R.R.; Fraker, D.L.; Iyer, P.; Lindeman, B.M.; Mejia, V.A.; Chen, H.; et al. Single Gland, Ectopic Location: Adenomas are Common Causes of Primary Hyperparathyroidism in Children and Adolescents. World J. Surg. 2020, 44, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Ghemigian, A.; Carsote, M.; Albu, S.E.; Terzea, D.; Valea, A. Thyroid nodules after initial evaluation by primary health care practitioners: An ultrasound pictorial essay. Arch. Balk. Med. Union 2016, 51, 434–438. [Google Scholar]
- Murugan, N.; Kandasamy, D.; Sharma, R.; Goyal, A.; Gupta, A.K.; Tandon, N.; Gupta, N.; Goswami, R.; Vurthaluru, S.; Damle, N.; et al. Comparison of 4DMRI and 4DCT for the preoperative evaluation of patients with primary hyperparathyroidism. Eur. J. Radiol. 2021, 138, 109625. [Google Scholar] [CrossRef] [PubMed]
- Bunch, P.M.; Randolph, G.W.; Brooks, J.A.; George, V.; Cannon, J.; Kelly, H.R. Parathyroid 4D CT: What the Surgeon Wants to Know. Radiographics 2020, 40, 1383–1394. [Google Scholar] [CrossRef]
- Muse, J.; Palmer, R.; Auriemma, J. A Giant Parathyroid Adenoma Presenting as Nausea, Vomiting, and Headaches in an Adolescent Male. Case Rep. Pediatr. 2023, 2023, 5530269. [Google Scholar] [CrossRef]
- Seo, Y.; Song, K.; Choi, H.S.; Suh, J.; Kwon, A.; Chae, H.W.; Kim, H.S. A case of primary hyperparathyroidism due to an intrathymic ectopic parathyroid adenoma in a 15-year-old boy. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 187–191. [Google Scholar] [CrossRef]
- Sahu, A.; Zameer, M.M.; Rao, S.; D’Cruz, A. Sestamibi-guided thoracoscopic excision of functional mediastinal parathyroid adenoma in a child: A case report and review of literature. J. Minim. Access. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, L.; Shao, M.; Li, P.; Liu, W.; Zhang, X.; Zhang, L.; Ma, Y.; Li, W. Primary hyperparathyroidism due to ectopic parathyroid adenoma in an adolescent: A case report and review of the literature. Endocrine 2019, 64, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Kollars, J.; Zarroug, A.E.; van Heerden, J.; Lteif, A.; Stavlo, P.; Suarez, L.; Moir, C.; Ishitani, M.; Rodeberg, D. Primary hyperparathyroidism in pediatric patients. Pediatrics 2005, 115, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Root, A.W.; Levine, M.A. One half-century of advances in the evaluation and management of disorders of bone and mineral metabolism in children and adolescents. J. Pediatr. Endocrinol. Metab. 2023, 36, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Boggs, E.; Szymusiak, J. Common in Adults and Often Overlooked in Pediatrics: A Case Report of Primary Hyperparathyroidism in an Adolescent Patient. Cureus 2023, 15, e38112. [Google Scholar] [CrossRef]
- Duval, M.; Haissaguerre, M. MEN for multiple endocrin neoplasms: When evokate MEN? Update 2022. Rev. Med. Int. 2023, 44, 12–18. [Google Scholar] [CrossRef]
- Blackburn, J.; Mulvey, I.; Nadar, R.; Dias, R.P.; Saraff, V.; Senniappan, S. Heterozygous CDC73 mutation causing hyperparathyroidism in children and adolescents: A report of 2 cases. J. Pediatr. Endocrinol. Metab. 2022, 35, 1547–1551. [Google Scholar] [CrossRef]
- Bernardor, J.; Flammier, S.; Salles, J.P.; Amouroux, C.; Castanet, M.; Lienhardt, A.; Martinerie, L.; Damgov, I.; Linglart, A.; Bacchetta, J. Off-label use of cinacalcet in pediatric primary hyperparathyroidism: A French multicenter experience. Front. Pediatr. 2022, 10, 926986. [Google Scholar] [CrossRef]
- Vierimaa, O.; Villablanca, A.; Alimov, A.; Georgitsi, M.; Raitila, A.; Vahteristo, P.; Larsson, C.; Ruokonen, A.; Eloranta, E.; Ebeling, T.M.; et al. Mutation analysis of MEN1, HRPT2, CASR, CDKN1B, and AIP genes in primary hyperparathyroidism patients with features of genetic predisposition. J. Endocrinol. Investig. 2009, 32, 512–528. [Google Scholar] [CrossRef]
- Machens, A.; Elwerr, M.; Lorenz, K.; Weber, F.; Dralle, H. 100-Year evolution of precision medicine and surgery for multiple endocrine neoplasia type 2A. Endocrine 2020, 68, 368–376. [Google Scholar] [CrossRef]
- Cetani, F.; Pardi, E.; Aretini, P.; Saponaro, F.; Borsari, S.; Mazoni, L.; Apicella, M.; Civita, P.; La Ferla, M.; Caligo, M.A.; et al. Whole exome sequencing in familial isolated primary hyperparathyroidism. J. Endocrinol. Investig. 2020, 43, 231–245. [Google Scholar] [CrossRef]
- Mariathasan, S.; Andrews, K.A.; Thompson, E.; Challis, B.G.; Wilcox, S.; Pierce, H.; Hale, J.; Spiden, S.; Fuller, G.; Simpson, H.L.; et al. Genetic testing for hereditary hyperparathyroidism and familial hypocalciuric hypercalcaemia in a large UK cohort. Clin. Endocrinol. 2020, 93, 409–418. [Google Scholar] [CrossRef]
- Masi, L. Primary Hyperparathyroidism. In Frontiers of Hormone Research; Karger: Basel, Switzerland, 2019; Volume 51, pp. 1–12. [Google Scholar] [CrossRef]
- McKenna, K.; Dunbar, N.S.; Parham, K. Why is primary hyperparathyroidism more severe in children? Med. Hypotheses 2021, 147, 110482. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.N.; Bhadada, S.K.; Bhansali, A.; Behera, A.; Mittal, B.R.; Bhavin, V. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J. Postgrad. Med. 2012, 58, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Memon, S.; Lila, A.R.; Sarathi, V.; Arya, S.; Jadhav, S.S.; Hira, P.; Garale, M.; Gosavi, V.; Karlekar, M.; et al. Genotype-Phenotype Correlations in Asian Indian Children and Adolescents with Primary Hyperparathyroidism. Calcif. Tissue Int. 2022, 111, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Szabo Yamashita, T.; Gudmundsdottir, H.; Foster, T.R.; Lyden, M.L.; Dy, B.M.; Tebben, P.J.; McKenzie, T. Pediatric primary hyperparathyroidism: Surgical pathology and long-term outcomes in sporadic and familial cases. Am. J. Surg. 2023, 225, 699–702. [Google Scholar] [CrossRef]
- Sharanappa, V.; Mishra, A.; Bhatia, V.; Mayilvagnan, S.; Chand, G.; Agarwal, G.; Agarwal, A.; Mishra, S.K. Pediatric Primary Hyperparathyroidism: Experience in a Tertiary Care Referral Center in a Developing Country Over Three Decades. World J. Surg. 2021, 45, 488–495. [Google Scholar] [CrossRef]
- Bin Yahib, S.M.; Algarni, B.; Alghamdi, A.; Nassan, S. Primary Hyperparathyroidism as a Rare Cause of Unexplained Recurrent Abdominal Pain: Case Presentation and Literature Review. Cureus 2021, 13, e19155. [Google Scholar] [CrossRef]
- Stokes, V.J.; Nielsen, M.F.; Hannan, F.M.; Thakker, R.V. Hypercalcemic Disorders in Children. J. Bone Miner. Res. 2017, 32, 2157–2170. [Google Scholar] [CrossRef]
- George, J.; Acharya, S.V.; Bandgar, T.R.; Menon, P.S.; Shah, N.S. Primary hyperparathyroidism in children and adolescents. Indian J. Pediatr. 2010, 77, 175–178. [Google Scholar] [CrossRef]
- Plunkett, A.; Beattie, R.M. Recurrent abdominal pain in childhood. J. R. Soc. Med. 2005, 98, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Belcher, R.; Metrailer, A.M.; Bodenner, D.L.; Stack, B.C., Jr. Characterization of hyperparathyroidism in youth and adolescents: A literature review. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Lee, Y.; Yoo, H.W.; Choi, J.H. Three pediatric patients with primary hyperparathyroidism caused by parathyroid adenoma. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 142–147. [Google Scholar] [CrossRef]
- Fukaya, Y.; Oto, Y.; Inoue, T.; Itabashi, H.; Shiraishi, M.; Nitta, A.; Murakami, N.; Soh, S.; Ogawa, T.; Matsubara, T. Primary hyperparathyroidism in a child with abdominal pain and hematuria. Clin. Pediatr. Endocrinol. 2021, 30, 111–113. [Google Scholar] [CrossRef]
- Tuli, G.; Munarin, J.; Tessaris, D.; Buganza, R.; Matarazzo, P.; De Sanctis, L. Primary Hyperparathyroidism (PHPT) in Children: Two Case Reports and Review of the Literature. Case Rep. Endocrinol. 2021, 2021, 5539349. [Google Scholar] [CrossRef] [PubMed]
- Gafar, S.M.; Fadlalbari, G.F.; Abdalla, A.T.; Mohammed, S.A.R.; Alrasheed, M.K.; Taha, I.A.; Abdullah, M.A. Pitfalls in the Diagnosis of Primary Hyperparathyroidism in a Sudanese Adolescent Boy; a case disguised as rickets. BMC Endocr. Disord. 2022, 22, 322. [Google Scholar] [CrossRef]
- Dikova, M.I.; Petkova, B.; Alexiev, V. Skeletal Deformity in Children with Primary Hyperparathyroidism. Acta Chir. Orthop. Traumatol. Cech. 2021, 88, 375–378. [Google Scholar] [CrossRef]
- Lee, S.P.; Chai, S.T.; Loh, L.T.; Ali, N.M. Bilateral Genu Valgum in an Adolescent with Primary Hyperparathyroidism: A Case Report and Review of Literature. J. ASEAN Fed. Endocr. Soc. 2020, 35, 220–223. [Google Scholar] [CrossRef]
- de Silva, N.L.; Jayalath, M.D.; Sampath, W.K.C.; Perera, R.; Karunathilake, C. Primary hyperparathyroidism in an adolescent presenting with genu valgus progressing to extensive bone disease; a case report. BMC Endocr. Disord. 2023, 23, 71. [Google Scholar] [CrossRef]
- Chuang, T.J.; Tang, W.H.; Hung, Y.J.; Kuo, F.C. Aggressive gyriform calcifications and seizures after ischemia stroke in a patient with primary hyperparathyroidism. QJM 2014, 107, 567–569. [Google Scholar] [CrossRef]
- de la Plaza, L.R.; Ramia Ángel, J.M.; Arteaga Peralta, V.; Hernández Cristóbal, J.; López Marcano, A.J. Brain calcifications and primary hyperparathyroidism. Cir. Esp. 2016, 94, e5–e7. [Google Scholar] [CrossRef]
- Landais, A.; Mallet, G.; Bourgeois-Beauvais, Q.; Parisi, J.; Mecharles, S.; Lannuzel, A. Gyriform calcifications after ischemic stroke in a patient with primary hyperparathyroidism. Press. Med. 2018, 47 Pt 1, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Dutta, A.; Agrawal, K.; Jain, N.; Dutta, P.; Bhansali, A.; Behera, A.; Bhadada, S.K. Primary Hyperparathyroidism Presenting as Posterior Reversible Encephalopathy Syndrome: A Report of Two Cases. J. Clin. Res. Pediatr. Endocrinol. 2020, 12, 432–438. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, Y.; Jin, S.; Wang, O.; Liao, Q.; Zhu, Q.; Liu, H. Can we skip technetium-99 m sestamibi scintigraphy in pediatric primary hyperparathyroidism patients with positive neck ultrasound results? Pediatr. Radiol. 2023, 53, 2253–2259. [Google Scholar] [CrossRef]
- Sharma, A.; Patil, V.; Sarathi, V.; Purandare, N.; Hira, P.; Memon, S.; Jadhav, S.S.; Karlekar, M.; Lila, A.R.; Bandgar, T. Dual-phase computed tomography for localization of parathyroid lesions in children and adolescents with primary hyperparathyroidism. Ann. Endocrinol. 2023, 84, 446–453. [Google Scholar] [CrossRef]
- Ramonell, K.M.; Fazendin, J.; Lovell, K.; Iyer, P.; Chen, H.; Lindeman, B.; Dream, S. Outpatient parathyroidectomy in the pediatric population: An 18-year experience. J. Pediatr. Surg. 2022, 57, 410–413. [Google Scholar] [CrossRef]
- Boro, H.; Khatiwada, S.; Alam, S.; Kubihal, S.; Dogra, V.; Malla, S.; Kumar, C. The spectrum of manifestations of primary hyperparathyroidism in children and adolescents. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 178–187. [Google Scholar] [CrossRef]
- Shariq, O.A.; Lines, K.E.; English, K.A.; Jafar-Mohammadi, B.; Prentice, P.; Casey, R.; Challis, B.G.; Selberherr, A.; Boon, H.; Cranston, T.; et al. Multiple endocrine neoplasia type 1 in children and adolescents: Clinical features and treatment outcomes. Surgery 2022, 171, 77–87. [Google Scholar] [CrossRef]
- El Allali, Y.; Hermetet, C.; Bacchetta, J.; Amouroux, C.; Rothenbuhler, A.; Porquet-Bordes, V.; Champigny, M.A.; Baron, S.; Barat, P.; Bony-Trifunovic, H.; et al. Presenting features and molecular genetics of primary hyperparathyroidism in the paediatric population. Eur. J. Endocrinol. 2021, 184, 347–355. [Google Scholar] [CrossRef]
- Jovanovic, M.; Paunovic, I.; Zdravkovic, V.; Djordjevic, M.; Rovcanin, B.; Tausanovic, K.; Slijepcevic, N.; Zivaljevic, V. Case-control study of primary hyperparathyroidism in juvenile vs. adult patients. Int. J. Pediatr. Otorhinolaryngol. 2020, 131, 109895. [Google Scholar] [CrossRef]
- Zivaljevic, V.; Jovanovic, M.; Diklic, A.; Zdravkovic, V.; Djordjevic, M.; Paunovic, I. Differences in primary hyperparathyroidism characteristics between children and adolescents. J. Pediatr. Surg. 2020, 55, 1660–1662. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Chung, Y.J. A germline c.1546dupC MEN1 mutation in an MEN1 family: A case report. Medicine 2021, 100, e26382. [Google Scholar] [CrossRef]
- Al-Salameh, A.; Cadiot, G.; Calender, A.; Goudet, P.; Chanson, P. Clinical aspects of multiple endocrine neoplasia type 1. Nat. Rev. Endocrinol. 2021, 17, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Pieterman, C.R.C.; Valk, G.D. Update on the clinical management of multiple endocrine neoplasia type 1. Clin. Endocrinol. 2022, 97, 409–423. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.F.M.; van Nesselrooij, B.P.M.; Pieterman, C.R.C.; Verrijn Stuart, A.A.; van de Ven, A.C.; de Herder, W.W.; Dekkers, O.M.; Drent, M.L.; Havekes, B.; Kerstens, M.N.; et al. Clues for Genetic Anticipation in Multiple Endocrine Neoplasia Type 1. J. Clin. Endocrinol. Metab. 2020, 105, dgaa257. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Giusti, F.; Cioppi, F.; Maraghelli, D.; Cavalli, T.; Tonelli, F.; Brandi, M.L. Bone and Mineral Metabolism Phenotypes in MEN1-Related and Sporadic Primary Hyperparathyroidism, before and after Parathyroidectomy. Cells 2021, 10, 1895. [Google Scholar] [CrossRef]
- Wang, W.; Kong, J.; Nie, M.; Jiang, Y.; Li, M.; Xia, W.; Meng, X.; Xing, X.; Wang, O. Primary hyperparathyroidism in Chinese children and adolescents: A single-centre experience at Peking Union Medical College Hospital. Clin. Endocrinol. 2017, 87, 865–873. [Google Scholar] [CrossRef]
- Roizen, J.; Levine, M.A. Primary hyperparathyroidism in children and adolescents. J. Chin. Med. Assoc. 2012, 75, 425–434. [Google Scholar] [CrossRef]
- Marx, S.J.; Lourenço, D.M., Jr. Familial Hyperparathyroidism–Disorders of Growth and Secretion in Hormone-Secretory Tissue. Horm. Metab. Res. 2017, 49, 805–815. [Google Scholar] [CrossRef]
- Srirangam Nadhamuni, V.; Iacovazzo, D.; Evanson, J.; Sahdev, A.; Trouillas, J.; McAndrew, L.R.; Kurzawinski, T.; Bryant, D.; Hussain, K.; Bhattacharya, S.; et al. GHRH secretion from a pancreatic neuroendocrine tumor causing gigantism in a patient with MEN1. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Stasiak, M.; Dedecjus, M.; Zawadzka-Starczewska, K.; Adamska, E.; Tomaszewska, M.; Lewiński, A. Novel Germline c.105_107dupGCT MEN1 Mutation in a Family with Newly Diagnosed Multiple Endocrine Neoplasia Type 1. Genes 2020, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Nagy, R. Genetic testing by cancer site: Endocrine system. Cancer J. 2012, 18, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Petriczko, E.; Marcinkiewicz, K.; Prokurat, A.; Sagan, L.; Malkowski, B.; Biczysko-Mokosa, A.; Horodnicka-Jozwa, A.; Andrysiak-Mamos, E.; Syrenicz, A.; Kostrzeba, E.; et al. Rare clinical manifestation of multiple endocrine neoplasia type 1. Neuro Endocrinol. Lett. 2022, 43, 199–207. [Google Scholar] [PubMed]
- Mamedova, E.; Kolodkina, A.; Vasilyev, E.V.; Petrov, V.; Belaya, Z.; Tiulpakov, A. Successful Use of Denosumab for Life-Threatening Hypercalcemia in a Pediatric Patient with Primary Hyperparathyroidism. Horm. Res. Paediatr. 2020, 93, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Oba, T.; Ichikawa, K.; Nakamura, C.; Hara, Y.; Kanai, T.; Sato, Y.; Uehara, T.; Ito, K.I. Hypercalcemic crisis caused by primary hyperparathyroidism in a 11-year-old boy: A rare case report and review of the literature. Gland Surg. 2022, 11, 1279–1286. [Google Scholar] [CrossRef]
- Belaid, R.; Oueslati, I.; Chihaoui, M.; Yazidi, M.; Grira, W.; Chaker, F. A Case of Von Hippel-Lindau Disease with Bilateral Pheochromocytoma and Ectopic Hypersecretion of Intact Parathyroid Hormone in an Adolescent Girl. Case Rep. Endocrinol. 2020, 2020, 8824640. [Google Scholar] [CrossRef]
- Flokas, M.E.; Ganieva, G.; Grieco, A.; Agdere, L. Ectopic Parathyroid Adenoma in an 11-Year-Old Girl: Case Report and Literature Review. AACE Clin. Case Rep. 2020, 7, 51–56. [Google Scholar] [CrossRef]
- Vitale, R.J.; Shieh, H.F.; Modi, B.P.; Gordon, R.J. Primary Hyperparathyroidism from Ectopic Parathyroid Adenoma in a 12-Year-Old With Slipped Capital Femoral Epiphysis. J. Endocr. Soc. 2022, 6, bvac071. [Google Scholar] [CrossRef]
- Boro, H.; Alam, S.; Kubihal, V.; Khatiwada, S.; Kubihal, S.; Agarwal, S.; Khadgawat, R. Atypical parathyroid adenoma: Severe manifestations in an adolescent girl. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 91–100. [Google Scholar] [CrossRef]
- Legault, O.; Inman, M.; Moolman, N.; Wiebe, S.; Poulin, A.; Nour, M.A. Severe hypercalcemia and a pelvic brown tumor in an adolescent with primary hyperparathyroidism: A case report. BMC Pediatr. 2020, 20, 547. [Google Scholar] [CrossRef]
- Lenherr-Taube, N.; Lam, C.K.; Vali, R.; Shammas, A.; Campisi, P.; Zawawi, F.; Somers, G.R.; Stimec, J.; Mete, O.; Wong, A.K.; et al. Severe Primary Hyperparathyroidism Caused by Parathyroid Carcinoma in a 13-Year-Old Child; Novel Findings from HRpQCT. JBMR Plus 2020, 4, e10324. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Shahbazi, R.; Nikuei, P.; Soleimani, S.; Moradkhani, A.; Atashabparvar, A.; Khajehrahimi, F.; Zoghi, G.; Kheirandish, M. A Pediatric Parathyroid Carcinoma: An Unusual Clinical Presentation and Mini-review. Int. J. Endocrinol. Metab. 2021, 19, e110234. [Google Scholar] [CrossRef] [PubMed]
- David, O.; Loewenthal, N.; Haim, A.; Makarov, V.; Hershkovitz, E. Diagnosis, Management, and Possible Prevention of Hungry Bone Syndrome in an Adolescent with Primary Hyperparathyroidism and Vitamin D Deficiency. Isr. Med. Assoc. J. 2020, 22, 122–124. [Google Scholar] [PubMed]
- Lenschow, C.; Wennmann, A.; Hendricks, A.; Germer, C.T.; Fassnacht, M.; Buck, A.; Werner, R.A.; Plassmeier, L.; Schlegel, N. Questionable value of [99mTc]-sestamibi scintigraphy in patients with pHPT and negative ultrasound. Langenbecks Arch. Surg. 2022, 407, 3661–3669. [Google Scholar] [CrossRef]
- Prakash, S.; Damle, N.A.; Kumar, P.; Dharmashaktu, Y.; Bal, C.S. Lincoln Sign in a Case of Primary Hyperparathyroidism on 18 F-Fluorocholine PET/CT. Clin. Nucl. Med. 2023, 48, e343–e344. [Google Scholar] [CrossRef]
- Roztoczyńska, D.; Wójcik, M.; Konturek, A.; Nogieć, A.; Hubalewska-Dydejczyk, A.; Starzyk, J. Bilateral slipped capital femoral epiphysis as first manifestation of primary hyperparathyroidism in a 15-year-old boy. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 220–224. [Google Scholar] [CrossRef]
- Omi, Y.; Yamamoto, T.; Nagashima, Y.; Abe, K.; Karasawa, K.; Tanaka, Y.; Okamoto, T. Parathyroid carcinoma in a 13-year-old girl with a long-term survival. Surg. Case Rep. 2020, 6, 145. [Google Scholar] [CrossRef]
- Hendricks, A.; Lenschow, C.; Kroiss, M.; Buck, A.; Kickuth, R.; Germer, C.T.; Schlegel, N. Evaluation of diagnostic efficacy for localization of parathyroid adenoma in patients with primary hyperparathyroidism undergoing repeat surgery. Langenbecks Arch. Surg. 2021, 406, 1615–1624. [Google Scholar] [CrossRef]
- Kim, R.C.; Roch, A.M.; Birdas, T.J.; Ritter, H.E.; McDow, A.D. Hypercalcemic Crisis Caused by a Parathyroid Mass Requiring Thoracoscopic Resection. AACE Clin. Case Rep. 2021, 7, 264–267. [Google Scholar] [CrossRef]
- Abdulsalam, M.S.; Devanayagam, S.; Santosham, R.; Ganapathy, V.; Menon, M.; Simon, S. Mediastinal parathyroid adenoma removal by video-assisted thoracoscopic surgery. Ann. Afr. Med. 2021, 20, 150–153. [Google Scholar] [CrossRef]
- España, M.; Sastre, I.; Ceballos, R.J.; Bustos, M.E.F. VATS parathyroidectomy for primary hyperparathyroidism. Multimed. Man. Cardiothorac. Surg. 2020, 2020. [Google Scholar] [CrossRef]
- Badhe, P.V.; Patil, S.; Vikram Reddy, G.; Sheshadri, H.; Jain, S.; Nikumbh, T. Slipped capital femoral epiphysis as primary presentation in an adolescent with primary hyperparathyroidism due to ectopic mediastinal parathyroid adenoma. Clin. Case Rep. 2023, 11, e7498. [Google Scholar] [CrossRef] [PubMed]
- Zenno, A.; Ramamoorthy, B.; Hammoud, D.A.; Quezado, M.; Zeiger, M.A.; Jha, S. Case Report: Nine-year-old with parathyroid adenoma within the piriform sinus. Front. Endocrinol. 2023, 14, 1171052. [Google Scholar] [CrossRef]
- Minelli, R.; Meoli, A.; Tiri, A.; Fanelli, U.; Iannarella, R.; Gismondi, P.; Esposito, S. An Atypical Presentation of Primary Hyperparathyroidism in an Adolescent: A Case Report of Hypercalcaemia and Neuropsychiatric Symptoms Due to a Mediastinal Parathyroid Adenoma. Front. Endocrinol. 2020, 11, 581765. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.L.; Frazee, L.A. Cinacalcet for the treatment of primary hyperparathyroidism. Am. J. Ther. 2011, 18, 313–322. [Google Scholar] [CrossRef]
- Peacock, M.; Bilezikian, J.P.; Bolognese, M.A.; Borofsky, M.; Scumpia, S.; Sterling, L.R.; Cheng, S.; Shoback, D. Cinacalcet HCl reduces hypercalcemia in primary hyperparathyroidism across a wide spectrum of disease severity. J. Clin. Endocrinol. Metab. 2011, 96, E9–E18. [Google Scholar] [CrossRef]
- Erickson, L.A.; Mete, O.; Juhlin, C.C.; Perren, A.; Gill, A.J. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr. Pathol. 2022, 33, 64–89. [Google Scholar] [CrossRef]
- Batte, A.; Kasirye, P.; Baluku, R.; Kiguli, S.; Kalyesubula, R.; John, C.C.; Schwaderer, A.L.; Imel, E.A.; Conroy, A.L. Mineral bone disorders and kidney disease in hospitalized children with sickle cell anemia. Front. Pediatr. 2023, 10, 1078853. [Google Scholar] [CrossRef]
- Prytula, A.; Shroff, R.; Krupka, K.; Deschepper, E.; Bacchetta, J.; Ariceta, G.; Awan, A.; Benetti, E.; Büscher, A.; Berta, L.; et al. Hyperparathyroidism Is an Independent Risk Factor for Allograft Dysfunction in Pediatric Kidney Transplantation. Kidney Int. Rep. 2022, 8, 81–90. [Google Scholar] [CrossRef]
- Middelkoop, K.; Walker, N.; Stewart, J.; Delport, C.; Jolliffe, D.A.; Nuttall, J.; Coussens, A.K.; Naude, C.E.; Tang, J.C.Y.; Fraser, W.D.; et al. Prevalence and Determinants of Vitamin D Deficiency in 1825 Cape Town Primary Schoolchildren: A Cross-Sectional Study. Nutrients 2022, 14, 1263. [Google Scholar] [CrossRef]
- Yıldız, G.; Torun Bayram, M.; Çinleti, T.; Koç, A.; Soylu, A.; Kavukçu, S. Late onset Bartter syndrome: Bartter syndrome type 2 presenting with isolated nephrocalcinosis and high parathyroid hormone levels mimicking primary hyperparathyroidism. J. Pediatr. Endocrinol. Metab. 2022, 35, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Paduraru, D.N.; Nica, A.E.; Valea, A. Parathyroidectomy: Is vitamin D a player for a good outcome? J. Med. Life 2016, 9, 348–352. [Google Scholar] [PubMed]
- Mouly, C.; Vargas-Poussou, R.; Lienhardt, A.; Silve, C.; Hureaux, M.; Magdelaine, C.; Buffet, A.; Grunenwald, S.; Kuhn, J.M.; Brue, T.; et al. Reference Centre for Rare Diseases of Calcium, Phosphate Metabolism. Clinical characteristics of familial hypocalciuric hypercalcaemia type 1: A multicentre study of 77 adult patients. Clin. Endocrinol. 2020, 93, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Gorvin, C.M. Genetic causes of neonatal and infantile hypercalcaemia. Pediatr. Nephrol. 2022, 37, 289–301. [Google Scholar] [CrossRef]
- Arshad, M.F.; McAllister, J.; Merchant, A.; Rab, E.; Cook, J.; Eastell, R.; Balasubramanian, S. Urinary calcium indices in primary hyperparathyroidism (PHPT) and familial hypocalciuric hypercalcaemia (FHH): Which test performs best? Postgrad. Med. J. 2021, 97, 577–582. [Google Scholar] [CrossRef]
- Bollerslev, J.; Rejnmark, L.; Zahn, A.; Heck, A.; Appelman-Dijkstra, N.M.; Cardoso, L.; Hannan, F.M.; Cetani, F.; Sikjær, T.; Formenti, A.M.; et al. European Expert Consensus on Practical Management of Specific Aspects of Parathyroid Disorders in Adults and in Pregnancy: Recommendations of the ESE Educational Program of Parathyroid Disorders. Eur. J. Endocrinol. 2022, 186, R33–R63. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Ertl, D.A.; Zillikens, M.C.; Rjenmark, L.; Winter, E.M. Hypercalcemia during pregnancy: Management and outcomes for mother and child. Endocrine 2021, 71, 604–610. [Google Scholar] [CrossRef]
- Sadacharan, D.; Mahadevan, S.; Rao, S.S.; Kumar, A.P.; Swathi, S.; Kumar, S.; Kannan, S. Neonatal Severe Primary Hyperparathyroidism: A Series of Four Cases and their Long-term Management in India. Indian J. Endocrinol. Metab. 2020, 24, 196–201. [Google Scholar] [CrossRef]
- Höppner, J.; Sinningen, K.; Raimann, A.; Obermayer-Pietsch, B.; Grasemann, C. Disorders of the Calcium Sensing Signaling Pathway: From Familial Hypocalciuric Hypercalcemia (FHH) to Life Threatening Conditions in Infancy. J. Clin. Med. 2022, 11, 2595. [Google Scholar] [CrossRef]
- Höppner, J.; Lais, S.; Roll, C.; Wegener-Panzer, A.; Wieczorek, D.; Högler, W.; Grasemann, C. Case Report: Severe Neonatal Course in Paternally Derived Familial Hypocalciuric Hypercalcemia. Front. Endocrinol. 2021, 12, 700612. [Google Scholar] [CrossRef]
- Shaukat, M.; Ahmad, H.M.; Shafiq, M.U. Neonatal severe hyperparathyroidism: A case report. J. Pak. Med. Assoc. 2022, 72, 2538–2541. [Google Scholar] [CrossRef] [PubMed]
- Özgüç Çömlek, F.; Demir, S.; Gürkan, H.; İnan, M.; Sezer, A.; Dilek, E.; Kökenli, F. The efficiency of cinacalcet treatment in delaying parathyroidectomy in a case with neonatal severe hyperparathyroidism caused by homozygous mutation in the CASR gene. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Tak, S.A.; Misgar, R.A.; Agarwala, S.; Jain, V.; Sharma, R. A Case of Neonatal Severe Hyperparathyroidism: Challenges in Management. Indian J. Pediatr. 2022, 89, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Kempers, M.; Lugtenberg, D.; Abdallah, A.T.; Musa, S.A.; Ibrahim, A.A.; Abdullah, M.A. Challenges in diagnosis and management of neonatal hyperparathyroidism in a resource-limited country: A case series from a Sudanese family. Pan. Afr. Med. J. 2021, 40, 105. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, T.; Korkmaz, M.; Kul, M.; Koray, N. A rare cause of neonatal hypercalcemia: Neonatal severe primary hyperparathyroidism: A case report and review of the literature. Int. J. Surg. Case Rep. 2020, 66, 365–369. [Google Scholar] [CrossRef]
- Sorapipatcharoen, K.; Mahachoklertwattana, P.; Tim-Aroon, T.; Wattanasirichaigoon, D.; Karanes, S.; Molagool, S.; Poomthavorn, P. Successful parathyroidectomy with intra-operative parathyroid hormone monitoring in a neonate with severe primary hyperparathyroidism caused by homozygous mutation in CASR gene. J. Paediatr. Child Health 2020, 56, 1144–1146. [Google Scholar] [CrossRef]
- Gulcan-Kersin, S.; Kirkgoz, T.; Eltan, M.; Rzayev, T.; Ata, P.; Bilgen, H.; Ozek, E.; Bereket, A.; Turan, S. Cinacalcet as a First-Line Treatment in Neonatal Severe Hyperparathyroidism Secondary to Calcium Sensing Receptor (CaSR) Mutation. Horm. Res. Paediatr. 2020, 93, 313–321. [Google Scholar] [CrossRef]
- Eremkina, A.; Krupinova, J.; Dobreva, E.; Gorbacheva, A.; Bibik, E.; Samsonova, M.; Ajnetdinova, A.; Mokrysheva, N. Denosumab for management of severe hypercalcemia in primary hyperparathyroidism. Endocr. Connect 2020, 9, 1019–1027. [Google Scholar] [CrossRef]
- Jalleh, R.; Basu, G.; Le Leu, R.; Jesudason, S. Denosumab-Induced Severe Hypocalcaemia in Chronic Kidney Disease. Case Rep. Nephrol. 2018, 2018, 7384763. [Google Scholar] [CrossRef]
- Morimoto, H.; Nakajima, H.; Mori, J.; Fukuhara, S.; Shigehara, K.; Adachi, S.; Hosoi, H. Decrement in bone mineral density after parathyroidectomy in a pediatric patient with primary hyperparathyroidism. Clin. Pediatr. Endocrinol. 2018, 27, 81–86. [Google Scholar] [CrossRef]
- Sankaran, S.; Gamble, G.; Bolland, M.; Reid, I.R.; Grey, A. Skeletal effects of interventions in mild primary hyperparathyroidism: A meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 1653–1662. [Google Scholar] [CrossRef]
- Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to manage osteoporosis before the age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Madhuchani, D.; Seneviratne, S.N.; Ward, L.M. Bone health in childhood and adolescence: An overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries. Front. Endocrinol. 2023, 14, 1082413. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, S.; Högler, W.; Sakkers, R.J.B.; Appelman-Dijkstra, N.M.; Boot, A.M.; Sas, T.C.J.; Renes, J.S. Osteoporosis in children and adolescents: How to treat and monitor? Eur. J. Pediatr. 2023, 182, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.D. Osteoporosis in children and young adults. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nie, M.; Jiang, Y.; Li, M.; Meng, X.; Xing, X.; Wang, O.; Xia, W. Impaired geometry, volumetric density, and microstructure of cortical and trabecular bone assessed by HR-pQCT in both sporadic and MEN1-related primary hyperparathyroidism. Osteoporos. Int. 2020, 31, 165–173. [Google Scholar] [CrossRef]
- Cusano, N.E.; Rubin, M.R.; Silva, B.C.; Tay, Y.D.; Williams, J.M.; Agarwal, S.; Omeragic, B.; Guo, X.E.; Bilezikian, J.P. Skeletal Microstructure and Estimated Bone Strength Improve Following Parathyroidectomy in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2018, 103, 196–205. [Google Scholar] [CrossRef]
- Fraga, M.M.; de Sousa, F.P.; Szejnfeld, V.L.; de Moura Castro, C.H.; de Medeiros Pinheiro, M.; Terreri, M.T. Trabecular bone score (TBS) and bone mineral density (BMD) analysis by dual X-ray absorptiometry (DXA) in healthy Brazilian children and adolescents: Normative data. Arch. Osteoporos. 2023, 18, 82. [Google Scholar] [CrossRef]
- Valenzuela Riveros, L.F.; Long, J.; Bachrach, L.K.; Leonard, M.B.; Kent, K. Trabecular Bone Score (TBS) Varies with Correction for Tissue Thickness Versus Body Mass Index: Implications When Using Pediatric Reference Norms. J. Bone Miner. Res. 2023, 38, 493–498. [Google Scholar] [CrossRef]
- Nistor, C.E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. [Google Scholar] [CrossRef]
- Alfadhli, E.M. Management of Primary Hyperparathyroidism with Severe Hypercalcemia During the COVID-19 Pandemic. Clin. Ther. 2021, 43, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients–Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Mathew, A.; Kaur, P.; Mishra, S.K. Denosumab can be used successfully as a bridge to surgery in patients with severe hypercalcemia due to primary hyperparathyroidism. Arch. Endocrinol. Metab. 2021, 65, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Aojula, N.; Ready, A.; Gittoes, N.; Hassan-Smith, Z. Management of Parathyroid Disease during the COVID-19 Pandemic. J. Clin. Med. 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.; Ristea, R.P.; Popescu, M.; Vasile, C.M.; Popa, F.L. Thyroid Carcinoma Showing Thymus-like Differentiation (CASTLE): A Case Report. Life 2022, 12, 1314. [Google Scholar] [CrossRef]
- Linquest, L.; Ackerman, K.; Dewan, K. Implications of COVID-19 in Airway and Swallowing Function. OTO Open 2023, 7, e74. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Chen, S.; Wang, S.; Lin, J. Risk factors for neck pain in college students: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1502. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studied population: children and teenagers (patients 18 years old or younger) | Reviews, editorials |
| PubMed access of the papers | Experimental studies |
| Full-length, English-language articles | Secondary (vitamin D-associated) hyperparathyroidism and/or rickets |
| Research keywords within the title and/or abstract: “pediatric primary hyperparathyroidism”, “child and primary hyperparathyroidism”, or “teenager and primary hyperparathyroidism” | Secondary or tertiary (renal) hyperparathyroidism |
| Original studies (studies, case series, case reports) of any design (regardless the level of statistical evidence) | Primary hyperparathyroidism in pregnancy |
| Time frame (by publication date): January 2020–July 2023 | Familial hypocalciuric hypercalcemia Bartter syndrome Inherited conditions of mineral metabolism |
| Mixed (adult and pediatric) studies, unless a specific analysis of the pediatric data was provided |
| First Author Year of Publication Reference Number | Study Design Studied Population | Outcome |
|---|---|---|
| He 2023 [45] | Single-center study (between 2003 and 2021) N = 32 patients with PHP Age: ≤18 years old (mean age of 14.7 ± 2.5 years) Parathyroid tumor size: 1–5.8 cm (mean size of 2.85 cm) | Cervical ultrasound: 100% sensitivity for single parathyroid tumors (none ectopic) 99mTc Sestamibi scintigraphy: concordant with ultrasound results in 98% of cases (N1 = 30) N2 = 2 patients with multi-glandular parathyroid disease according to 99mTc Sestamibi, but not with ultrasound assessment |
| Sharma 2023 [46] | Single-center imaging study N = 23 children and teenagers with PHP (4/23 with germline mutations: 3 with CDC73 and 1 with CASR) | Dual-phase computed tomography provided good sensitivity (91.3%) and specificity (99.5%) in single-gland disease |
| Szabo Yamashita 2022 [27] | Single-center retrospective study (between 1994 and 2020) N = 66 with PHP (61% females) Age: ≤21 years old (mean age of 17.3 years) | 71% symptomatic PHP 32% known with genetic PHP (mostly MEN1) 5% of apparently sporadic cases were genetic PHP Sporadic vs. familial PHP:
|
| Sharma 2022 [26] | Single-center retrospective study (between January 2020 and January 2021) N = 36 patients with PHP (55% males) Age < 20 years (median age of 17 years) N1 = 10 genetic/familial PHP N2 = 16 apparently sporadic PHP | N1: 90% with pathogenic variants: MEN1 gene (8/10), CDC73 (1/10) N2 = 26.9% with pathogenic variants/homozygotes: CDC73 (4/26), CASR (3/26) N1 vs. N2:
|
| Bernardor 2022 [18] | Multicenter study N = 18 patients with PHP receiving cinacalcet Median age of 10.2 years (N1 = 10 genetic PHP involving CASR, CDC73, and MEN1 genes) |
Median duration of therapy: 2.2 years Dose: 0.7 (0.6–1) mg/kg/day → 1 (0.9–1.4) mg/kg/day PTH drop (p = 0.01) Hypercalcemia drop (p = 0.002) Nephrolithiasis: 1/13 |
| Ramonell 2022 [47] | Single-center (single surgeon) study N = 19 patients who underwent parathyroidectomy (as outpatients) for PHP Mean age of 14.1 years N1 = 1 case with MEN1 N2 = 1 case with MEN2 | 8/19 patients had unilateral parathyroidectomies 9/19 patients had trans-cervical thymectomies 1/19 subject had transitory hypocalcemia 1/19 subject had permanent hypoparathyroidism |
| Boro 2022 [48] | Single-center study N = 10 patients who underwent parathyroidectomy Mean age of 16.7 years | 90% (of the patients) had muscle and skeletal complaints 50%: bone deformities 50%: kidney stones 30%: fractures 40%: gastrointestinal complaints 30%: pancreatitis 40%: hungry bone syndrome |
| Shariq 2021 [49] | Retrospective study N = 80 patients with MEN1 Age: ≤18 years old (median age of 14 years) | 80% of the patients had PHP (70% of them underwent a parathyroidectomy) |
| El Allali 2021 [50] | Retrospective study N = 63 patients with PHP who underwent genetic analysis | 52% had genetic PHP Younger group (94%) had CASR mutations Older group presented other mutations (MEN1, CDC73, RET, and CDKN1B) |
| Sharanappa 2020 [28] | Retrospective study (between 1989 and 2019) N = 35 patients with PHP Mean age of 15.2 years | 94% of the patients had symptomatic PHP 83%: skeletal anomalies 29%: renal complications 8%: familial PHP 97%: cure rate via parathyroidectomy 2.8%: hypercalcemic crisis 34%: hungry bone syndrome |
| Rampp 2020 [5] | Triple-center retrospective study (between 1997 and 2017) N = 83 patients with PHP who underwent parathyroidectomy (64% females) Age: ≤21 years old (mean age of 17 years) | 25% of the patients had ectopic adenomas (59% of them were intra-thymic) 98% of the patients had a 6-month cure rate |
| Jovanovic 2020 [51] | Case–control, single-center (high-volume surgery) study N1 = 14 patients with PHP (age ≤ 20 years) N2 = 28 adults with PHP | N1: high frequency of bone disease (42% of the patients from N1) N2: high frequency of asymptomatic presentation (39% of the patients from N2) Female to male ratio: 1 to 1 (N1); 8 to 1 (N2) (p = 0.005) |
| Zivaljevic 2020 [52] | Retrospective study Out of 1363 patients, N = 14 patients with PHP (age < 20 years representing 1% of entire cohort) N1 = 6 children with PHP (aged ≤ 15 years) N2 = 8 teenagers with PHP (age: >15 and ≤20 years) | N1 vs. N2:
|
| Pathogenic Variant | First Author Year of Publication Reference Number | Index Case |
|---|---|---|
| MEN1 | Petriczko 2022 [64] | 17-year-old male diagnosed with PHP and pancreatic neuroendocrine tumor Family data: father with PHP; sister with PHP, pancreatic neuroendocrine tumor, and central ganglioglioma |
| Srirangam Nadhamuni 2021 [61] | At the age of 10: resection of an insulinoma At the age of 15: parathyroidectomy for PHP At the age of 18: gigantism due to ectopic GHRH production (pancreatic neuroendocrine tumor) | |
| Cho # 2021 [53] | 12-year-old female was the daughter of the proband PHP, prolactinoma, pancreatic neuroendocrine tumor; Frame-shift mutation: NM_130799.1:c.1546dupC (p.Arg516Profs∗15) | |
| Stasiak 2020 [62] | 16-year-old female diagnosed with PHP and pituitary microadenoma Novel MEN1 germline pathogenic variant (heterozygous variant c.105_107dupGCT) Family data: father with the same pathogenic variant (PHP and pituitary neuroendocrine tumor) | |
| CDC73 | Blackburn 2022 [17] | 14-year-old male with heterogeneous gene deletion (symptomatic hypercalcemia due to a single PT adenoma) 10-year-old female with known autosomal dominant mutation (symptomatic hypercalcemia and bilateral PT adenoma) |
| Mamedova 2020 [65] | 16-year-old female admitted for fractures, nausea, vomiting, weight loss (heterozygote status) 60 mg denosumab → parathyroidectomy → hypocalcemia | |
| VHL | Belaid 2020 [67] | 16-year-old female admitted for diabetes mellitus and high blood pressure due to pheochromocytoma Ectopic PTH (adrenal) production → remission of PHP after bilateral adrenalectomy |
| First Authors Reference Number | Year of Publication | Patient | Preoperative Findings | Postoperative Findings and Outcome |
|---|---|---|---|---|
| Muse [9] | 2023 | 16-year-old male | Nausea, vomiting, headache | Giant PT adenoma This is the first report of a PHP-related brain calcification at the frontal lobe in a child |
| Sahu [11] | 2023 | 12-year-old female | History of limb deformities, multiple fragility fractures, kidney stones | Ectopic PT adenoma (intra-thymus) Sestamibi-guided thoracoscopic left thymectomy (CT with radioisotope scans) |
| Boggs [15] | 2023 | 12-year-old female | Chest pain and dyspnea (complication: one osteolytic rib lesion) | Cystic left inferior PT adenoma Hungry bone syndrome following parathyroidectomy |
| Badhe [83] | 2023 | 13-year-old male | Unilateral slipped capital femoral epiphysis | Ectopic mediastinal PT adenoma |
| Zenno [84] | 2023 | 9-year-old female | Symptomatic hypercalcemia | Ectopic PT adenoma (pyriform sinus) → reperformed parathyroidectomy |
| de Silva [40] | 2023 | 17-year-old male | Multiple fractures (brown tumors) | Good outcome after parathyroidectomy |
| Prakash [76] | 2023 | 14-year-old female | PHP | Lincoln sign (“black beard sign”) due to mandibular uptake of the tracer during 8 F-fluorocholine PE/CT |
| Gafar [37] | 2022 | 12-year-old male | A 6-year history of bone pain, loss of appetite, fatigue, progressive lower limb deformity | Removal of inferior parathyroid adenoma → postoperative hypocalcemia → calcium and alfacalcidol replacements Association with genu valgum due to rickets |
| Hayashi [66] | 2022 | 11-year-old male | Hypercalcemic crisis | Poor response to standard care (including calcitonine → pamidronate) → successful emergency parathyroidectomy |
| Vitale [69] | 2022 | 12-year-old male | Bilateral slipped capital femoral epiphysis | Ectopic PT adenoma (intra-thymus) Thoracoscopic resection → post-parathyroidectomy hungry bone syndrome |
| Boro [70] | 2022 | 16-year-old female | Severe clinical picture | Atypical PT adenoma → postoperative hungry bone disease |
| Oh [34] | 2022 | 9-year-old female (C1) 14-year-old male (C2) 14-year-old female (C3) | Abdominal pain due to pancreatitis (C1) Abdominal pain due to ureter stone (C2) Gait disturbance, weakness (C3) | Postoperative PTH normalization (C3: ectopic PT adenoma) |
| Bin Yahib [29] | 2021 | 13-year-old female | 8-month history of recurrent abdominal pain, nausea, vomiting, bone pain | Ectopic PT adenoma (intra-thymus) Thoracoscopic resection |
| Dikova [38] | 2021 | 12-year-old female (C1) 15-year-old female (C2) | Admission for genu valgum (orthopedic assessment) | C1 associated a local brown tumor C2 associated a local bone cyst |
| Tuli [36] | 2021 | 16-year-old female (C1) 14-year-old female (C1) | Brown tumor at the heel (C1) Recurrent abdominal pain, emotional lability, asymptomatic nephrolithiasis (C2) | Preoperative cinacalcet (3 months) use (C2) Successful conventional parathyroidectomy in both cases (hungry bone syndrome in C2 that required calcitriol for 2 years) |
| Flokas [68] | 2021 | 11-year-old female | Unexpected detection of hypercalcemia during admission for peritonsillar cellulitis | Ectopic PT adenoma (intra-thymic) Thoracoscopic thymectomy (5 months after initial presentation) |
| Fukaya [35] | 2021 | 12-year-old female | Recurrent abdominal pain, macroscopic hematuria | Parathyroidectomy of a large PT adenoma (1.8 g) |
| Rahimi [73] | 2021 | 15-year-old female | 8-year history of bone pain and progressive limping | Parathyroid carcinoma |
| Roztoczyńska [77] | 2020 | 15-year-old male | Bilateral slipped capital femoral epiphysis, polydipsia, polyuria, weight loss | Orthopedic correction (brown tumors) → medical therapy for hypercalcemia (including pamidronate) → left inferior parathyroidectomy |
| Legault [71] | 2020 | 14-year-old male | Abdominal pain, vomiting, constipation, pelvic brown tumor | Parathyroidectomy → hungry bone syndrome Postoperative brown tumor remission |
| Minelli [85] | 2020 | 17-year-old female | Psychiatric manifestations | Ectopic PT adenoma (intra-thymus) Robot-assisted surgery for tumor removal |
| Seo [10] | 2020 | 15-year-old male | Adenoma localization required SPECT/CT | Ectopic PT adenoma (intra-thymus) → VATS |
| Lenherr-Taube [72] | 2020 | 13-year-old male | Muscle and bone pain, brown tumors | Parathyroid carcinoma (loss of parafibromin) Therapy with pamidronate, denosumab → parathyroidectomy → hungry bone syndrome (intravenous calcium for 3 weeks) → no relapse for 18 months Postoperative brown tumor remission |
| Pal [44] | 2020 | 12-year-old male 16-year-old male | Posterior reversible encephalopathy syndrome | Neurological symptom remission after parathyroidectomy |
| David [74] | 2020 | 15-year-old female | Brown tumors | Parathyroidectomy → hungry bone syndrome |
| Lee [39] | 2020 | 15-year-old male | Bilateral genu valgum | Parathyroidectomy → calcium normalization within 2 months |
| Omi [78] | 2020 | 13-year-old female | Fibular fracture | Parathyroidectomy (confirmation of parathyroid carcinoma) → at age of 22: femoral fracture → resection of bilateral lung metastases → at age of 33: re-resection of pulmonary metastases → at age of 57 → suspected neck recurrence → en bloc resection of parathyroid adenoma → 11C-methionine-positive tumor recurrence → removal → relapse 8 months later → denosumab for hypercalcemia + radiotherapy to control the recurrence |
| First Author Reference Number | Year of Publication | Patient | Clinical Presentation | Management |
|---|---|---|---|---|
| Shaukat [102] | 2022 | 6-month-old male | Lethargy, bradycardia, hyperreflexia (his sibling died of the same condition) | CASR pathogenic variant Poor control of hypercalcemia through pamidronate, cinacalcet → parathyroidectomy+ self-transplantation of half of the left inferior PT gland → calcium and alfacalcidol supplements |
| Özgüç Çömlek [103] | 2022 | 6-day-old male | Hypotonia, poor feeding → weight loss | Therapy with cinacalcet for 13 months → hypercalcemia after 2 months since stopping cinacalcet→ parathyroidectomy |
| Gupta [104] | 2022 | 20-day-old female | Growth and developmental delays | Parathyroidectomy at 7 months |
| Hassan [105] | 2022 | 3 infant siblings | Polyuria, failure to thrive, fractures | 2/3 patients (a 7-month-old female and an 8-month-old male) were tested and found positive for homozygous missense CASR pathogenic variant: c 2038 C T p (Arg680Cys) Patients underwent parathyroidectomies |
| Höppner [101] | 2021 | 2 infant siblings | Hypercalcemia + preterm (C1) Hypercalcemia + severe muscular hypotonia + thrombocytopenia + multiple fractures (C2) | Heterozygous CASR pathogenic variant: c.554G>A; p. (Arg185Gln) Both patients were stabilized under medical therapy (C2: therapy with cinacalcet was used for 3 months) |
| Gulcan-Kersin [108] | 2020 | 2-day-old female | Severe hypercalcemia during the first day of life | Homozygous CASR pathogenic variant: c.1836G>A (p.G613E) Therapy with cinacalcet since day 2 → 18 months (heterozygote status of her father and sister) |
| Abdullayev [106] | 2020 | 7-month-old male | Multiple episodes of hypercalcemia early after birth | Therapy with calcitonin, cinacalcet, pamidronate → parathyroidectomy twice at 7 months (first removal of all 4 PT glands → persistent high PTH → thymectomy) |
| Sorapipatcharoen [107] | 2020 | 10-day-old female | Severe hypercalcemia early after birth | Homozygous CASR pathogenic variant 1630 (c.1630C > T) Therapy with calcitonin, cinacalcet, pamidronate, zoledronate → parathyroidectomy (day 70) → hungry bone syndrome |
| Sadacharan [99] | 2020 | 4 cases (mean age of 28.7 days) | Severe hypercalcemia early after birth (hypotonia, respiratory insufficiency, failure to thrive) | Medical therapy → parathyroidectomy + trans-cervical thymectomy (+hemi-thyroidectomy in one case) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsote, M.; Stanciu, M.; Popa, F.L.; Gheorghe, A.-M.; Ciuche, A.; Nistor, C. Pediatric Neuroendocrine Neoplasia of the Parathyroid Glands: Delving into Primary Hyperparathyroidism. Biomedicines 2023, 11, 2810. https://doi.org/10.3390/biomedicines11102810
Carsote M, Stanciu M, Popa FL, Gheorghe A-M, Ciuche A, Nistor C. Pediatric Neuroendocrine Neoplasia of the Parathyroid Glands: Delving into Primary Hyperparathyroidism. Biomedicines. 2023; 11(10):2810. https://doi.org/10.3390/biomedicines11102810
Chicago/Turabian StyleCarsote, Mara, Mihaela Stanciu, Florina Ligia Popa, Ana-Maria Gheorghe, Adrian Ciuche, and Claudiu Nistor. 2023. "Pediatric Neuroendocrine Neoplasia of the Parathyroid Glands: Delving into Primary Hyperparathyroidism" Biomedicines 11, no. 10: 2810. https://doi.org/10.3390/biomedicines11102810







