Role of Akt/Protein Kinase B in Cancer Metastasis
Abstract
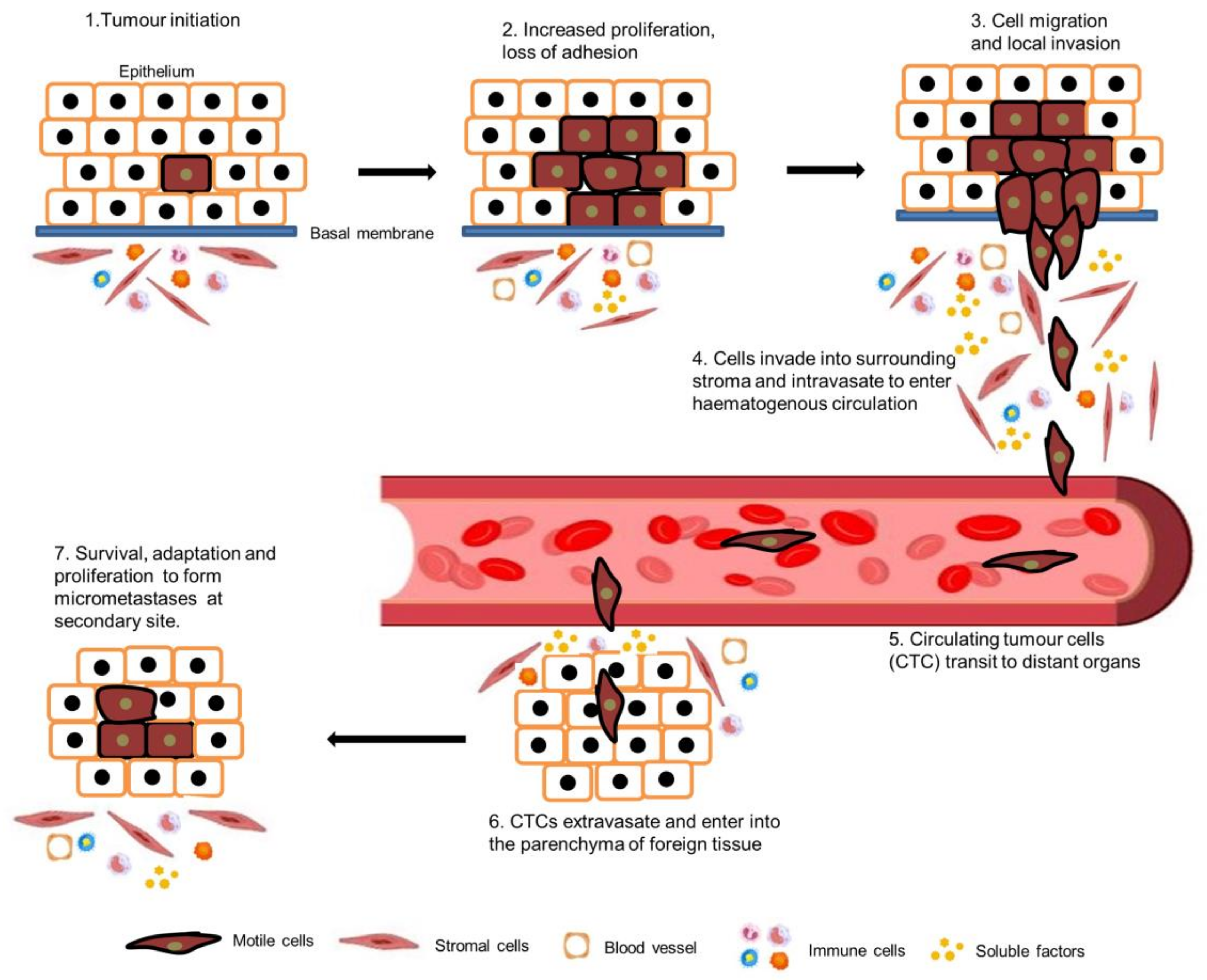
1. Introduction
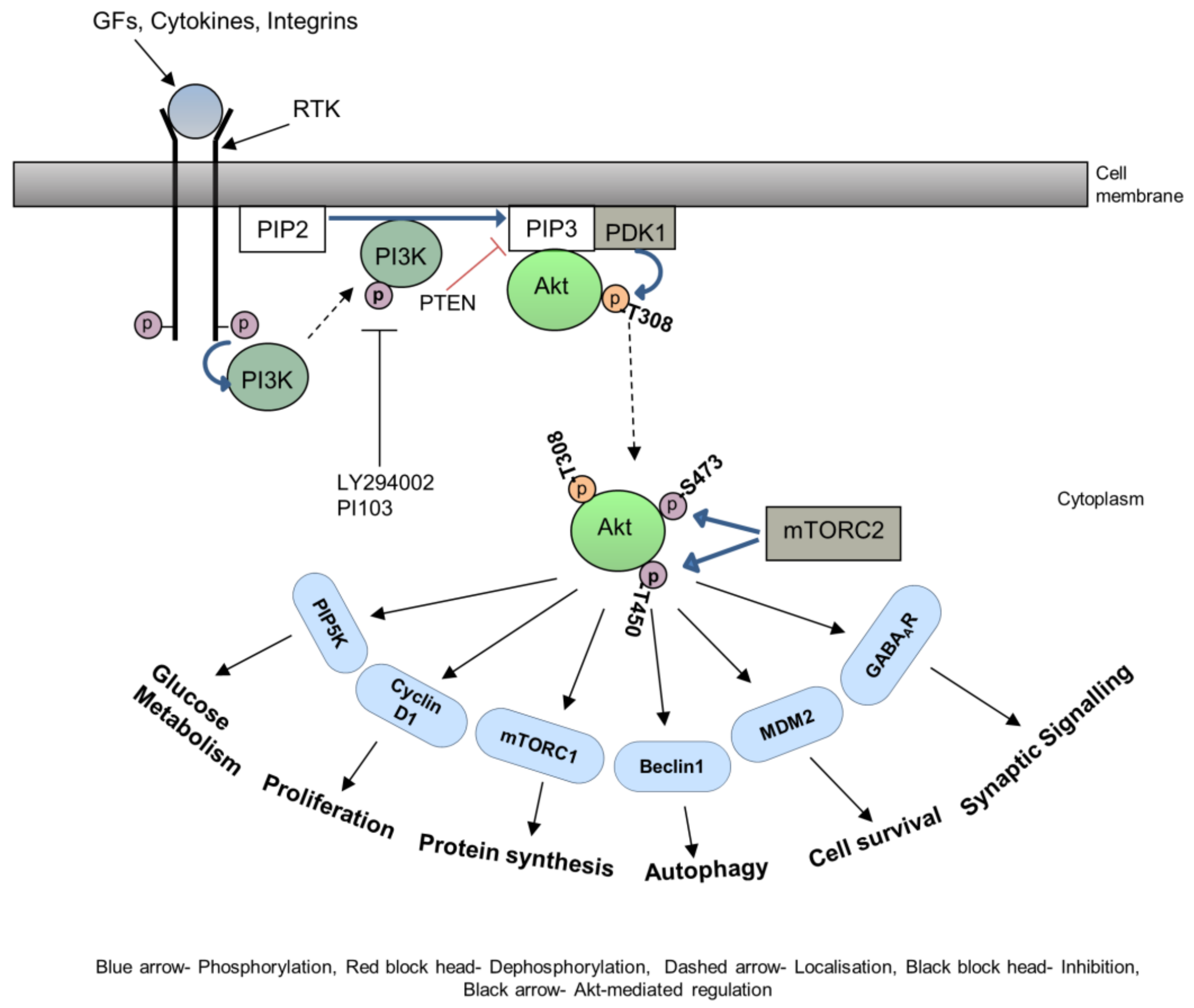
2. Akt in Cytoskeletal Rearrangements
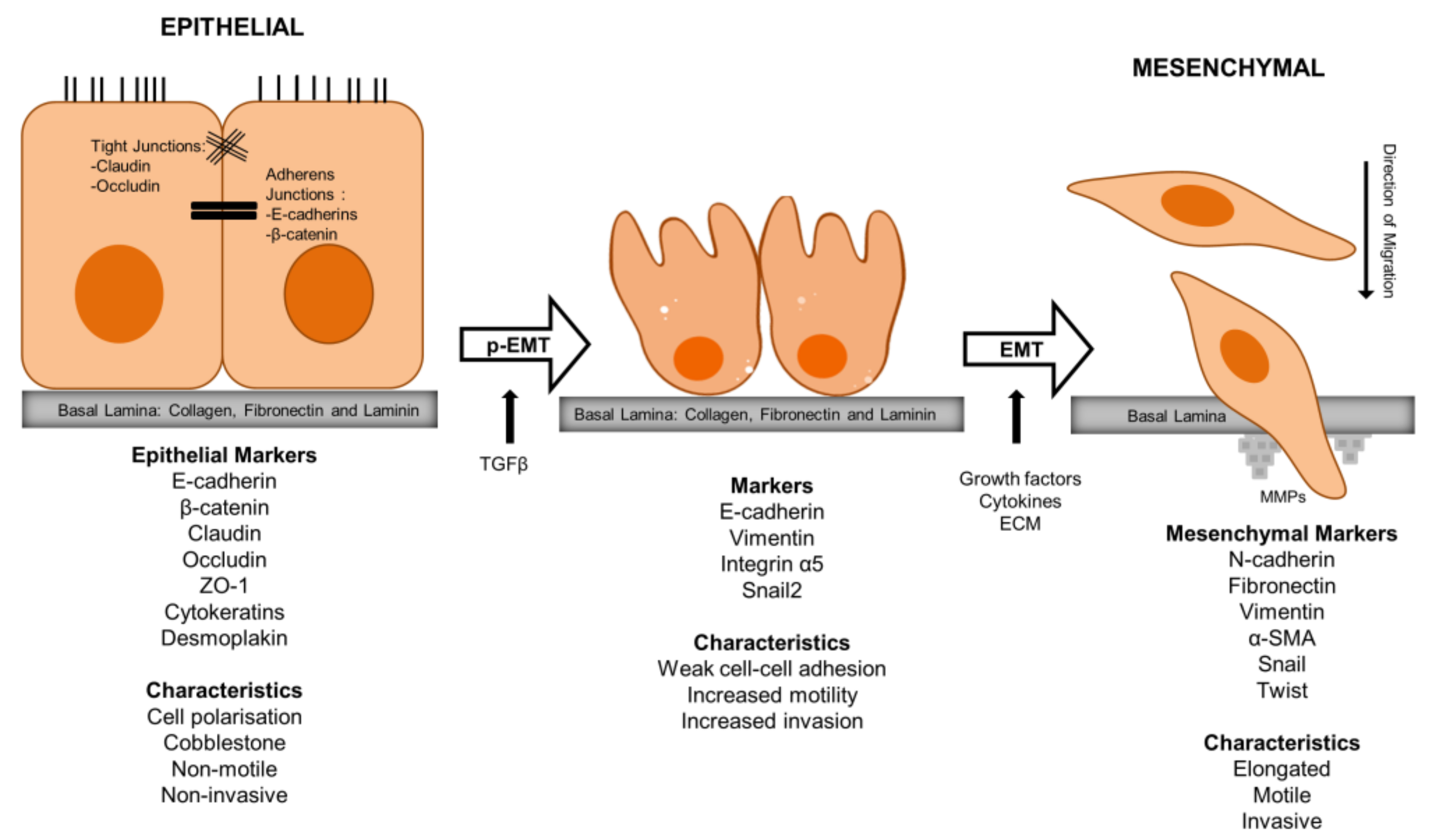
3. Akt in EMT
4. Akt in HNSCC Metastasis
5. PI3K/Akt Inhibitors in Clinical Trials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Palmer, T.D.; Ashby, W.J.; Lewis, J.D.; Zijlstra, A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011, 63, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 2009, 34, 881–895. [Google Scholar] [PubMed]
- Robert, J. Biology of cancer metastasis. Bull. Cancer 2013, 100, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, E.C.; Chuaqui, R.F.; Liotta, L.A. General mechanisms of metastasis. Cancer 1997, 80, 1529–1537. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as multidimensional spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Friedl, P.; Brocker, E.B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell. Mol. Life Sci. 2000, 57, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, S. Role of mTOR Signaling in Tumor Cell Motility, Invasion and Metastasis. Curr. Protein Pept. Sci. 2011, 12, 30–42. [Google Scholar] [PubMed]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Mitchison, T.J.; Cramer, L.P. Actin-based cell motility and cell locomotion. Cell 1996, 84, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, M.N. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998, 14, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Ghoshal, A.; Jones, S.; Ellis, I.; Islam, M. Head and Neck Cancer Metastasis and the Effect of the Local Soluble Factors, from the Microenvironment, on Signalling Pathways: Is It All about the Akt? Cancers 2020, 12, 2093. [Google Scholar] [CrossRef] [PubMed]
- Ellis, I.R.; Islam, M.R.; Aljorani, L.; Jones, S.J. Fibronectin: The N-terminal region and its role in cell migration- implications for disease and healing. In Fibronectin: Current Concepts in Structure, Function and Pathology; Beattie, J., Ed.; Protein Biochemistry, Synthesis, Structure and Cellular Functions; Nova Science Publishers: New York, NY, USA, 2012; pp. 35–69. [Google Scholar]
- Friedl, P. Prespecification and plasticity: Shifting mechanisms of cell migration. Curr. Opin. Cell Biol. 2004, 16, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Inaki, M.; Vishnu, S.; Cliffe, A.; Rorth, P. Effective guidance of collective migration based on differences in cell states. Proc. Natl. Acad. Sci. USA 2012, 109, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Rorth, P. Whence directionality: Guidance mechanisms in solitary and collective cell migration. Dev. Cell 2011, 20, 9–18. [Google Scholar] [CrossRef]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Cohen, P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998, 8, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bozulic, L.; Hemmings, B.A. PIKKing on PKB: Regulation of PKB activity by phosphorylation. Curr. Opin. Cell Biol. 2009, 21, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Park, J.; Cron, P.; Hess, D.; Hemmings, B.A. Identification of a PKB/Akt Hydrophobic Motif Ser-473 Kinase as DNA-dependent Protein Kinase. J. Biol. Chem. 2004, 279, 41189–41196. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Chan, T.O.; Ahmed, N.N.; Datta, K.; Malstrom, S.; Stokoe, D.; McCormick, F.; Feng, J.; Tsichlis, P. Akt activation by growth factors is a multiple-step process: The role of the PH domain. Oncogene 1998, 17, 313–325. [Google Scholar] [CrossRef]
- Hart, J.R.; Vogt, P.K. Phosphorylation of AKT: A mutational analysis. Oncotarget 2011, 2, 467–476. [Google Scholar] [CrossRef]
- Ikenoue, T.; Inoki, K.; Yang, Q.; Zhou, X.; Guan, K.-L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008, 27, 1919–1931. Available online: http://www.nature.com/emboj/journal/v27/n14/suppinfo/emboj2008119a_S1.html (accessed on 5 September 2023). [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef]
- De Marco, C.; Rinaldo, N.; Bruni, P.; Malzoni, C.; Zullo, F.; Fabiani, F.; Losito, S.; Scrima, M.; Marino, F.Z.; Franco, R.; et al. Multiple genetic alterations within the PI3K pathway are responsible for AKT activation in patients with ovarian carcinoma. PLoS ONE 2013, 8, e55362. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Ferrer-Lozano, J.; Stemke-Hale, K.; Sahin, A.; Liu, S.; Barrera, J.A.; Burgues, O.; Lluch, A.M.; Chen, H.; Hortobagyi, G.N. PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol. Cancer Ther. 2011, 10, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Baker, S.J.; Hu, T.C.; Norman, K.M.; Fearon, E.R.; Cho, K.R. Type I to Type II Ovarian Carcinoma Progression: Mutant Trp53 or Pik3ca Confers a More Aggressive Tumor Phenotype in a Mouse Model of Ovarian Cancer. Am. J. Pathol. 2013, 182, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hemmings, B.A. PKB/Akt-Dependent Regulation of Cell Motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Yoeli-Lerner, M.; Toker, A. Akt/PKB Signaling in Cancer: A Function in Cell Motility and Invasion. Cell Cycle 2006, 5, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Frixione, E. Recurring views on the structure and function of the cytoskeleton: A 300-year epic. Cell Motil. Cytoskelet. 2000, 46, 73–94. [Google Scholar] [CrossRef]
- Bonello, T.; Coombes, J.; Schevzov, G.; Gunning, P.; Stehn, J. Therapeutic Targeting of the Actin Cytoskeleton in Cancer. In Cytoskeleton and Human Disease; Kavallaris, M., Ed.; Humana Press: New York, NY, USA, 2012; pp. 181–200. [Google Scholar]
- Bugyi, B.; Carlier, M.F. Control of actin filament treadmilling in cell motility. Annu. Rev. Biophys. 2010, 39, 449–470. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef]
- Morales-Ruiz, M.; Fulton, D.; Sowa, G.; Languino, L.R.; Fujio, Y.; Walsh, K.; Sessa, W.C. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ. Res. 2000, 86, 892–896. [Google Scholar] [CrossRef]
- Dimmeler, S.; Dernbach, E.; Zeiher, A.M. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000, 477, 258–262. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Corum, L.; Meng, Q.; Blenis, J.; Zheng, J.Z.; Shi, X.; Flynn, D.C.; Jiang, B.H. PI3K induced actin filament remodeling through Akt and p70S6K1: Implication of essential role in cell migration. Am. J. Physiol. Cell Physiol. 2004, 286, C153–C163. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhong, X.; Flynn, D.C.; Zheng, J.Z.; Qiao, M.; Wu, C.; Dedhar, S.; Shi, X.; Jiang, B.H. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene 2005, 24, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.K.M.; Cheung, A.N.Y.; Ngan, H.Y.S.; Wong, A.S.T. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 2011, 30, 2420–2432. [Google Scholar] [CrossRef]
- Chodniewicz, D.; Zhelev, D.V. Chemoattractant receptor-stimulated F-actin polymerization in the human neutrophil is signaled by 2 distinct pathways. Blood 2003, 101, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dan, H.C.; Sun, M.; Liu, Q.; Sun, X.M.; Feldman, R.I.; Hamilton, A.D.; Polokoff, M.; Nicosia, S.V.; Herlyn, M.; et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004, 64, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Noei, F.; Jeganathan, S.; Kulkarni, G.; Pinke, D.E.; Lee, J.M. eEF1A2 activates Akt and stimulates Akt-dependent actin remodeling, invasion and migration. Oncogene 2007, 26, 3027–3040. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Sirri, A.; Riccio, M.; Lattanzi, G.; Santi, S.; de Pol, A.; Maraldi, N.M.; Marmiroli, S. Targeting of the Akt/PKB kinase to the actin skeleton. Cell Mol. Life Sci. 2003, 60, 2710–2720. [Google Scholar] [CrossRef]
- Ho, Y.P.; Kuo, C.W.; Hsu, Y.T.; Huang, Y.S.; Yew, L.P.; Huang, W.F.; Lin, K.C.; Hsu, J.H. beta-Actin is a downstream effector of the PI3K/AKT signaling pathway in myeloma cells. Mol. Cell Biochem. 2011, 348, 129–139. [Google Scholar] [CrossRef]
- Zhou, G.L.; Zhuo, Y.; King, C.C.; Fryer, B.H.; Bokoch, G.M.; Field, J. Akt phosphorylation of serine 21 on Pak1 modulates Nck binding and cell migration. Mol. Cell Biol. 2003, 23, 8058–8069. [Google Scholar] [CrossRef]
- Chung, C.Y.; Potikyan, G.; Firtel, R.A. Control of cell polarity and chemotaxis by Akt/PKB and PI3 kinase through the regulation of PAKa. Mol. Cell 2001, 7, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, E.S.; Higgs, H.N. The many faces of actin: Matching assembly factors with cellular structures. Nat. Cell Biol. 2007, 9, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Murakami, H.; Asai, N.; Morone, N.; Watanabe, T.; Kawai, K.; Murakumo, Y.; Usukura, J.; Kaibuchi, K.; Takahashi, M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell 2005, 9, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Enomoto, A.; Jijiwa, M.; Kato, T.; Hasegawa, T.; Ishida, M.; Sato, T.; Asai, N.; Murakumo, Y.; Takahashi, M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008, 68, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Kato, T.; Kinjo, S.; Enomoto, A.; Toda, H.; Shimato, S.; Ohka, F.; Motomura, K.; Kondo, Y.; Miyata, T.; et al. Girdin maintains the stemness of glioblastoma stem cells. Oncogene 2012, 31, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Enomoto, A.; Ishida-Takagishi, M.; Asai, N.; Takahashi, M. Girding for migratory cues: Roles of the Akt substrate Girdin in cancer progression and angiogenesis. Cancer Sci. 2010, 101, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Matsuo, Y.; Shamoto, T.; Hirokawa, T.; Tsuboi, K.; Takahashi, H.; Ishiguro, H.; Kimura, M.; Takeyama, H.; Inagaki, H. Girdin, a regulator of cell motility, is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol. Rep. 2013, 29, 2127–2132. [Google Scholar] [CrossRef][Green Version]
- Yamamura, Y.; Asai, N.; Enomoto, A.; Kato, T.; Mii, S.; Kondo, Y.; Ushida, K.; Niimi, K.; Tsunoda, N.; Nagino, M.; et al. Akt–Girdin Signaling in Cancer-Associated Fibroblasts Contributes to Tumor Progression. Cancer Res. 2015, 75, 813–823. [Google Scholar] [CrossRef]
- Cenni, V.; Bavelloni, A.; Beretti, F.; Tagliavini, F.; Manzoli, L.; Lattanzi, G.; Maraldi, N.M.; Cocco, L.; Marmiroli, S. Ankrd2/ARPP is a novel Akt2 specific substrate and regulates myogenic differentiation upon cellular exposure to H(2)O(2). Mol. Biol. Cell 2011, 22, 2946–2956. [Google Scholar] [CrossRef]
- Feng, Y.; Walsh, C.A. The many faces of filamin: A versatile molecular scaffold for cell motility and signalling. Nat. Cell Biol. 2004, 6, 1034–1038. [Google Scholar] [CrossRef]
- Ravid, D.; Chuderland, D.; Landsman, L.; Lavie, Y.; Reich, R.; Liscovitch, M. Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Exp. Cell Res. 2008, 314, 2762–2773. [Google Scholar] [CrossRef]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ravid, D.; Maor, S.; Werner, H.; Liscovitch, M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene 2005, 24, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Nallapalli, R.K.; Ibrahim, M.X.; Zhou, A.X.; Bandaru, S.; Sunkara, S.N.; Redfors, B.; Pazooki, D.; Zhang, Y.; Boren, J.; Cao, Y.; et al. Targeting filamin A reduces K-RAS-induced lung adenocarcinomas and endothelial response to tumor growth in mice. Mol. Cancer 2012, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Meima, M.E.; Webb, B.A.; Witkowska, H.E.; Barber, D.L. The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J. Biol. Chem. 2009, 284, 26666–26675. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Barber, D.L. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 2002, 159, 1087–1096. [Google Scholar] [CrossRef]
- Martin, C.; Pedersen, S.F.; Schwab, A.; Stock, C. Intracellular pH gradients in migrating cells. Am. J. Physiol. Cell Physiol. 2011, 300, C490–C495. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Schwab, A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol. 2006, 187, 149–157. [Google Scholar] [CrossRef]
- Stuwe, L.; Muller, M.; Fabian, A.; Waning, J.; Mally, S.; Noel, J.; Schwab, A.; Stock, C. pH dependence of melanoma cell migration: Protons extruded by NHE1 dominate protons of the bulk solution. J. Physiol. 2007, 585, 351–360. [Google Scholar] [CrossRef]
- Clement, D.L.; Mally, S.; Stock, C.; Lethan, M.; Satir, P.; Schwab, A.; Pedersen, S.F.; Christensen, S.T. PDGFRalpha signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J. Cell Sci. 2013, 126, 953–965. [Google Scholar] [CrossRef]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Helfand, B.T.; Chang, L.; Goldman, R.D. Intermediate filaments are dynamic and motile elements of cellular architecture. J. Cell Sci. 2004, 117, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.S.; Rosenblatt, K.; Huang, K.L.; Lahat, G.; Brobey, R.; Bolshakov, S.; Nguyen, T.; Ding, Z.; Belousov, R.; Bill, K.; et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene 2011, 30, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Zhu, Q.S.; Huang, K.L.; Wang, S.; Bolshakov, S.; Liu, J.; Torres, K.; Langley, R.R.; Lazar, A.J.; Hung, M.C.; et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS ONE 2010, 5, e10105. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Inuzuka, H.; Tseng, A.; Chin, R.Y.; Toker, A.; Wei, W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat. Cell Biol. 2009, 11, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Wang, G.; Chen, Z.; Teruya-Feldstein, J.; Liu, Y.; Chan, C.H.; Yang, W.L.; Erdjument-Bromage, H.; Nakayama, K.I.; Nimer, S.; et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat. Cell Biol. 2009, 11, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.O.; Kim, J.H.; Hong, J.S.; Yoon, H.J.; Lee, J.I.; Hong, S.P.; Hong, S.D. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J. Exp. Clin. Cancer Res. CR 2009, 28, 28. [Google Scholar] [CrossRef]
- McPhee, T.R.; McDonald, P.C.; Oloumi, A.; Dedhar, S. Integrin-linked kinase regulates E-cadherin expression through PARP-1. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 2737–2747. [Google Scholar] [CrossRef]
- Onishi, K.; Higuchi, M.; Asakura, T.; Masuyama, N.; Gotoh, Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells Devoted Mol. Cell. Mech. 2007, 12, 535–546. [Google Scholar] [CrossRef]
- Ridnour, L.A.; Barasch, K.M.; Windhausen, A.N.; Dorsey, T.H.; Lizardo, M.M.; Yfantis, H.G.; Lee, D.H.; Switzer, C.H.; Cheng, R.Y.; Heinecke, J.L.; et al. Nitric oxide synthase and breast cancer: Role of TIMP-1 in NO-mediated Akt activation. PLoS ONE 2012, 7, e44081. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Thangada, S.; Paik, J.H.; Sapkota, G.P.; Ancellin, N.; Chae, S.S.; Wu, M.; Morales-Ruiz, M.; Sessa, W.C.; Alessi, D.R.; et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol. Cell 2001, 8, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Hla, T.; Lee, M.J. Sphingosine-1-phosphate signaling in endothelial activation. J. Atheroscler. Thromb. 2003, 10, 125–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miao, H.; Li, D.Q.; Mukherjee, A.; Guo, H.; Petty, A.; Cutter, J.; Basilion, J.P.; Sedor, J.; Wu, J.; Danielpour, D.; et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 2009, 16, 9–20. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Kobayashi, M.; Hiramoto-Yamaki, N.; Harada, K.; Negishi, M.; Katoh, H. Ephexin4-mediated promotion of cell migration and anoikis resistance is regulated by serine 897 phosphorylation of EphA2. FEBS Open Bio 2013, 3, 78–82. [Google Scholar] [CrossRef]
- Li, J.; Ballif, B.A.; Powelka, A.M.; Dai, J.; Gygi, S.P.; Hsu, V.W. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev. Cell 2005, 9, 663–673. [Google Scholar] [CrossRef]
- Rowland, A.F.; Larance, M.; Hughes, W.E.; James, D.E. Identification of RhoGAP22 as an Akt-dependent regulator of cell motility in response to insulin. Mol. Cell Biol. 2011, 31, 4789–4800. [Google Scholar] [CrossRef]
- Berven, L.A.; Willard, F.S.; Crouch, M.F. Role of the p70(S6K) pathway in regulating the actin cytoskeleton and cell migration. Exp. Cell Res. 2004, 296, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, K.; Liu, B.; Hollenbeck, S.; Kent, K.C. Rapamycin inhibits fibronectin-induced migration of the human arterial smooth muscle line (E47) through the mammalian target of rapamycin. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2861–H2868. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, F.; Cardelli, J.A.; Martin, K.A.; Blenis, J.; Huang, S. Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene 2006, 25, 7029–7040. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, L.; Chung, J.; Huang, S. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene 2008, 27, 4998–5010. [Google Scholar] [CrossRef] [PubMed]
- Benefield, J.; Meisinger, J.; Petruzzelli, G.J.; Young, M.R. Endothelial cell response to human head and neck squamous cell carcinomas involves downregulation of protein phosphatases-1/2A, cytoskeletal depolymerization and increased motility. Invasion Metastasis 1997, 17, 210–220. [Google Scholar] [PubMed]
- Jackson, J.L.; Young, M.R. Protein phosphatase-2A regulates protein tyrosine phosphatase activity in Lewis lung carcinoma tumor variants. Clin. Exp. Metastasis 2003, 20, 357–364. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yue, P.; Tao, H.; Ramalingam, S.S.; Owonikoko, T.K.; Deng, X.; Wang, Y.; Fu, H.; Khuri, F.R.; et al. Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced Akt phosphorylation. J. Biol. Chem. 2013, 288, 13215–13224. [Google Scholar] [CrossRef]
- Liu, L.; Chen, L.; Luo, Y.; Chen, W.; Zhou, H.; Xu, B.; Han, X.; Shen, T.; Huang, S. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS ONE 2010, 5, e10578. [Google Scholar] [CrossRef]
- Wlodarski, P.; Grajkowska, W.; Lojek, M.; Rainko, K.; Jozwiak, J. Activation of Akt and Erk pathways in medulloblastoma. Folia Neuropathol. 2006, 44, 214–220. [Google Scholar]
- Fong, Y.-C.; Hsu, S.-F.; Wu, C.-L.; Li, T.-M.; Kao, S.-T.; Tsai, F.-J.; Chen, W.-C.; Liu, S.-C.; Wu, C.-M.; Tang, C.-H. Transforming growth factor-β1 increases cell migration and β1 integrin up-regulation in human lung cancer cells. Lung Cancer 2009, 64, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-Y.; Chiao, C.-C.; Kuo, W.-Y.; Hsiao, Y.-C.; Chen, Y.-J.; Wei, Y.-Y.; Lai, T.-H.; Fong, Y.-C.; Tang, C.-H. TGF-β1 increases motility and αvβ3 integrin up-regulation via PI3K, Akt and NF-κB-dependent pathway in human chondrosarcoma cells. Biochem. Pharmacol. 2008, 75, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Renaud, S.J.; Schleussner, E.; Graham, C.H.; Markert, U.R. mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3. Exp. Cell Res. 2009, 315, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, N.; Roy, B.C.; Zhu, Y.; Wang, Y.; Kiyama, R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J. Cell Biol. 2008, 181, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.R.; Toker, A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Mol. Cell 2010, 38, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.R.; Toker, A. Akt2 regulates expression of the actin-bundling protein palladin. FEBS Lett. 2010, 584, 4769–4774. [Google Scholar] [CrossRef]
- Liu, H.; Radisky, D.C.; Nelson, C.M.; Zhang, H.; Fata, J.E.; Roth, R.A.; Bissell, M.J. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc. Natl. Acad. Sci. USA 2006, 103, 4134–4139. [Google Scholar] [CrossRef]
- Nieto, M.A. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Larue, L.; Bellacosa, A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 2005, 24, 7443–7454. [Google Scholar] [CrossRef]
- Zheng, H.; Kang, Y. Multilayer control of the EMT master regulators. Oncogene 2013, 33, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Mani, S.A.; Levine, H. Hybrid epithelial/mesenchymal phenotype(s): The ‘fittest’ for metastasis? Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624. [Google Scholar] [CrossRef]
- Thompson, E.W.; Williams, E.D. EMT and MET in carcinoma--clinical observations, regulatory pathways and new models. Clin. Exp. Metastasis 2008, 25, 591–592. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; Gonzalez-Baron, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Ringel, M.D.; Hayre, N.; Saito, J.; Saunier, B.; Schuppert, F.; Burch, H.; Bernet, V.; Burman, K.D.; Kohn, L.D.; Saji, M. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001, 61, 6105–6111. [Google Scholar] [PubMed]
- Testa, J.R.; Bellacosa, A. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 10983–10985. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Ko, S.Y.; Fong, J.H.; Chang, K.W.; Liu, T.Y.; Kao, S.Y. Expression of phosphorylated Akt in oral carcinogenesis and its induction by nicotine and alkaline stimulation. J. Oral Pathol. Med. 2009, 38, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; de Feo, D.; Godwin, A.K.; Bell, D.W.; Cheng, J.Q.; Altomare, D.A.; Wan, M.; Dubeau, L.; Scambia, G.; Masciullo, V.; et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 1995, 64, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; Van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Larue, L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Larue, L. PI3K/AKT Pathway and the Epithelial–Mesenchymal Transition. In Cancer Genome and Tumor Microenvironment; Thomas-Tikhonenko, A., Ed.; Springer Science+Business Media: New York, NY, USA, 2010; pp. 11–31. [Google Scholar]
- Katoh, M.; Katoh, M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 2006, 5, 1059–1064. [Google Scholar] [CrossRef]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef]
- Ha, G.-H.; Park, J.-S.; Breuer, E.-K.Y. TACC3 promotes epithelial–mesenchymal transition (EMT) through the activation of PI3K/Akt and ERK signaling pathways. Cancer Lett. 2013, 332, 63–73. [Google Scholar] [CrossRef]
- Smith, A.; Teknos, T.N.; Pan, Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.-S.; Zhou, B.-H.; Li, C.-L.; Zhang, F.; Wang, X.-F.; Zhang, G.; Bu, X.-Z.; Cai, S.-H.; Du, J. Epithelial–Mesenchymal Transition (EMT) Induced by TNF-α Requires AKT/GSK-3β-Mediated Stabilization of Snail in Colorectal Cancer. PLoS ONE 2013, 8, e56664. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Zhang, L.; Ning, Z.; Zeng, J.; Zhou, J.; Li, L.; Chen, Y.; Zhang, T.; Wang, X.; et al. PI3K/Akt to GSK3β/β-catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell. Signal. 2012, 24, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Ni, H.; Shen, X. COL11A1-Driven Epithelial–Mesenchymal Transition and Stemness of Pancreatic Cancer Cells Induce Cell Migration and Invasion by Modulating the AKT/GSK-3β/Snail Pathway. Biomolecules 2022, 12, 391. [Google Scholar]
- Evdokimova, V.; Tognon, C.; Ng, T.; Ruzanov, P.; Melnyk, N.; Fink, D.; Sorokin, A.; Ovchinnikov, L.P.; Davicioni, E.; Triche, T.J.; et al. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell 2009, 15, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Villagrasa, P.; Diaz, V.M.; Vinas-Castells, R.; Peiro, S.; Del Valle-Perez, B.; Dave, N.; Rodriguez-Asiain, A.; Casal, J.I.; Lizcano, J.M.; Dunach, M.; et al. Akt2 interacts with Snail1 in the E-cadherin promoter. Oncogene 2012, 31, 4022–4033. [Google Scholar] [CrossRef]
- Cheng, G.Z.; Chan, J.; Wang, Q.; Zhang, W.; Sun, C.D.; Wang, L.H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007, 67, 1979–1987. [Google Scholar] [CrossRef]
- Xue, G.; Restuccia, D.F.; Lan, Q.; Hynx, D.; Dirnhofer, S.; Hess, D.; Ruegg, C.; Hemmings, B.A. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2012, 2, 248–259. [Google Scholar] [CrossRef]
- Yao, K.; Ye, P.P.; Tan, J.; Tang, X.J.; Shen Tu, X.C. Involvement of PI3K/Akt pathway in TGF-beta2-mediated epithelial mesenchymal transition in human lens epithelial cells. Ophthalmic Res. 2008, 40, 69–76. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kimoto, K.; Itoh, Y.; Nakatsuka, K.; Matsuo, N.; Yoshioka, H.; Kubota, T. The PI3K/Akt pathway mediates the expression of type I collagen induced by TGF-beta2 in human retinal pigment epithelial cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 15–23. [Google Scholar] [CrossRef]
- Yang, M.H.; Hsu, D.S.; Wang, H.W.; Wang, H.J.; Lan, H.Y.; Yang, W.H.; Huang, C.H.; Kao, S.Y.; Tzeng, C.H.; Tai, S.K.; et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat. Cell Biol. 2010, 12, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Nacerddine, K.; Beaudry, J.B.; Ginjala, V.; Westerman, B.; Mattiroli, F.; Song, J.Y.; van der Poel, H.; Ponz, O.B.; Pritchard, C.; Cornelissen-Steijger, P.; et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J. Clin. Investig. 2012, 122, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.H.; Feng, Y.; Zhang, R.; Xu, L.H.; Li, M.Z.; Kung, H.F.; Song, L.B.; Zeng, M.S. Bmi-1 promotes invasion and metastasis, and its elevated expression is correlated with an advanced stage of breast cancer. Mol. Cancer 2011, 10, 10. [Google Scholar] [CrossRef]
- Song, L.B.; Li, J.; Liao, W.T.; Feng, Y.; Yu, C.P.; Hu, L.J.; Kong, Q.L.; Xu, L.H.; Zhang, X.; Liu, W.L.; et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J. Clin. Investig. 2009, 119, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sanz-Moreno, V.; Marshall, C.J. The metastasis gene NEDD9 product acts through integrin beta3 and Src to promote mesenchymal motility and inhibit amoeboid motility. J. Cell Sci. 2012, 125, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moreno, V. Tumour invasion: A new twist on Rac-driven mesenchymal migration. Curr. Biol. 2012, 22, R449–R451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, W.-H.; Lan, H.-Y.; Huang, C.-H.; Tai, S.-K.; Tzeng, C.-H.; Kao, S.-Y.; Wu, K.-J.; Hung, M.-C.; Yang, M.-H. RAC1 activation mediates Twist1-induced cancer cell migration. Nat. Cell Biol. 2012, 14, 366–374. Available online: http://www.nature.com/ncb/journal/v14/n4/abs/ncb2455.html#supplementary-information (accessed on 7 September 2023). [CrossRef]
- Hill, L.; Browne, G.; Tulchinsky, E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int. J. Cancer 2013, 132, 745–754. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Polytarchou, C.; Hatziapostolou, M.; Kottakis, F.; Maroulakou, I.G.; Struhl, K.; Tsichlis, P.N. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci. Signal. 2009, 2, ra62. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Ellis, I.R.; Macluskey, M.; Cochrane, L.; Jones, S.J. Activation of Akt at T308 and S473 in alcohol, tobacco and HPV-induced HNSCC: Is there evidence to support a prognostic or diagnostic role? Exp. Hematol. Oncol. 2014, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Jones, S.J.; Macluskey, M.; Ellis, I.R. Is there a pAkt between VEGF and oral cancer cell migration? Cell. Signal. 2014, 26, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Amornphimoltham, P.; Sriuranpong, V.; Patel, V.; Benavides, F.; Conti, C.J.; Sauk, J.; Sausville, E.A.; Molinolo, A.A.; Gutkind, J.S. Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: A potential target for UCN-01. Clin. Cancer Res. 2004, 10, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Amornphimoltham, P.; Patel, V.; Molinolo, A.; Gutkind, J.S. Head and Neck Cancer and PI3K/Akt/mTOR Signaling Network: Novel Molecular Targeted Therapy. In Signaling Pathways in Squamous Cancer; Glick, A.B., Van Waes, C., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2011; pp. 407–430. [Google Scholar]
- Marquard, F.E.; Jücker, M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Liu, D.D.; Lee, J.J.; El-Naggar, A.K.; Lo Muzio, L.; Staibano, S.; De Placido, S.; Myers, J.N.; Papadimitrakopoulou, V.A. Akt activation correlates with adverse outcome in tongue cancer. Cancer 2005, 104, 2430–2436. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Weinberger, P.M.; Sasaki, C.; Egleston, B.L.; Speier, W.F.t.; Haffty, B.; Kowalski, D.; Camp, R.; Rimm, D.; Vairaktaris, E.; et al. Phosphorylation of Akt (Ser473) predicts poor clinical outcome in oropharyngeal squamous cell cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 553–558. [Google Scholar] [CrossRef]
- Pontes, H.A.; de Aquino Xavier, F.C.; da Silva, T.S.; Fonseca, F.P.; Paiva, H.B.; Pontes, F.S.; dos Santos Pinto, D., Jr. Metallothionein and p-Akt proteins in oral dysplasia and in oral squamous cell carcinoma: An immunohistochemical study. J. Oral Pathol. Med. 2009, 38, 644–650. [Google Scholar] [CrossRef]
- Miyazawa, J.; Mitoro, A.; Kawashiri, S.; Chada, K.K.; Imai, K. Expression of Mesenchyme-Specific Gene HMGA2 in Squamous Cell Carcinomas of the Oral Cavity. Cancer Res. 2004, 64, 2024–2029. [Google Scholar] [CrossRef]
- Maeda, G.; Chiba, T.; Okazaki, M.; Satoh, T.; Taya, Y.; Aoba, T.; Kato, K.; Kawashiri, S.; Imai, K. Expression of SIP1 in oral squamous cell carcinomas: Implications for E-cadherin expression and tumor progression. Int. J. Oncol. 2005, 27, 1535–1541. [Google Scholar] [PubMed]
- Yokoyama, K.; Kamata, N.; Hayashi, E.; Hoteiya, T.; Ueda, N.; Fujimoto, R.; Nagayama, M. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001, 37, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Kamata, N.; Yokoyama, K.; Fujimoto, R.; Tsutsumi, S.; Nagayama, M. Down-regulation of Wnt-4 and up-regulation of Wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci. 2003, 94, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Puig, I.; Caretti, E.; Bonaventure, J.; Nelles, L.; van Roy, F.; Dargemont, C.; de Herreros, A.G.; Bellacosa, A.; Larue, L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene 2007, 26, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Ling, M.T.; Wong, Y.C.; Leung, S.C.; Wang, X. Anti-apoptotic role of TWIST and its association with Akt pathway in mediating taxol resistance in nasopharyngeal carcinoma cells. Int. J. Cancer 2007, 120, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Onoue, T.; Uchida, D.; Begum, N.M.; Tomizuka, Y.; Yoshida, H.; Sato, M. Epithelial-mesenchymal transition induced by the stromal cell-derived factor-1/CXCR4 system in oral squamous cell carcinoma cells. Int. J. Oncol. 2006, 29, 1133–1138. [Google Scholar] [CrossRef]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.D.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.V.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The mutational landscape of head and neck squamous cell carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Z.; Xiong, X.; Zhong, Y.; Zhang, W.; Dong, Y.; Li, J.; Zhu, Z.; Zhang, W.; Wu, H.; et al. Membrane-tethered Notch1 exhibits oncogenic property via activation of EGFR–PI3K–AKT pathway in oral squamous cell carcinoma. J. Cell. Physiol. 2019, 234, 5940–5952. [Google Scholar] [CrossRef]
- Vasko, V.; Saji, M.; Hardy, E.; Kruhlak, M.; Larin, A.; Savchenko, V.; Miyakawa, M.; Isozaki, O.; Murakami, H.; Tsushima, T.; et al. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J. Med. Genet. 2004, 41, 161–170. [Google Scholar] [CrossRef]
- Wang, R.; Brattain, M.G. AKT can be activated in the nucleus. Cell. Signal. 2006, 18, 1722–1731. [Google Scholar] [CrossRef]
- Alkhadar, H.; Macluskey, M.; White, S.; Ellis, I. Nerve growth factor-induced migration in oral and salivary gland tumour cells utilises the PI3K/Akt signalling pathway: Is there a link to perineural invasion? J. Oral Pathol. Med. 2020, 49, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Alghamdi, A.; Sriramula, P.; Shalgm, B.; Jones, S.; Ellis, I. Is it all just an Akt—you’d be SMAD to believe it! Role of TGFβ1 in oral cancer metastasis. Sci. Repos. Dent. Oral Biol. Craniofacial Res. 2018, 1, 9. [Google Scholar] [CrossRef]
- Thwe, A.M.; Mossey, P.; Ellis, I.R. Effect of tyrosine kinase inhibitors on cell migration and epithelial-to-mesenchymal transition in Asian head and neck cancer cell lines. J. Oral Pathol. Med. 2021, 50, 1031–1039. [Google Scholar] [CrossRef]
- Khattri, A.; Sheikh, N.; Acharya, R.; Tan, Y.-H.C.; Kochanny, S.; Lingen, M.W.; Vokes, E.E.; Seiwert, T.Y. Mechanism of acquired resistance to cetuximab in head and neck cancer. J. Clin. Oncol. 2018, 36, e18061. [Google Scholar] [CrossRef]
- Montagut, C.; Dalmases, A.; Bellosillo, B.; Crespo, M.; Pairet, S.; Iglesias, M.; Salido, M.; Gallen, M.; Marsters, S.; Tsai, S.P.; et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat. Med. 2012, 18, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Zaryouh, H.; De Pauw, I.; Baysal, H.; Pauwels, P.; Peeters, M.; Vermorken, J.B.; Lardon, F.; Wouters, A. The Role of Akt in Acquired Cetuximab Resistant Head and Neck Squamous Cell Carcinoma: An In Vitro Study on a Novel Combination Strategy. Front. Oncol. 2021, 11, 697967. [Google Scholar] [CrossRef] [PubMed]
- Caforio, M.; de Billy, E.; De Angelis, B.; Iacovelli, S.; Quintarelli, C.; Paganelli, V.; Folgiero, V. PI3K/Akt Pathway: The Indestructible Role of a Vintage Target as a Support to the Most Recent Immunotherapeutic Approaches. Cancers 2021, 13, 4040. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S. The role of PI3K in immune cells. Nat. Immunol. 2003, 4, 313–319. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1(+) Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e626. [Google Scholar] [CrossRef]
- Lai, Y.J.; Yu, W.N.; Kuo, S.C.; Ho, C.T.; Hung, C.M.; Way, T.D.; Chen, C.T. CSC-3436 inhibits TWIST-induced epithelial-mesenchymal transition via the suppression of Twist/Bmi1/Akt pathway in head and neck squamous cell carcinoma. J. Cell Physiol. 2019, 234, 9118–9129. [Google Scholar] [CrossRef]
- National Cancer Institute. Akt Inhibitor MK2206 in Treating Patients with Recurrent or Metastatic Head and Neck Cancer. Available online: https://ClinicalTrials.gov/show/NCT01349933 (accessed on 4 April 2022).
- Ma, B.B.; Goh, B.C.; Lim, W.T.; Hui, E.P.; Tan, E.H.; Lopes Gde, L.; Lo, K.W.; Li, L.; Loong, H.; Foster, N.R.; et al. Multicenter phase II study of the AKT inhibitor MK-2206 in recurrent or metastatic nasopharyngeal carcinoma from patients in the mayo phase II consortium and the cancer therapeutics research group (MC1079). Investig. New Drugs 2015, 33, 985–991. [Google Scholar] [CrossRef]
- Ho, A.L.; Foster, N.R.; Meyers, J.P.; Vasudeva, S.D.; Katabi, N.; Antonescu, C.R.; Pfister, D.G.; Horvath, L.E.; Erlichman, C.; Schwartz, G.K. Alliance A091104: A phase II trial of MK-2206 in patients (pts) with progressive, recurrent/metastatic adenoid cystic carcinoma. J. Clin. Oncol. 2015, 33, 6039. [Google Scholar] [CrossRef]
- Yonsei University. An Open Label, Single Arm, Multicenter Phase II Study of BYL719 in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of Head and Neck Who Failed to Respond to Platinum-Based Therapy. Available online: https://ClinicalTrials.gov/show/NCT02145312 (accessed on 10 September 2023).
- Kim, H.R.; Kang, H.N.; Yun, M.R.; Lim, S.M.; Kim, C.G.; Ahn, M.-J.; Sun, J.-M.; Kim, J.-H.; Paik, S.; Cho, B.C. Clinical trials outcomes of combined BKM120 and cetuximab compared to BKM120 in recurrent and/or metastatic squamous cell carcinoma of head and neck (R/M-SCCHN). J. Clin. Oncol. 2015, 33, 6049. [Google Scholar] [CrossRef]
- Rodon, J.; Curigliano, G.; Delord, J.-P.; Harb, W.; Azaro, A.; Han, Y.; Wilke, C.; Donnet, V.; Sellami, D.; Beck, T. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018, 9, 31709–31718. [Google Scholar] [CrossRef] [PubMed]
- Dana-Farber Cancer Institute.; Novartis Pharmaceuticals. Phase Ib Study of BKM120 With Cisplatin and XRT in High Risk Locally Advanced Squamous Cell Cancer of Head and Neck. Available online: https://ClinicalTrials.gov/show/NCT02113878 (accessed on 10 September 2023).
- Seoul National University Hospital; Korean Cancer Study Group; Chungnam National Cancer Hospital. Korean Cancer Study Group: Translational bIomarker Driven UMbrella Project for Head and Neck (TRIUMPH), Esophageal Squamous Cell Carcinoma—Part 1 (HNSCC). Available online: https://ClinicalTrials.gov/show/NCT03292250 (accessed on 10 September 2023).
- University of Chicago; National Cancer Institute. PI3K Inhibitor BKM120 and Cetuximab in Treating Patients with Recurrent or Metastatic Head and Neck Cancer. Available online: https://ClinicalTrials.gov/show/NCT01816984 (accessed on 10 September 2023).
- Genentech, Inc. A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0973 in Combination with GDC-0068 When Administered in Participants with Locally Advanced or Metastatic Solid Tumors. Available online: https://classic.clinicaltrials.gov/show/NCT01562275 (accessed on 10 September 2023).
- AstraZeneca. Investigating Safety, Tolerability and Efficacy of AZD5363 When Combined with Paclitaxel in Breast Cancer Patients. Available online: https://classic.clinicaltrials.gov/show/NCT01625286 (accessed on 10 September 2023).
- Genentech, Inc. A Study Assessing the Safety and Efficacy of Adding Ipatasertib to Paclitaxel Treatment in Participants with Breast Cancer That Has Spread Beyond the Initial Site, and the Cancer Does Not Have Certain Hormonal Receptors. Available online: https://classic.clinicaltrials.gov/show/NCT02162719 (accessed on 10 September 2023).
- National Cancer Institute. MK2206 in Treating Younger Patients with Recurrent or Refractory Solid Tumors or Leukemia. Available online: https://classic.clinicaltrials.gov/show/NCT01231919 (accessed on 10 September 2023).
- National Cancer Institute. Selumetinib and Akt Inhibitor MK2206 or mFOLFOX Therapy Comprising Oxaliplatin and Fluorouracil in Treating Patients With Metastatic Pancreatic Cancer Previously Treated With Chemotherapy. Available online: https://classic.clinicaltrials.gov/show/NCT01658943 (accessed on 10 September 2023).
- EMD Serono Research & Development Institute, Inc. First-in-Human Dose Escalation Trial in Subjects with Advanced Malignancies. Available online: https://classic.clinicaltrials.gov/show/NCT01971515 (accessed on 10 September 2023).
- National Cancer Institute. MK2206 in Combination with Anastrozole, Fulvestrant, or Anastrozole and Fulvestrant in Treating Postmenopausal Women with Metastatic Breast Cancer. Available online: https://classic.clinicaltrials.gov/show/NCT01344031 (accessed on 10 September 2023).
- National Cancer Institute. Akt Inhibitor MK2206 in Treating Patients with Previously Treated Colon or Rectal Cancer That Is Metastatic or Locally Advanced and Cannot Be Removed by Surgery. Available online: https://classic.clinicaltrials.gov/show/NCT01802320 (accessed on 10 September 2023).
- National Cancer Institute. Akt Inhibitor MK2206 in Combination with Lapatinib Ditosylate in Patients with Advanced or Metastatic Solid Tumors or Breast Cancer. Available online: https://classic.clinicaltrials.gov/show/NCT01245205 (accessed on 11 September 2023).
- National Cancer Institute. Dinaciclib and Akt Inhibitor MK2206 in Treating Patients with Pancreatic Cancer That Cannot Be Removed by Surgery. Available online: https://classic.clinicaltrials.gov/show/NCT01783171 (accessed on 11 September 2023).
- National Cancer Institute; National Institutes of Health Clinical, Center. MK-2206 and AZD6244 in Patients with Advanced Colorectal Carcinoma. Available online: https://classic.clinicaltrials.gov/show/NCT01333475 (accessed on 11 September 2023).
- National Cancer Institute. Trametinib with or without GSK2141795 in Treating Patients with Metastatic Uveal Melanoma. Available online: https://classic.clinicaltrials.gov/show/NCT01979523 (accessed on 11 September 2023).
- National Cancer Institute. Akt Inhibitor MK2206 in Treating Patients with Progressive, Recurrent, or Metastatic Adenoid Cyst Carcinoma. Available online: https://classic.clinicaltrials.gov/show/NCT01604772 (accessed on 11 September 2023).
- Genentech, Inc. A Study of Ipatasertib (GDC-0068) in Combination with Fluoropyrimidine Plus Oxaliplatin in Participants with Advanced or Metastatic Gastric or Gastroesophageal Junction Cancer. Available online: https://classic.clinicaltrials.gov/show/NCT01896531 (accessed on 11 September 2023).
- National Cancer Institute. MK2206 and Paclitaxel in Treating Patients with Locally Advanced or Metastatic Solid Tumors or Metastatic Breast Cancer. Available online: https://classic.clinicaltrials.gov/show/NCT01263145 (accessed on 11 September 2023).
- National Cancer Institute; GlaxoSmithKline. Trametinib and Akt Inhibitor GSK2141795 in Treating Patients with Metastatic Triple-Negative Breast Cancer. Available online: https://classic.clinicaltrials.gov/show/NCT01964924 (accessed on 11 September 2023).
- Gustave Roussy, Cancer Campus Grand Paris. A Phase Ib Study of the Safety, Tolerability and Efficacy of LY2780301 in Combination with Gemcitabine. Available online: https://classic.clinicaltrials.gov/show/NCT02018874 (accessed on 11 September 2023).

| Trial Identifier | Phase | Type of Cancer | PI3K/Akt Inhibitor | Combination | Result | Ref |
|---|---|---|---|---|---|---|
| NCT01349933 | II | IV/recurrent NPC | MK2206 (Akt inhibitor) | None | CR—0%, PR—4.8%, stable disease 52.4%, OS—10 months, PFS—3.5 months | [186] |
| NCT01370070 | II | Recurrent NPC | MK2206 | None | CR—0%, PR—5%, Stable disease—52%, OS—10 months, PFS—3.5 months | [187] |
| NCT01604772 | II | IV/recurrent ADCC | MK2206 | None | CR/PR—0%, Stable disease—81%, PFS—9.7 months, OS—18 months | [188] |
| NCT02145312 | II | Recurrent/metastatic HNSCC | BYL719/Alpelisib (PI3K inhibitor) | None | Not published | [189] |
| NCT01527877 | II | Recurrent/metastatic HNSCC | BKM120/Buparlisib (PI3K inhibitor) | None | RR—3%, Stable disease—49%, PFS—63 days, OS—143 days | [190] |
| NCT02021751 | Ib | Recurrent/metastatic HNSCC | BYL719 | Paclitaxel | Challenging safety profile, dose expansion phase was not initiated | [191] |
| NCT02113878 | Ib | Locally advanced HNSCC | BKM120 | Cisplatin/RT | Not published yet | [192] |
| NCT03292250 | II | HNSCC | BYL719 | Poziotinib (EGFR inhibitor) | Not published yet | [193] |
| NCT01816984 | I/II | Recurrent/metastatic HNSCC | BKM120 | Cetuximab | OS—9.3 months, PFS—2 months, RR—8–9% | [194] |
| NCT01562275 | Ib | Locally advanced/metastatic solid tumours | GDC0068 (Ipatasertib) | GDC0973 (MEK1 inhibitor) | PFS—not measured due to very few participants with measurable response | [195] |
| NCT01625286 | II | Advanced/metastatic breast cancer | AZD5363 (Capivasertib) | Paclitaxel | Adding capivasertib did not prolong PFS in the overall population | [196] |
| NCT01625286 | II | Advanced/metastatic breast cancer | Ipatasertib | Paclitaxel | Not completed due to high number of patient death | [197] |
| NCT01231919 | I | Recurrent solid tumours and leukaemia | MK2206 | Not published | [198] | |
| NCT01658943 | II | Metastatic pancreatic cancer | MK2206 | Selumetinib | OS—3.9 months, PFS—1.9 months, Disease increased—19% | [199] |
| NCT01971515 | I | Advanced malignancy | MSC2363318A (p70S6K/Akt inhibitor) | Trastuzumab | Not published | [200] |
| NCT1344031 | I | Postmenopausal women with metastatic breast cancer | MK2206 | Anastrozole, Fulvestrant | Not published | [201] |
| NCT01802320 | II | Recurrent/metastatic colon cancer | MK2206 | OS—6.8 months, PFS—1.8 month, ORR—O% | [202] | |
| NCT01245205 | I | Metastatic solid tumour/breast cancer | MK2206 | Lapatinib | Not published | [203] |
| NCT01783171 | I | Pancreatic cancer | MK2206 | Dinaciclib | Not published | [204] |
| NCT01333475 | Pilot | Advanced colorectal cancer | MK2206 | Selumetinib | Biomarker analysis | [205] |
| NCT01979523 | II | Metastatic uveal melanoma | GSK2141795 | Trametinib | PFS—15.6 wks, OS—88 wks, disease progressed—22% | [206] |
| NCT01604772 | II | Adenoid cyst carcinoma | MK2206 | PFS—9 months, PS—18 months, Grade 3 adverse event—62% | [207] | |
| NCT01896531 | II | Metastatic gastro-oesophageal cancer | Ipatasertib | mFOLFOX6 | pFS—6.57 months, OS—12 months, ORR—52% | [208] |
| NCT01349933 | II | Metastatic HNSCC | MK2206 | Disease progressed or dead: 57%, PR—4.8% | [186] | |
| NCT01263145 | I | Metastatic solid or breast cancer | MK2206 | Paclitaxel | Not published | [209] |
| NCT01964924 | II | Metastatic triple-negative breast cancer | GSK2141795 | Trametinib | ORR—5.4%, CBR—31.2% | [210] |
| NCT02018874 | Ib | Solid tumours and non-Hodgkin’s Lymphoma | LY2780301(p70S6K/Akt inhibitor) | Gemcitabine | Not published | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Jones, S.; Ellis, I. Role of Akt/Protein Kinase B in Cancer Metastasis. Biomedicines 2023, 11, 3001. https://doi.org/10.3390/biomedicines11113001
Islam M, Jones S, Ellis I. Role of Akt/Protein Kinase B in Cancer Metastasis. Biomedicines. 2023; 11(11):3001. https://doi.org/10.3390/biomedicines11113001
Chicago/Turabian StyleIslam, Mohammad, Sarah Jones, and Ian Ellis. 2023. "Role of Akt/Protein Kinase B in Cancer Metastasis" Biomedicines 11, no. 11: 3001. https://doi.org/10.3390/biomedicines11113001
APA StyleIslam, M., Jones, S., & Ellis, I. (2023). Role of Akt/Protein Kinase B in Cancer Metastasis. Biomedicines, 11(11), 3001. https://doi.org/10.3390/biomedicines11113001








