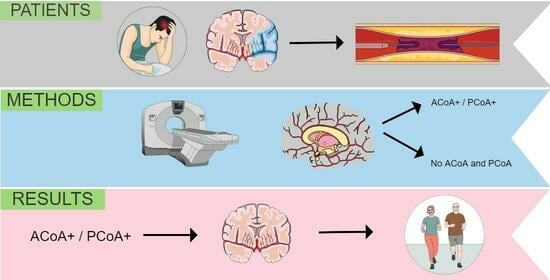
The Presence of Communicating Arteries in the Circle of Willis Is Associated with Higher Rate of Functional Recovery after Anterior Circulation Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
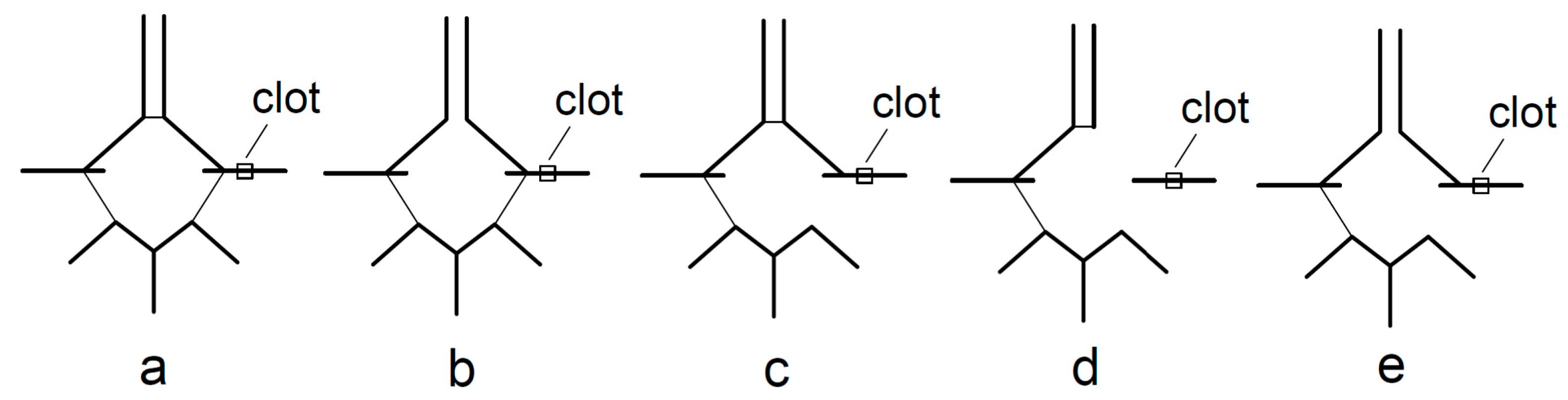
2.2. Imaging Data and Analysis
2.3. Endovascular Treatment
2.4. Main Outcomes and Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, 143–152. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Hendrix, P.; Sofoluke, N. Risk Factors for Acute Ischemic Stroke Caused by Anterior Large Vessel Occlusion. Stroke 2019, 50, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.; Carter, K. Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurol. 2006, 5, 130–139. [Google Scholar] [CrossRef]
- De Silva, D.A.; Woon, F.P. Metabolic syndrome is associated with intracranial large artery disease among ethnic Chinese patients with stroke. J. Stroke Cerebrovasc. Dis. 2009, 18, 424–427. [Google Scholar] [CrossRef]
- Casella, I.B.; Sotelo, F.J. Comparison of common carotid artery intima-media thickness between Brazilian Euro-descendants and Afro-descendants with atherosclerosis risk factors. Clinics 2009, 64, 657–664. [Google Scholar] [CrossRef]
- Schneider, A.T.; Kissela, B. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke 2004, 35, 1552–1556. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, P.; Kubis, N. Collateral Supply in Preclinical Cerebral Stroke Models. Transl. Stroke Res. 2022, 13, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J. Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collaterals in acute stroke: Beyond the clot. Neuroimaging Clin. N. Am. 2005, 15, 553–573. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Bonnin, P.; Mazighi, M. Early Collateral Recruitment after Stroke in Infants and Adults. Stroke 2019, 50, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Bergui, M.; Cerrato, P. Stroke attributable to acute basilar occlusion. Curr. Treat. Options Neurol. 2007, 9, 126–135. [Google Scholar] [CrossRef]
- Morita, Y.; Fukuuchi, Y. Rapid changes in pial arterial diameter and cerebral blood flow caused by ipsilateral carotid artery occlusion in rats. Keio J. Med. 1997, 46, 120–127. [Google Scholar] [CrossRef]
- Hindenes, L.B.; Håberg, A.K. Variations in the Circle of Willis in a large population sample using 3D TOF angiography: The Tromsø Study. PLoS ONE 2020, 15, e0241373. [Google Scholar] [CrossRef]
- Nyasa, C.; Mwakikunga, A. Distribution of variations in anatomy of the circle of Willis: Results of a cadaveric study of the Malawian population and review of literature. Pan. Afr. Med. J. 2021, 38, 11. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, M.; Scheau, C. Circle of Willis: Anatomical variations of configuration. A magnetic resonance angiography study. Folia Morphol. 2023, 82, 24–29. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Eva, L. Anatomical study of circle of Willis on fresh autopsied brains. A study of a Romanian population. Rom. J. Morphol. Embryol. 2022, 63, 395–406. [Google Scholar] [CrossRef]
- Westphal, L.P.; Lohaus, N. Circle of Willis variants and their association with outcome in patients with middle cerebral artery-M1-occlusion stroke. Eur. J. Neurol. 2021, 28, 3682–3691. [Google Scholar] [CrossRef] [PubMed]
- Seifert-Held, T.; Eberhard, K. Circle of Willis variants are not associated with thrombectomy outcomes. Stroke Vasc. Neurol. 2021, 6, 310–313. [Google Scholar] [CrossRef] [PubMed]
- De Caro, J.; Ciacciarelli, A. Variants of the circle of Willis in ischemic stroke patients. J. Neurol. 2021, 268, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Kamel, H. Incomplete circle of Willis variants and stroke outcome. Eur. J. Radiol. 2022, 153, 110383. [Google Scholar] [CrossRef]
- Sadeh-Gonik, U.; Budylev, A. Circle of Willis integrity in acute middle cerebral artery occlusion: Does the posterior communicating artery matter? J. Neurointerv. Surg. 2023. [Google Scholar] [CrossRef]
- Rangus, I.; Milles, L.S. Reclassifications of ischemic stroke patterns due to variants of the Circle of Willis. Int. J. Stroke 2022, 17, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, J. Correlation Between the Integrity of the Circle of Willis and the Severity of Initial Noncardiac Cerebral Infarction and Clinical Prognosis. Medicine 2016, 95, e2892. [Google Scholar] [CrossRef]
- Barber, P.A.; Demchuk, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef]
- Weiss, D.; Kraus, B. Systematic evaluation of computed tomography angiography collateral scores for estimation of long-term outcome after mechanical thrombectomy in acute ischaemic stroke. Neuroradiol. J. 2019, 32, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lee, K. Value of utilizing both ASPECTS and CT angiography collateral score for outcome prediction in acute ischemic stroke. Int. J. Stroke 2015, 10, 1018–1023. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Tomsick, T.A. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014, 45, 759–764. [Google Scholar] [CrossRef]
- Higashida, R.T.; Furlan, A.J. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003, 34, e109–e137. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Lev, M.H. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke 2009, 40, 3001–3005. [Google Scholar] [CrossRef]
- Nannoni, S.; Cereda, C.W. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 2019, 61, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, J. Association of Collateral Status and Ischemic Core Growth in Patients with Acute Ischemic Stroke. Neurology 2021, 96, e161–e170. [Google Scholar] [CrossRef]
- Leng, X.; Lan, L. Good collateral circulation predicts favorable outcomes in intravenous thrombolysis: A systematic review and meta-analysis. Eur. J. Neurol. 2016, 23, 1738–1749. [Google Scholar] [CrossRef]
- Tan, I.Y.; Demchuk, A.M. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am. J. Neuroradiol. 2009, 30, 525–531. [Google Scholar] [CrossRef]
- Gerber, J.C.; Petrova, M. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. 2016, 6, e00513. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, F.; Motta, C. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. J. Neurointerv. Surg. 2017, 9, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Anadani, M.; Finitsis, S. Collateral status reperfusion and outcomes after endovascular therapy: Insight from the Endo-vascular Treatment in Ischemic Stroke (ETIS) Registry. J. Neurointerv. Surg. 2022, 14, 551–557. [Google Scholar] [CrossRef]
- Rusanen, H.; Saarinen, J.T. Collateral Circulation Predicts the Size of the Infarct Core and the Proportion of Salvageable Penumbra in Hyperacute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Cerebrovasc. Dis. 2015, 40, 182–190. [Google Scholar] [CrossRef]
- Wufuer, A.; Wubuli, A. Impact of collateral circulation status on favorable outcomes in thrombolysis treatment: A system-atic review and meta-analysis. Exp. Ther. Med. 2018, 15, 707–718. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Saber, H. Collateral Circulation in Thrombectomy for Stroke After 6 to 24 Hours in the DAWN Trial. Stroke 2022, 53, 742–748. [Google Scholar] [CrossRef] [PubMed]
- de Havenon, A.; Mlynash, M. Results From DEFUSE 3: Good Collaterals Are Associated With Reduced Ischemic Core Growth but Not Neurologic Outcome. Stroke 2019, 50, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasuqi, K.; Payabvash, S. Effects of Collateral Status on Infarct Distribution Following Endovascular Therapy in Large Vessel Occlusion Stroke. Stroke 2020, 51, e193–e202. [Google Scholar] [CrossRef]
- Renú, A.; Laredo, C. Greater infarct growth limiting effect of mechanical thrombectomy in stroke patients with poor col-laterals. J. Neurointerv. Surg. 2019, 11, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Robichon, E.; Maïer, B.; Mazighi, M. Endovascular therapy for acute ischemic stroke: The importance of blood pressure control, sedation modality and anti-thrombotic management to improve functional outcomes. Rev. Neurol. 2022, 178, 175–184. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H. Prognostic significance of blood pressure parameters after mechanical thrombectomy according to col-lateral status. BMC Neurol. 2023, 23, 123. [Google Scholar] [CrossRef]
- Lu, Y.; Shen, R. Association between blood pressure variability and clinical outcomes after successful recanalization in pa-tients with large vessel occlusion stroke after mechanical thrombectomy. Front. Neurol. 2022, 13, 967395. [Google Scholar] [CrossRef]
- Amaral, S.; Duloquin, G. Symptomatic Intracranial Hemorrhage after Ischemic Stroke Treated with Bridging Revasculari-zation Therapy. Life 2023, 13, 1593. [Google Scholar] [CrossRef]
- Hoffman, H.; Cote, J.R. The influence of pre-reperfusion blood pressure on outcomes following mechanical thrombectomy for anterior circulation large vessel occlusion. J. Clin. Neurosci. 2023, 113, 99–107. [Google Scholar] [CrossRef]
- Iancu, A.; Buleu, F. Early Hemorrhagic Transformation after Reperfusion Therapy in Patients with Acute Ischemic Stroke: Analysis of Risk Factors and Predictors. Brain Sci. 2023, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Cui, J.S. Effect of blood pressure on the prognosis of acute ischemic stroke patients caused by anterior circulation large vessel occlusion without recanalization. Clin. Neurol. Neurosurg. 2023, 224, 107540. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Saver, J.L. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011, 42, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.C. The core/penumbra model: Implications for acute stroke treatment and patient selection in 2021. Eur. J. Neurol. 2021, 28, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
| Collateral-Positive Group (n = 104) | Collateral-Negative Group (n = 54) | p Value | |
|---|---|---|---|
| Age, years (median, IQR) | 78.5 (71–83) | 76.5 (67–84) | 0.657 * |
| Women (no, %) | 65 (62.5%) | 33 (61.1%) | 0.865 † |
| Arterial hypertension (no, %) | 71 (68.3%) | 39 (72.2%) | 0.710 † |
| Diabetes (no, %) | 24 (23%) | 11 (20.3%) | 0.839 † |
| Atrial fibrillation (no, %) | 51 (49%) | 33 (61.1%) | 0.169 † |
| Antiplatelet/anticoagulation therapy (no, %) | 34 (32.7%) | 26 (48.1%) | 0.082 † |
| Baseline NIHSS (median, IQR) | 15 (14–17) | 15 (11.5–17) | 0.397 † |
| Systolic blood pressure (median, IQR) | 140 (130–170) | 145 (120–162.5) | 0.576 * |
| Diastolic blood pressure (median, IQR) | 80 (70–95) | 80 (70–90) | 0.406 * |
| IVT | 25/38 (65.8%) | 13/19 (68.4%) | 0.844 † |
| Hemorrhage on control CT | 21 (20.2%) | 15 (27.7%) | 0.322 † |
| Collateral-Positive Group (n = 104) | Collateral-Negative Group (n = 54) | |
|---|---|---|
| Localisation of thrombus | ||
| M1 segment | 76/104 (73.1%) | 34/54 (63%) |
| M2 segment | 12/104 (11.5%) | 3/54 (5.5%) |
| T-occlusion | 15/104 (14.4%) | 16/54 (29.6%) |
| tandem occlusion | 1/104 (1%) | 1/54 (1.9%) |
| EVT technique | ||
| aspiration only | 70/104 (67.3%) | 37/54 (68.5%) |
| aspiration and stent retriever | 18/104 (17.3%) | 5/54 (9.3%) |
| unsuccessful | 16/104 (15.4%) | 12/54 (22.2%) |
| Good reperfusion (TICI 2B, 2C or 3) | 74/104 (71.1%) | 33/54 (61.1%) |
| TICI 3 (%) | 63/104 (60.6%) | 27/54 (50%) |
| Ischemic lesion on control CT | ||
| 0 | 14/103 (13.6%) | 6/54 (11.1%) |
| 1 | 46/103 (44.7%) | 15/54 (27.8%) |
| 2 | 29/103 (28.1%) | 17/54 (31.5%) |
| 3 | 14/103 (13.6%) | 16/54 (29.6%) |
| Hemorrhage on control CT | ||
| SAH | 10/20 (50%) | 6/15 (40%) |
| ICH | 10/20 (50%) | 9/15 (60%) |
| Modified Rankin Scale Score | ||
| 0–2 | 49/92 (53.3%) | 15/52 (28.8%) |
| 3–5 | 19/92 (20.6%) | 12/52 (23.1%) |
| 6 | 24/92 (26.1%) | 25/52 (48.1%) |
| Outcomes | OR or β | 95% CI | p Value |
|---|---|---|---|
| NIHSS score | −1.164 | −2.784 to 0.4555 | 0.1567 |
| Successful recanalization (TICI score 2B, 2C, 3) | 6.844 | 1.278 to 52.21 | 0.036 |
| Control CT score | −0.3520 | −0.6569 to −0.04699 | 0.024 |
| Favorable functional recovery (mRS 0–2) | 11.87 | 2.952 to 61.60 | 0.001 |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Age | 0.9436 | 0.8494 to 1.028 | 0.2211 |
| Sex | 0.6433 | 0.1344 to 2.908 | 0.5668 |
| Present collaterals | 2.617 | 1.047 to 7.521 | 0.0505 |
| Antiplatelet therapy | 0.7220 | 0.1486 to 3.512 | 0.6800 |
| NIHSS score | 0.9537 | 0.7739 to 1.178 | 0.6498 |
| SAH or ICH | 0.4691 | 0.08010 to 2.373 | 0.3697 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sablić, S.; Dolić, K.; Kraljević, I.; Budimir Mršić, D.; Čičmir-Vestić, M.; Benzon, B.; Lovrić Kojundžić, S.; Marinović Guić, M. The Presence of Communicating Arteries in the Circle of Willis Is Associated with Higher Rate of Functional Recovery after Anterior Circulation Ischemic Stroke. Biomedicines 2023, 11, 3008. https://doi.org/10.3390/biomedicines11113008
Sablić S, Dolić K, Kraljević I, Budimir Mršić D, Čičmir-Vestić M, Benzon B, Lovrić Kojundžić S, Marinović Guić M. The Presence of Communicating Arteries in the Circle of Willis Is Associated with Higher Rate of Functional Recovery after Anterior Circulation Ischemic Stroke. Biomedicines. 2023; 11(11):3008. https://doi.org/10.3390/biomedicines11113008
Chicago/Turabian StyleSablić, Sara, Krešimir Dolić, Ivan Kraljević, Danijela Budimir Mršić, Mate Čičmir-Vestić, Benjamin Benzon, Sanja Lovrić Kojundžić, and Maja Marinović Guić. 2023. "The Presence of Communicating Arteries in the Circle of Willis Is Associated with Higher Rate of Functional Recovery after Anterior Circulation Ischemic Stroke" Biomedicines 11, no. 11: 3008. https://doi.org/10.3390/biomedicines11113008
APA StyleSablić, S., Dolić, K., Kraljević, I., Budimir Mršić, D., Čičmir-Vestić, M., Benzon, B., Lovrić Kojundžić, S., & Marinović Guić, M. (2023). The Presence of Communicating Arteries in the Circle of Willis Is Associated with Higher Rate of Functional Recovery after Anterior Circulation Ischemic Stroke. Biomedicines, 11(11), 3008. https://doi.org/10.3390/biomedicines11113008