Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. AF (Cell-Depleted Amniotic Fluid) Preparation
2.3. Western Blotting Analysis
2.4. RNA Isolation, Reverse Transcription (RT)-Quantitative (q)PCR Analysis
2.5. Dosage of Cytokines
2.6. Malondialdehyde (MDA) Determination in AF
2.7. Nitrite Determination in AF
2.8. Caspase 3 and Caspase 9 Activities in AFC
2.9. Senescence-Associated β-Galactosidase Staining (SA-βGal)
2.10. Catalytic Subunit of the Telomerase Reverse Transcriptase (TERT) Activity Detection
2.11. Amino Acid and Acylcarnitine Profiling by Targeted LC-MS/MS
2.12. Statistics
3. Results
3.1. Population Characteristics
3.2. ER Protein Expression in AFCs
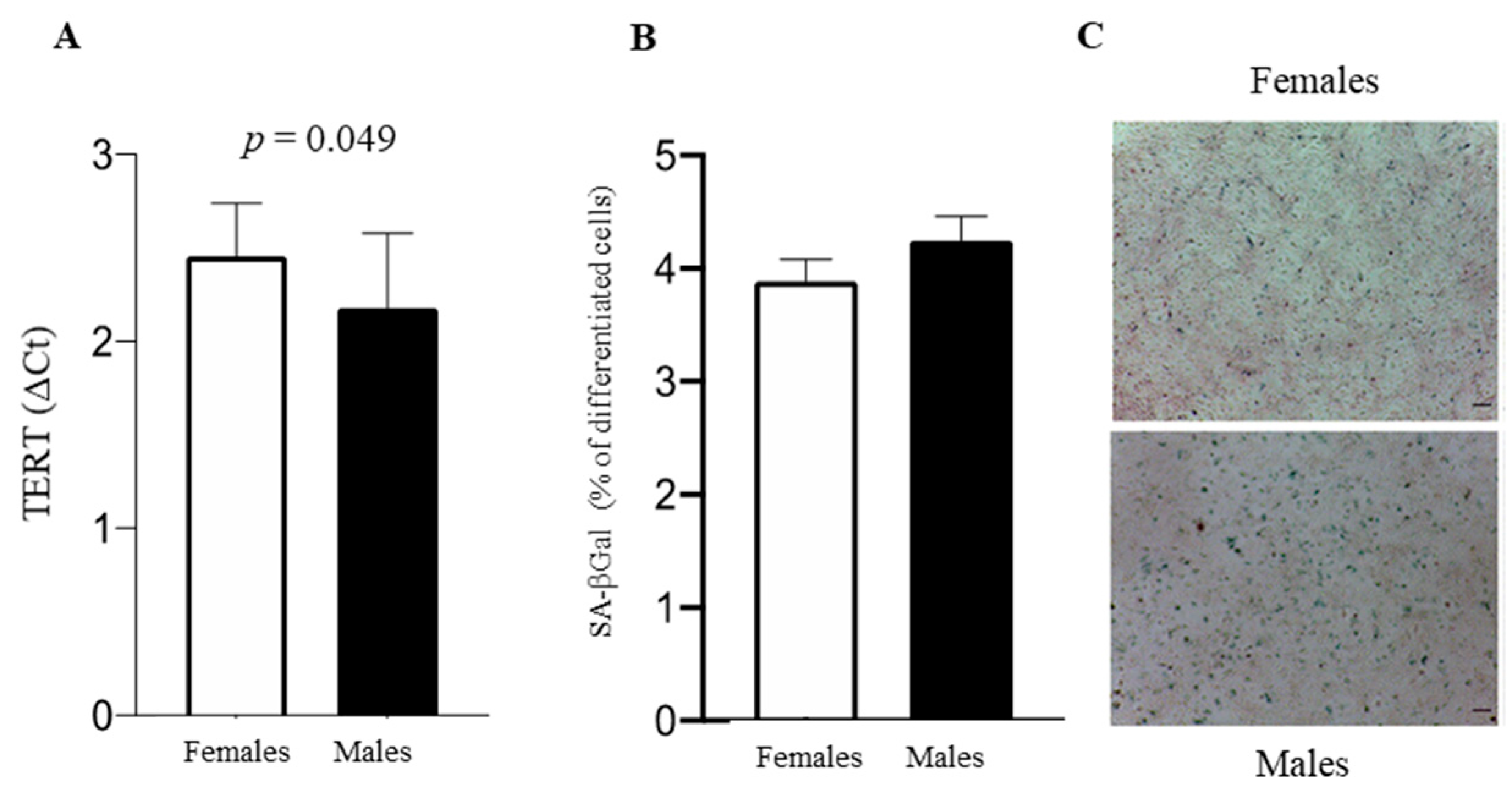
3.3. Cell Fate: Autophagy, Apoptosis, and Senescence
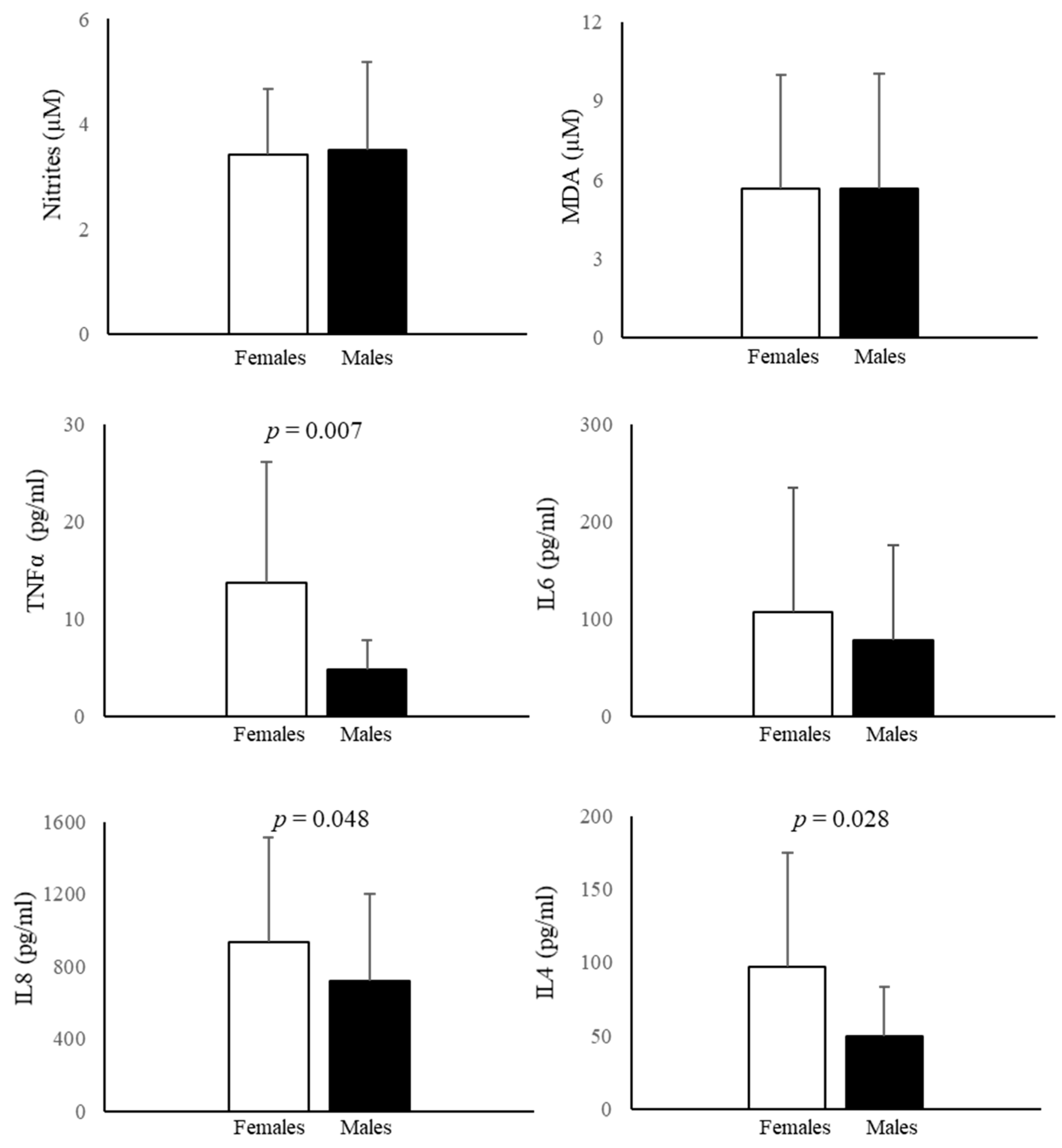
3.4. Nitrite and MDA Determination in AF
3.5. Cytokine Measurement in AF
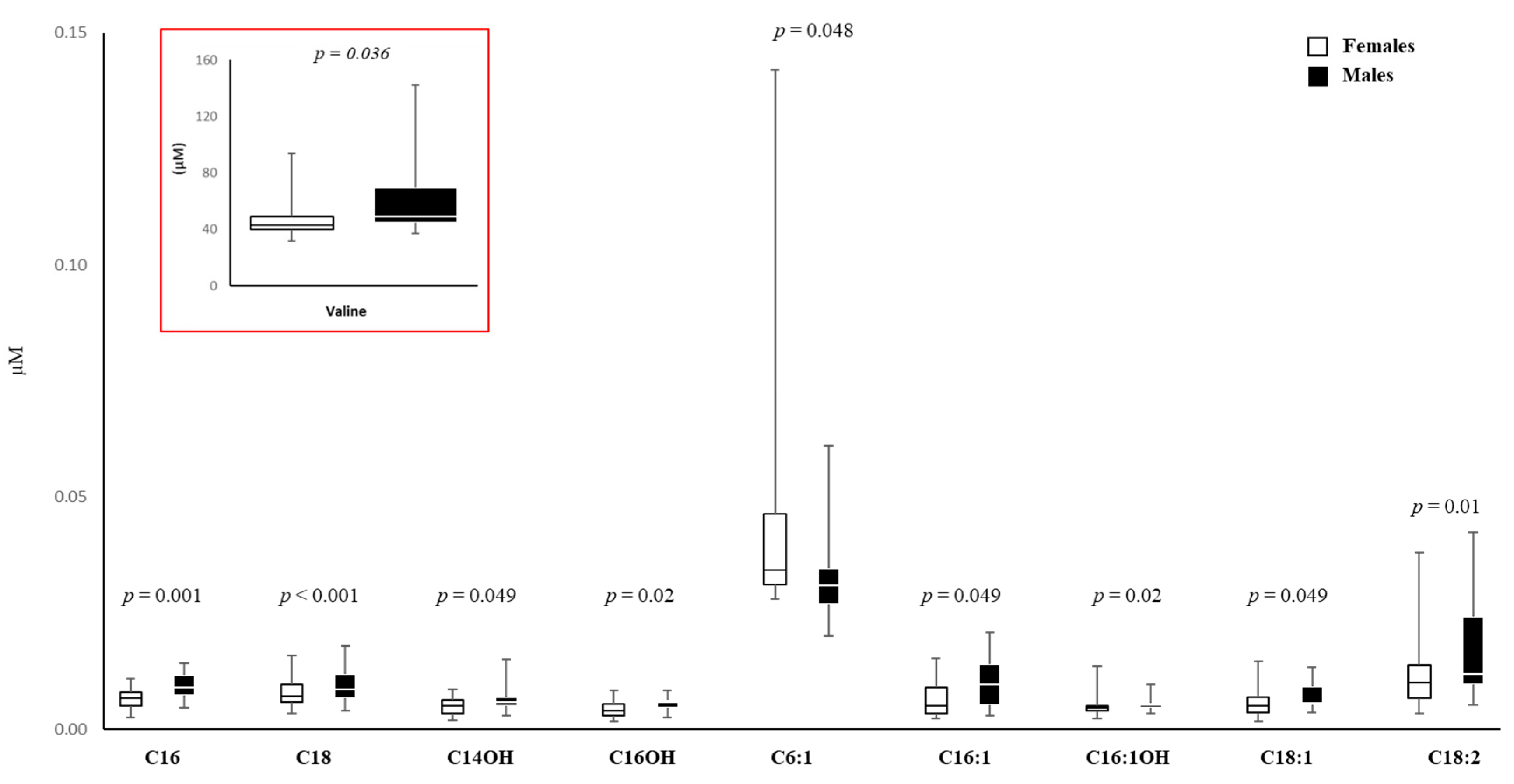
3.6. Amino Acid and Carnitine Levels in AF
3.7. Cluster Analysis of Metabolomic Parameters
4. Discussion
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beloosesky, R.; Ross, M.G. Amniotic Fluid. Encycl. Reprod. 2018, 3, 380–386. [Google Scholar] [CrossRef]
- Ten Broek, C.M.A.; Bots, J.; Varela-Lasheras, I.; Bugiani, M.; Galis, F.; van Dongen, S. Amniotic Fluid Deficiency and Congenital Abnormalities Both Influence Fluctuating Asymmetry in Developing Limbs of Human Deceased Fetuses. PLoS ONE 2013, 8, e81824. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.L.; Wang, L.; Gao, T.B.; Qin, Y.G.; Qi, Y.Q.; Xu, Y.P. Potential Function of Amniotic Fluid in Fetal Development—Novel Insights by Comparing the Composition of Human Amniotic Fluid with Umbilical Cord and Maternal Serum at Mid and Late Gestation. J. Chin. Med. Assoc. 2009, 72, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Romero, R.; Pique-Regi, R.; Pacora, P.; Done, B.; Kacerovsky, M.; Bhatti, G.; Jaiman, S.; Hassan, S.S.; Hsu, C.D.; et al. Amniotic Fluid Cell-Free Transcriptome: A Glimpse into Fetal Development and Placental Cellular Dynamics during Normal Pregnancy. BMC Med. Genom. 2020, 13, 25. [Google Scholar] [CrossRef]
- Vasani, A.; Kumar, M.S. Advances in the Proteomics of Amniotic Fluid to Detect Biomarkers for Chromosomal Abnormalities and Fetomaternal Complications during Pregnancy. Expert Rev. Proteom. 2019, 16, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, O.; Levitt, L.; Lilleri, D.; Vainer, G.W.; Kaplan, O.; Schreiber, L.; Arossa, A.; Spinillo, A.; Furione, M.; Alfi, O.; et al. Amniotic Fluid Biomarkers Predict the Severity of Congenital Cytomegalovirus Infection. J. Clin. Investig. 2022, 132, e157415. [Google Scholar] [CrossRef]
- Bhatti, G.; Romero, R.; Gomez-Lopez, N.; Pique-Regi, R.; Pacora, P.; Jung, E.; Yeo, L.; Hsu, C.D.; Kavdia, M.; Tarca, A.L. The Amniotic Fluid Cell-Free Transcriptome in Spontaneous Preterm Labor. Sci. Rep. 2021, 11, 13481. [Google Scholar] [CrossRef]
- Kolvatzis, C.; Tsakiridis, I.; Kalogiannidis, I.A.; Tsakoumaki, F.; Kyrkou, C.; Dagklis, T.; Daniilidis, A.; Michaelidou, A.-M.; Athanasiadis, A. Utilizing Amniotic Fluid Metabolomics to Monitor Fetal Well-Being: A Narrative Review of the Literature. Cureus 2023, 15, e36986. [Google Scholar] [CrossRef]
- Dobreva, M.P.; Pereira, P.N.G.; Deprest, J.; Zwijsen, A. On the Origin of Amniotic Stem Cells: Of Mice and Men. Int. J. Dev. Biol. 2010, 54, 761–777. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Hosper, N.; Luginbühl, J.; Biedermann, T.; Reichmann, E.; Meuli, M. Human Amniotic Fluid Derived Cells Can Competently Substitute Dermal Fibroblasts in a Tissue-Engineered Dermo-Epidermal Skin Analog. Pediatr. Surg. Int. 2013, 29, 61–69. [Google Scholar] [CrossRef]
- Legato, M. Principles of Gender-Specific Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Campesi, I.; Racagni, G.; Franconi, F. Just a Reflection: Does Drug Repurposing Perpetuate Sex-Gender Bias in the Safety Profile? Pharmaceuticals 2021, 14, 730. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Franconi, F.; Montella, A.; Dessole, S.; Capobianco, G. Human Umbilical Cord: Information Mine in Sex-Specific Medicine. Life 2021, 11, 52. [Google Scholar] [CrossRef]
- Galjaard, S.; Ameye, L.; Lees, C.C.; Pexsters, A.; Bourne, T.; Timmerman, D.; Devlieger, R. Sex Differences in Fetal Growth and Immediate Birth Outcomes in a Low-Risk Caucasian Population. Biol. Sex Differ. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.; Newnham, J.; Petraglia, F.; Yeganegi, M.; Bocking, A. Fetal Sex and Preterm Birth. Placenta 2013, 34, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Caterino, M.; Ruoppolo, M.; Costanzo, M.; Albano, L.; Crisci, D.; Sotgiu, G.; Saderi, L.; Montella, A.; Franconi, F.; Campesi, I. Sex Affects Human Premature Neonates’ Blood Metabolome According to Gestational Age, Parenteral Nutrition, and Caffeine Treatment. Metabolites 2021, 11, 158. [Google Scholar] [CrossRef]
- Anand-Ivell, R.; Ivell, R.; Driscoll, D.; Manson, J. Insulin-like Factor 3 Levels in Amniotic Fluid of Human Male Fetuses. Hum. Reprod. 2008, 23, 1180–1186. [Google Scholar] [CrossRef][Green Version]
- Cagnacci, A.; Arangino, S.; Caretto, S.; Mazza, V.; Volpe, A. Sexual Dimorphism in the Levels of Amniotic Fluid Leptin in Pregnancies at 16 Weeks of Gestation: Relation to Fetal Growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 53–57. [Google Scholar] [CrossRef]
- Poggi, S.H.; Spong, C.Y.; Ghidini, A.; Ossandon, M. Gender Differences in Amniotic Fluid Cytokine Levels. J. Matern. Fetal Neonatal Med. 2004, 15, 367–371. [Google Scholar] [CrossRef]
- Bamberg, C.; Fotopoulou, C.; Linder, M.; Roehr, C.C.; Dudenhausen, J.W.; Henrich, W.; Kalache, K. Mid-Trimester Amniotic Fluid Concentrations of the Proinflammatory Cytokines IL-6, IL-8, TNF-α, and Lipopolysaccharide Binding Protein in Normal Pregnancies: A Prospective Evaluation According to Parity, Gestational Age, and Fetal Gender. J. Perinat. Med. 2011, 39, 403–409. [Google Scholar] [CrossRef]
- Weissenbacher, T.; Laubender, R.P.; Witkin, S.S.; Gingelmaier, A.; Schiessl, B.; Kainer, F.; Friese, K.; Jeschke, U.; Dian, D.; Karl, K. Influence of Maternal Age, Gestational Age and Fetal Gender on Expression of Immune Mediators in Amniotic Fluid. BMC Res. Notes 2012, 5, 375. [Google Scholar] [CrossRef]
- Bernardi, S.; Toffoli, B.; Tonon, F.; Francica, M.; Campagnolo, E.; Ferretti, T.; Comar, S.; Giudici, F.; Stenner, E.; Fabris, B. Sex Differences in Proatherogenic Cytokine Levels. Int. J. Mol. Sci. 2020, 21, 3861. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Díaz, M.; Lerner, A.D.; Yu, D.H.; Thiboutot, J.P.; Liu, M.C.; Yarmus, L.B.; Bose, S.; Heller, N.M. Sex Differences in M2 Polarization, Chemokine and IL-4 Receptors in Monocytes and Macrophages from Asthmatics. Cell. Immunol. 2021, 360, 104252. [Google Scholar] [CrossRef] [PubMed]
- Marzi, M.; Vigano, A.; Trabattoni, D.; Villa, M.L.; Salvaggio, A.; Clerici, E.; Clerici, M. Characterization of Type 1 and Type 2 Cytokine Production Profile in Physiologic and Pathologic Human Pregnancy. Clin. Exp. Immunol. 1996, 106, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, S.; Pritz, S.; Amann, N.; Meister, S.; Beyer, S.; Jegen, M.; Kuhn, C.; Hutter, S.; Knabl, J.; Mahner, S.; et al. Sex Specific Expression of Interleukin 7, 8 and 15 in Placentas of Women with Gestational Diabetes. Int. J. Mol. Sci. 2020, 21, 8026. [Google Scholar] [CrossRef]
- O’Neill, K.; Alexander, J.; Azuma, R.; Xiao, R.; Snyder, N.W.; Mesaros, C.A.; Blair, I.A.; Pinney, S.E. Gestational Diabetes Alters the Metabolomic Profile in 2nd Trimester Amniotic Fluid in a Sex-Specific Manner. Int. J. Mol. Sci. 2018, 19, 2696. [Google Scholar] [CrossRef]
- Campesi, I.; Occhioni, S.; Capobianco, G.; Montella, A.; Dessole, S.; Franconi, F. Sex-Specific Pharmacological Modulation of Autophagic Process in Human Umbilical Artery Smooth Muscle Cells. Pharmacol. Res. 2016, 113, 166–174. [Google Scholar] [CrossRef]
- Addis, R.; Campesi, I.; Fois, M.; Capobianco, G.; Dessole, S.; Fenu, G.; Montella, A.; Cattaneo, M.G.; Vicentini, L.M.; Franconi, F. Human Umbilical Endothelial Cells (HUVECs) Have a Sex: Characterisation of the Phenotype of Male and Female Cells. Biol. Sex Differ. 2014, 5, 18. [Google Scholar] [CrossRef]
- Mank, J.E.; Rideout, E.J. Developmental Mechanisms of Sex Differences: From Cells to Organisms. Development 2021, 148, dev199750. [Google Scholar] [CrossRef]
- Khan-Dawood, F.S.; Dawood, M.Y. Estrogen and Progesterone Receptor and Hormone Levels in Human Myometrium and Placenta in Term Pregnancy. Am. J. Obstet. Gynecol. 1984, 150, 501–505. [Google Scholar] [CrossRef]
- Bukovsky, A.; Caudle, M.; Cekanova, M.; Fernando, R.; Wimalasena, J.; Foster, J.; Henley, D.; Elder, R. Placental Expression of Estrogen Receptor Beta and Its Hormone Binding Variant—Comparison with Estrogen Receptor Alpha and a Role for Estrogen Receptors in Asymmetric Division and Differentiation of Estrogen-Dependent Cells. Reprod. Biol. Endocrinol. 2003, 1, 36. [Google Scholar] [CrossRef]
- Pavan, B.; Paganetto, G.; Dalpiaz, A.; Biondi, C.; Lunghi, L. Estrogen Metabolites in the Release of Inflammatory Mediators from Human Amnion-Derived Cells. Life Sci. 2011, 88, 551–558. [Google Scholar] [CrossRef]
- Marinoni, E.; Di Iorio, R.; Scucchi, L.; Cosmi, E.V. Immunohistochemical Localization of Nitric Oxide Synthase in Human Fetal Membranes. Acta Obstet. Gynecol. Scand. 1997, 76, 725–727. [Google Scholar] [CrossRef]
- Vrachnis, N.; Karavolos, S.; Iliodromiti, Z.; Sifakis, S.; Siristatidis, C.; Mastorakos, G.; Creatsas, G. Review: Impact of Mediators Present in Amniotic Fluid on Preterm Labour—PubMed. In Vivo 2012, 26, 799–812. [Google Scholar]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, V.; Salvatori, I.; Costanzo, M.; Capozzi, A.; Caissutti, D.; Caterino, M.; Valle, C.; Ferri, A.; Sorice, M.; Ruoppolo, M.; et al. Overexpression of Neuroglobin Promotes Energy Metabolism and Autophagy Induction in Human Neuroblastoma SH-SY5Y Cells. Cells 2021, 10, 3394. [Google Scholar] [CrossRef] [PubMed]
- Balzano, F.; Campesi, I.; Cruciani, S.; Garroni, G.; Bellu, E.; Dei Giudici, S.; Angius, A.; Oggiano, A.; Rallo, V.; Capobianco, G.; et al. Epigenetics, Stem Cells, and Autophagy: Exploring a Path Involving MiRNA. Int. J. Mol. Sci. 2019, 20, 5091. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Hickey, R.W.; Bayir, H.; Watkins, S.C.; Tyurin, V.A.; Guo, F.; Kochanek, P.M.; Jenkins, L.W.; Ren, J.; Gibson, G.; et al. Starving Neurons Show Sex Difference in Autophagy. J. Biol. Chem. 2009, 284, 2383–2396. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Baker, P.N.; Symonds, E.M. Placental Apoptosis in Normal Human Pregnancy. Am. J. Obstet. Gynecol. 1997, 177, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Muralimanoharan, S.; Maloyan, A. Effect of Preeclampsia on Placental Function: Influence of Sexual Dimorphism, MicroRNA’s and Mitochondria. In Advances in Fetal and Neonatal Physiology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 814, pp. 133–146. [Google Scholar] [CrossRef]
- Cattaneo, M.G.; Banfi, C.; Brioschi, M.; Lattuada, D.; Vicentini, L.M. Sex-Dependent Differences in the Secretome of Human Endothelial Cells. Biol. Sex Differ. 2020, 12, 7. [Google Scholar] [CrossRef]
- Behnia, F.; Taylor, B.D.; Woodson, M.; Kacerovsky, M.; Hawkins, H.; Fortunato, S.J.; Saade, G.R.; Menon, R. Chorioamniotic Membrane Senescence: A Signal for Parturition? Am. J. Obstet. Gynecol. 2015, 213, 359.E1–359.E16. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espín, D.; Cañamero, M.; Maraver, A.; Gómez-López, G.; Contreras, J.; Murillo-Cuesta, S.; Rodríguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155, 1104. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence—Size Matters Not. EBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Liu, Y.; Robinson, W.P. Placental Telomere Length Decline with Gestational Age Differs by Sex and TERT, DNMT1, and DNMT3A DNA Methylation. Placenta 2016, 48, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Goumy, C.; Veronese, L.; Stamm, R.; Domas, Q.; Hadjab, K.; Gallot, D.; Laurichesse, H.; Delabaere, A.; Gouas, L.; Salaun, G.; et al. Reduced Telomere Length in Amniocytes: An Early Biomarker of Abnormal Fetal Development? Hum. Mol. Genet. 2022, 31, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Maiti, K.; Dedman, L.; Smith, R. Is There a Role for Placental Senescence in the Genesis of Obstetric Complications and Fetal Growth Restriction? Am. J. Obstet. Gynecol. 2018, 218, S762–S773. [Google Scholar] [CrossRef]
- Cho, C.K.J.; Shan, S.J.; Winsor, E.J.; Diamandis, E.P. Proteomics Analysis of Human Amniotic Fluid. Mol. Cell Proteom. 2007, 6, 1406–1415. [Google Scholar] [CrossRef]
- Kamath-Rayne, B.D.; Du, Y.; Hughes, M.; Wagner, E.A.; Muglia, L.J.; DeFranco, E.A.; Whitsett, J.A.; Salomonis, N.; Xu, Y. Systems Biology Evaluation of Cell-Free Amniotic Fluid Transcriptome of Term and Preterm Infants to Detect Fetal Maturity. BMC Med. Genom. 2015, 8, 67. [Google Scholar] [CrossRef]
- Ghi, T.; Sotiriadis, A.; Calda, P.; Da Silva Costa, F.; Raine-Fenning, N.; Alfirevic, Z.; McGillivray, G. ISUOG Practice Guidelines: Invasive Procedures for Prenatal Diagnosis. Ultrasound Obstet. Gynecol. 2016, 48, 256–268. [Google Scholar] [CrossRef]
- Comitato Nazionale per la Biosicurezza e le Biotecnologie. Della Presidenza del Consiglio dei Ministri. In Linee Guida per Test Genetici; Istituto Superiore di Sanita: Rome, Italy, 1998. [Google Scholar]
- Cambosu, F.; Capobianco, G.; Fogu, G.; Bandiera, P.; Pirino, A.; Moro, M.A.; Sanna, R.; Soro, G.; Dessole, M.; Montella, A. Partial Trisomy of the Long Arm of Chromosome 1: Prenatal Diagnosis, Clinical Evaluation and Cytogenetic Findings. Case Report and Review of the Literature. J. Obstet. Gynaecol. Res. 2013, 39, 592–597. [Google Scholar] [CrossRef]
- Campesi, I.; Occhioni, S.; Tonolo, G.; Cherchi, S.; Basili, S.; Carru, C.; Zinellu, A.; Franconi, F. Ageing/Menopausal Status in Healthy Women and Ageing in Healthy Men Differently Affect Cardiometabolic Parameters. Int. J. Med. Sci. 2016, 13, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and Nitrate Measurement by Griess Reagent in Human Plasma: Evaluation of Interferences and Standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Melo, M.; Fontana, A.O.; Viertl, D.; Allenbach, G.; Prior, J.O.; Rotman, S.; Feichtinger, R.G.; Mayr, J.A.; Costanzo, M.; Caterino, M.; et al. A Knock-in Rat Model Unravels Acute and Chronic Renal Toxicity in Glutaric Aciduria Type I. Mol. Genet. Metab. 2021, 134, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Giacco, A.; Paoli, G.D.; Senese, R.; Cioffi, F.; Silvestri, E.; Moreno, M.; Ruoppolo, M.; Caterino, M.; Costanzo, M.; Lombardi, A.; et al. The Saturation Degree of Fatty Acids and Their Derived Acylcarnitines Determines the Direct Effect of Metabolically Active Thyroid Hormones on Insulin Sensitivity in Skeletal Muscle Cells. FASEB J. 2019, 33, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.; Romero, R.; Rice, G.E.; Fitzgerald, W.; Pacora, P.; Gomez-Lopez, N.; Kavdia, M.; Tarca, A.L.; Margolis, L. Compartmentalized Profiling of Amniotic Fluid Cytokines in Women with Preterm Labor. PLoS ONE 2020, 15, e0227881. [Google Scholar] [CrossRef] [PubMed]
- Vesce, F.; Scapoli, C.; Giovannini, G.; Tralli, L.; Gotti, G.; Valerio, A.; Piffanelli, A. Cytokine Imbalance in Pregnancies with Fetal Chromosomal Abnormalities. Hum. Reprod. 2002, 17, 803–808. [Google Scholar] [CrossRef]
- Costanzo, M.; Caterino, M.; Sotgiu, G.; Ruoppolo, M.; Franconi, F.; Campesi, I. Sex Differences in the Human Metabolome. Biol. Sex. Differ. 2022, 13, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhang, L.; Zhu, H.; Chen, J.; Lin, Z.; Zhou, B.; Liu, S.; Wang, H.; Sun, H. Sex Differences in Protein Expression and Their Perturbations in Amniotic Fluid Cells of Down Syndrome Fetuses. ACS Omega 2022, 7, 35981–35992. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 Cytokines: Mechanisms and Therapeutic Strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Burns, C.; Hall, S.T.; Smith, R.; Blackwell, C. Cytokine Levels in Late Pregnancy: Are Female Infants Better Protected against Inflammation? Front. Immunol. 2015, 6, 318. [Google Scholar] [CrossRef]
- Kale, A.; Kale, E. Are Alanine, Cysteine, Glycine and Valine Amino Acids the Cause of Non-Immune Hydrops Fetalis? Clin. Exp. Obstet. Gynecol. 2012, 39, 341–342. [Google Scholar] [PubMed]
- Beikoghli Kalkhoran, S.; Kararigas, G. Oestrogenic Regulation of Mitochondrial Dynamics. Int. J. Mol. Sci. 2022, 23, 1118. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xiao, B.; Zhang, H.; Ye, J.; Qiu, W.; Zhu, H.; Wang, L.; Liang, L.; Zhan, X.; Ji, W.; et al. Biochemical and Genetic Approaches to the Prenatal Diagnosis of Propionic Acidemia in 78 Pregnancies. Orphanet J. Rare Dis. 2020, 15, 276. [Google Scholar] [CrossRef]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes Can Oxidize Medium- and Long-Chain Fatty Acids through a Pathway Involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Straface, E.; Vona, R.; Campesi, I.; Franconi, F. Mitochondria Can Orchestrate Sex Differences in Cell Fate of Vascular Smooth Muscle Cells from Rats. Biol. Sex. Differ. 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Bukovsky, A.; Cekanova, M.; Caudle, M.R.; Wimalasena, J.; Foster, J.S.; Henley, D.C.; Elder, R.F. Expression and Localization of Estrogen Receptor-Alpha Protein in Normal and Abnormal Term Placentae and Stimulation of Trophoblast Differentiation by Estradiol. Reprod. Biol. Endocrinol. 2003, 1, 13. [Google Scholar] [CrossRef]
- Felzen, V.; Hiebel, C.; Koziollek-Drechsler, I.; Reißig, S.; Wolfrum, U.; Kögel, D.; Brandts, C.; Behl, C.; Morawe, T. Estrogen Receptor α Regulates Non-Canonical Autophagy That Provides Stress Resistance to Neuroblastoma and Breast Cancer Cells and Involves BAG3 Function. Cell Death Dis. 2015, 6, e1812. [Google Scholar] [CrossRef]
- Liu, J. Studies of the Molecular Mechanisms in the Regulation of Telomerase Activity. FASEB J. 1999, 13, 2091–2104. [Google Scholar] [CrossRef]
- De Jesus, B.B.; Blasco, M.A. Assessing Cell and Organ Senescence Biomarkers. Circ. Res. 2012, 111, 97–109. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The Essence of Senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef]
- Prašnikar, E.; Borišek, J.; Perdih, A. Senescent Cells as Promising Targets to Tackle Age-Related Diseases. Ageing Res. Rev. 2021, 66, 101251. [Google Scholar] [CrossRef] [PubMed]
- Zapalska-Sozoniuk, M.; Chrobak, L.; Kowalczyk, K.; Kankofer, M. Is It Useful to Use Several “Omics” for Obtaining Valuable Results? Mol. Biol. Rep. 2019, 46, 3597–3606. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, F.; Yi, M.; Wang, Y.; Stephens, R.; Sonntag, K.C. Evidence for Gender-Specific Transcriptional Profiles of Nigral Dopamine Neurons in Parkinson Disease. PLoS ONE 2010, 5, e8856. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing Caspase-3 and Caspase-9 Substrates in and out of Apoptosis with Deep Substrate Profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef]
- An, H.K.; Chung, K.M.; Park, H.; Hong, J.; Gim, J.E.; Choi, H.; Lee, Y.W.; Choi, J.; Mun, J.Y.; Yu, S.W. CASP9 (Caspase 9) Is Essential for Autophagosome Maturation through Regulation of Mitochondrial Homeostasis. Autophagy 2020, 16, 1598–1617. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; de la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Srivastava, M.; Ahlawat, N.; Srivastava, A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. J. Obstet. Gynecol. India 2018, 68, 15–19. [Google Scholar] [CrossRef]
- Jou, H.J.; Lo, P.H.; Ling, P.Y. Recent Advances of Microfluidic Platform for Cell Based Non-Invasive Prenatal Diagnosis. Int. J. Mol. Sci. 2023, 24, 991. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.C.; Lv, G.J.; Liu, J.H.; Sun, C.; Mu, K. Amniotic Fluid Karyotype Analysis and Prenatal Diagnosis Strategy of 3117 Pregnant Women with Amniocentesis Indication. J. Comp. Eff. Res. 2023, 12, e220168. [Google Scholar] [CrossRef]
- Shi, C.; Li, S.; Gao, Y.; Deng, Z.; Hao, H.; Xiao, X. Prenatal Diagnosis of Two Common Inborn Errors of Metabolism by Genetic and Mass Spectrometric Analysis of Amniotic Fluid. Front. Pediatr. 2022, 10, 824399. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campesi, I.; Capobianco, G.; Cano, A.; Lodde, V.; Cruciani, S.; Maioli, M.; Sotgiu, G.; Idda, M.L.; Puci, M.V.; Ruoppolo, M.; et al. Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health. Biomedicines 2023, 11, 2830. https://doi.org/10.3390/biomedicines11102830
Campesi I, Capobianco G, Cano A, Lodde V, Cruciani S, Maioli M, Sotgiu G, Idda ML, Puci MV, Ruoppolo M, et al. Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health. Biomedicines. 2023; 11(10):2830. https://doi.org/10.3390/biomedicines11102830
Chicago/Turabian StyleCampesi, Ilaria, Giampiero Capobianco, Antonella Cano, Valeria Lodde, Sara Cruciani, Margherita Maioli, Giovanni Sotgiu, Maria Laura Idda, Mariangela Valentina Puci, Margherita Ruoppolo, and et al. 2023. "Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health" Biomedicines 11, no. 10: 2830. https://doi.org/10.3390/biomedicines11102830
APA StyleCampesi, I., Capobianco, G., Cano, A., Lodde, V., Cruciani, S., Maioli, M., Sotgiu, G., Idda, M. L., Puci, M. V., Ruoppolo, M., Costanzo, M., Caterino, M., Cambosu, F., Montella, A., & Franconi, F. (2023). Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health. Biomedicines, 11(10), 2830. https://doi.org/10.3390/biomedicines11102830














