Effects of CYP3A5 Genotypes on Thrombocytopenia in Liver Transplantation Patients Treated with Tacrolimus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection and Grouping
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
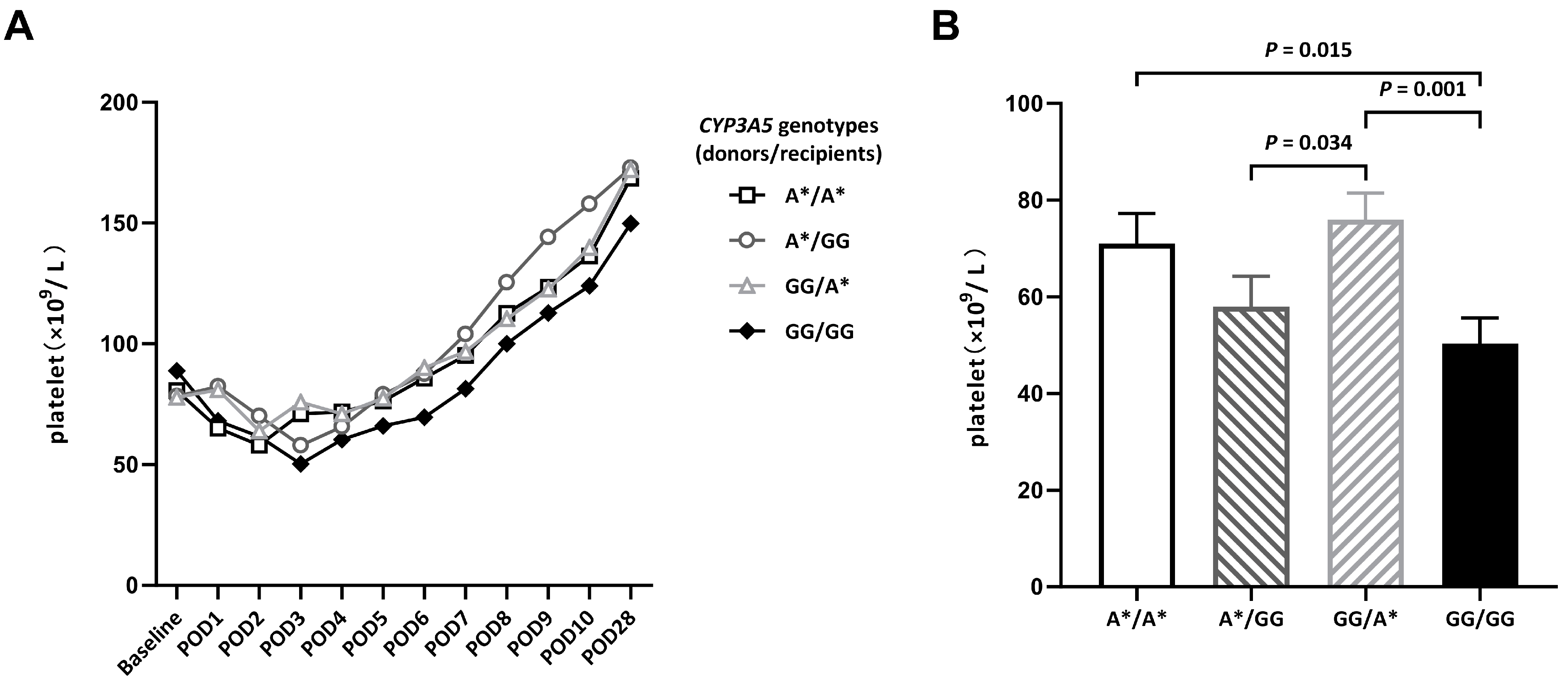
3.2. Changes in Platelet Counts in the First Month after DDLT
3.3. Correlation of Risk Factors for Thrombocytopenia
4. Discussion
- Liver failure. As an important regulator of endogenous platelet production, thrombopoietin (TPO) is synthesized by the liver. Chronic liver diseases such as cirrhosis can lead to a reduction in TPO concentrations, which will affect megakaryocyte proliferation and differentiation and eventually lead to thrombocytopenia [27].
- Excessive platelet consumption caused by massive blood loss during surgery. Combined organ transplantation and secondary organ transplantation may increase the degree of injury of patients, resulting in platelet consumption [28].
- Fluid resuscitation may cause hemodilution, [29] but massive blood loss and secondary liver transplantations were excluded in this study.
- Anticoagulation drugs can lead to a reduction in platelets [30].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, N. Liver Transplantation Criteria for Hepatocellular Carcinoma, Including Posttransplant Management. Clin. Liver Dis. 2021, 17, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Millson, C.; Considine, A.; Cramp, M.E.; Holt, A.; Hubscher, S.; Hutchinson, J.; Jones, K.; Leithead, J.; Masson, S.; Menon, K.; et al. Adult liver transplantation: UK clinical guideline—Part 2: Surgery and post-operation. Frontline Gastroenterol. 2020, 11, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nagai, S.; Safwan, M.; Liang, C.; Ohkohchi, N. Thrombocytopenia after liver transplantation: Should we care? World J. Gastroenterol. 2018, 24, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Gwiasda, J.; Schrem, H.; Klempnauer, J.; Kaltenborn, A. Identifying independent risk factors for graft loss after primary liver transplantation. Langenbecks Arch. Surg. 2017, 402, 757–766. [Google Scholar] [CrossRef]
- Takahashi, K.; Nagai, S.; Putchakayala, K.G.; Safwan, M.; Gosho, M.; Li, A.Y.; Kane, W.J.; Singh, P.L.; Rizzari, M.D.; Collins, K.M.; et al. Prediction of biliary anastomotic stricture after deceased donor liver transplantation: The impact of platelet counts—A retrospective study. Transpl. Int. 2017, 30, 1032–1040. [Google Scholar] [CrossRef]
- Akamatsu, N.; Sugawara, Y.; Kanako, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N. Low Platelet Counts and Prolonged Prothrombin Time Early After Operation Predict the 90 Days Morbidity and Mortality in Living-donor Liver Transplantation. Ann. Surg. 2017, 265, 166–172. [Google Scholar] [CrossRef]
- Armstrong, V.W.; Oellerich, M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin. Biochem. 2001, 34, 9–16. [Google Scholar] [CrossRef]
- Andrews, L.M.; Li, Y.; De Winter, B.C.M.; Shi, Y.Y.; Baan, C.C.; Van Gelder, T.; Hesselink, D.A. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert. Opin. Drug. Metab. Toxicol. 2017, 13, 1225–1236. [Google Scholar] [CrossRef]
- Kershner, R.P.; Fitzsimmons, W.E. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996, 62, 920–926. [Google Scholar] [CrossRef]
- Nelson, J.; Alvey, N.; Bowman, L.; Schulte, J.; Segovia Maria, C.; McDermott, J.; Te, H.S.; Kapila, N.; Levine, D.J.; Gottlieb, R.L.; et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 599–633. [Google Scholar] [CrossRef]
- Sam, W.J.; Tham, L.S.; Holmes, M.J.; Aw, M.; Quak, S.H.; Lee, K.H.; Lim, S.G.; Prabhakaran, K.; Chan, S.Y.; Ho, P.C. Population pharmacokinetics of tacrolimus in whole blood and plasma in asian liver transplant patients. Clin. Pharmacokinet. 2006, 45, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Looy, S.; Verplancke, T.; Benoit, D.; Hoste, E.; Maele, G.; De Turck, F.; Decruyenaere, J. A novel approach for prediction of tacrolimus blood concentration in liver transplantation patients in the intensive care unit through support vector regression. Crit. Care 2007, 11, R83. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Song, T.; Jiang, Y.; Li, X.; Fan, Y.; Lin, T. Tacrolimus Trough Level at the First Month May Predict Renal Transplantation Outcomes Among Living Chinese Kidney Transplant Patients: A Propensity Score-Matched Analysis. Ther. Drug. Monit. 2019, 41, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Urata, S.; Yasuda, K.; Sekiya, H.; Hirabara, Y.; Okumura, M.; Ikeda, R. Retrospective analysis of the correlation between tacrolimus concentrations measured in whole blood and variations of blood cell counts in patients undergoing allogeneic haematopoietic stem cell transplantation. Eur. J. Hosp. Pharm. 2020, 27, e7–e11. [Google Scholar] [CrossRef]
- Sikma, M.A.; van Maarseveen, E.M.; van de Graaf, E.A.; Kirkels, J.H.; Verhaar, M.C.; Donker, D.W.; Kesecioglu, J.; Meulenbelt, J. Pharmacokinetics and Toxicity of Tacrolimus Early After Heart and Lung Transplantation. Am. J. Transplant. 2015, 15, 2301–2313. [Google Scholar] [CrossRef]
- Bouamar, R.; Shuker, N.; Hesselink, D.A.; Weimar, W.; Ekberg, H.; Kaplan, B.; Bernasconi, C.; van Gelder, T. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: A pooled analysis from three randomized-controlled clinical trials. Am. J. Transplant. 2013, 13, 1253–1261. [Google Scholar] [CrossRef]
- Ihara, H.; Shinkuma, D.; Ichikawa, Y.; Nojima, M.; Nagano, S.; Ikoma, F. Intra- and interindividual variation in the pharmacokinetics of tacrolimus (FK506) in kidney transplant recipients—Importance of trough level as a practical indicator. Int. J. Urol. 1995, 2, 151–155. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Kuppusamy, G.; Ponnusankar, S.; Mutalik, S. Towards next-generation personalization of tacrolimus treatment: A review on advanced diagnostic and therapeutic approaches. Pharmacogenomics 2021, 22, 1151–1175. [Google Scholar] [CrossRef]
- Eng, H.S.; Mohamed, Z.; Calne, R.; Lang, C.C.; Mohd, M.A.; Seet, W.T.; Tan, S.Y. The influence of CYP3A gene polymorphisms on cyclosporine dose requirement in renal allograft recipients. Kidney Int. 2006, 69, 1858–1864. [Google Scholar] [CrossRef]
- Sun, S.; Ren, Q.; Zheng, G.; Han, T.; Feng, F. The relationship between metabolic enzyme genetic polymorphisms and anti-tuberculosis drug-induced hepatotoxicity. Int. J. Clin. Exp. Med. 2019, 12, 4321–4329. [Google Scholar]
- Nagrebetsky, A.; Al-Samkari, H.; Davis, N.M.; Kuter, D.J.; Wiener-Kronish, J.P. Perioperative thrombocytopenia: Evidence, evaluation, and emerging therapies. Br. J. Anaesth. 2019, 122, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F.; Azarpira, N.; Kaviani, M. Effect of CYP3A5*1 expression on tacrolimus required dose after liver transplantation: A systematic review and meta-analysis. Clin. Transplant. 2018, 32, e13306. [Google Scholar] [CrossRef]
- Navarro, V.; Herrine, S.; Katopes, C.; Colombe, B.; Spain, C.V. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: An analysis of the OPTN database. Liver Transpl. 2006, 12, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Capron, A.; Musuamba, F.; Latinne, D.; Mourad, M.; Lerut, J.; Haufroid, V.; Wallemacq, P.E. Validation of a liquid chromatography-mass spectrometric assay for tacrolimus in peripheral blood mononuclear cells. Ther. Drug. Monit. 2009, 31, 178–186. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, G.W.; Herkes, R.; Powers, B.; Rickard, K.; Gallagher, N.D.; Thompson, J.F.; Sheil, A.G. Thrombocytopenia post liver transplantation. Correlations with pre-operative platelet count, blood transfusion requirements, allograft function and outcome. J. Hepatol. 1992, 16, 16–22. [Google Scholar] [CrossRef]
- Saab, S.; Brown, R.S., Jr. Management of Thrombocytopenia in Patients with Chronic Liver Disease. Dig. Dis. Sci. 2019, 64, 2757–2768. [Google Scholar] [CrossRef]
- Unal, D.; Senayli, Y.; Polat, R.; Spahn, D.R.; Toraman, F.; Alkis, N. Peri-operative blood transfusion in elective major surgery: Incidence, indications and outcome—An observational multicentre study. Blood Transfus 2020, 18, 261–279. [Google Scholar] [PubMed]
- Kurokawa, T.; Ohkohchi, N. Platelets in liver disease, cancer and regeneration. World J. Gastroenterol. 2017, 23, 3228–3239. [Google Scholar] [CrossRef]
- Assfalg, V.; Huser, N. Heparin-induced thrombocytopenia in solid organ transplant recipients: The current scientific knowledge. World J. Transplant. 2016, 6, 165–173. [Google Scholar] [CrossRef]
- Aster, R.H. Splenic platelet pooling as a cause of “hypersplenic” thrombocytopenia. Trans. Assoc. Am. Phys. 1965, 78, 362–373. [Google Scholar]
- Chatzipetrou, M.A.; Tsaroucha, A.K.; Weppler, D.; Pappas, P.A.; Kenyon, N.S.; Nery, J.R.; Khan, M.F.; Kato, T.; Pinna, A.D.; O’Brien, C.; et al. Thrombocytopenia after liver transplantation. Transplantation 1999, 67, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Egritas, O.; Dalgic, B. Tacrolimus-Induced Autoimmune Hemolytic Anemia in a Previously Reported Child With History of Thrombocytopenia Following Liver Transplant. Exp. Clin. Transplant. 2018, 16, 355–356. [Google Scholar] [PubMed]
- Jodele, S.; Fukuda, T.; Vinks, A.; Mizuno, K.; Laskin, B.L.; Goebel, J.; Dixon, B.P.; Teusink, A.; Pluthero, F.G.; Lu, L.; et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol. Blood Marrow Transpl. 2014, 20, 518–525. [Google Scholar]
- Nosari, A.; Marbello, L.; De Carlis, L.G.; De Gasperi, A.; Muti, G.; Mancini, V.; Morra, E. Bone marrow hypoplasia complicating tacrolimus (FK506) therapy. Int. J. Hematol. 2004, 79, 130–132. [Google Scholar]
- De Simone, P.; Carrai, P.; Coletti, L.; Ghinolfi, D.; Petruccelli, S.; Precisi, A.; Campani, D.; Marchetti, P.; Filipponi, F. Everolimus vs Mycophenolate Mofetil in Combination with Tacrolimus: A Propensity Score-matched Analysis in Liver Transplantation. Transplant. Proc. 2018, 50, 3615–3620. [Google Scholar]
- Bradbury, C.A.; Pell, J.; Hill, Q.; Bagot, C.; Cooper, N.; Ingram, J.; Breheny, K.; Kandiyali, R.; Rayment, R.; Evans, G.; et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. New Engl. J. Med. 2021, 385, 885–895. [Google Scholar]
| Variables | A*/A* (n = 22) | A*/GG (n = 20) | GG/A* (n = 31) | GG/GG (n = 27) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 54.09 ± 9.78 | 51.75 ± 10.28 | 51.71 ± 11.21 | 52.04 ± 12.22 | 0.875 |
| Gender | 0.665 | ||||
| Male (n, %) | 17 (77.3%) | 18 (90%) | 24 (77.4%) | 23 (85.2%) | |
| Female (n, %) | 5 (22.7%) | 2 (10%) | 7 (22.6%) | 4 (14.8%) | |
| BMI (kg/m2) | 23.29 ± 3.74 | 23.13 ± 4.52 | 23.22 ± 5.95 | 23.42 ± 3.44 | 0.996 |
| Etiology of liver disease | |||||
| HCC (n, %) | 12 (54.5%) | 12 (60.0%) | 12 (38.7%) | 10 (37.0%) | |
| HBV cirrhosis (n, %) | 4 (18.2%) | 5 (25%) | 11 (35.5%) | 8 (29.6%) | |
| Alcoholic cirrhosis (n, %) | 2 (9.1%) | 2 (10%) | 5 (16.1%) | 5 (18.5%) | |
| ALD (n, %) | 3 (13.6%) | 1 (5%) | 2 (6.5%) | 3 (11.1%) | |
| Other (n, %) | 1 (4.5%) | 0 (0.0%) | 1 (3.2%) | 1 (3.7%) | |
| Platelet (×109/L) | 80.53 ± 43.39 | 78.30 ± 53.81 | 77.81 ± 49.23 | 88.78 ± 62.59 | 0.888 |
| WBC (×109/L) | 3.21 ± 0.55 | 3.68 ± 0.39 | 2.96 ± 1.07 | 3.15 ± 0.92 | 0.348 |
| Hb (g/dL) | 10.55 ± 1.88 | 11.10 ± 1.72 | 10.69 ± 1.28 | 9.98 ± 1.36 | 0.683 |
| INR (IQR) | 1.36 (0.92) | 1.33 (0.61) | 1.48 (0.57) | 1.48 (0.62) | 0.736 |
| ALT (U/L) | 68.74 ± 52.35 | 39.89 ± 47.51 | 60.03 ± 64.58 | 88.32 ± 21.54 | 0.635 |
| AST (U/L) | 87.44 ± 28.60 | 61.11 ± 27.60 | 82.63 ± 21.31 | 88.41 ± 25.33 | 0.875 |
| TB (mg/dL) | 7.46 ± 2.39 | 6.22 ± 2.07 | 12.59 ± 2.58 | 10.54 ± 2.29 | 0.307 |
| Albumin (g/dL) | 3.28 ± 0.52 | 3.69 ± 1.08 | 3.77 ± 0.62 | 3.33 ± 0.69 | 0.473 |
| Creatinine (mg/dL) | 1.08 ± 0.18 | 1.17 ± 0.38 | 0.95 ± 0.45 | 1.05 ± 0.39 | 0.413 |
| Spleen size (mm) | 173.36 ± 11.42 | 179.45 ± 10.08 | 173.32 ± 9.46 | 179.29 ± 11.84 | 0.055 |
| PVT (n, %) | 2 (9.1%) | 1 (5.0%) | 3 (9.7%) | 2 (7.4%) | 0.458 |
| MELD (n, %) | 14.23 ± 8.35 | 13.10 ± 7.39 | 16.58 ± 11.52 | 16.89 ± 10.94 | 0.051 |
| Child–Pugh (IQR) | 8 (3) | 9 (3) | 8 (2) | 8 (3) | 0.380 |
| Complications in 180 days after DDLT | |||||
| Infection (n, %) | 2 (9.1%) | 0 (0.0%) | 2 (6.5%) | 6 (22.2%) | 0.162 |
| Graft rejection (n, %) | 1 (4.5%) | 0 (0.0%) | 1 (3.2%) | 1 (3.7%) | 0.509 |
| Biliary leak (n, %) | 2 (9.1%) | 3 (15.0%) | 8 (25.8%) | 7 (25.9%) | 0.383 |
| Thromboembolism (n, %) | 1 (4.5%) | 2 (10.0%) | 3 (9.7%) | 4 (14.8%) | 0.394 |
| Hemorrhage (n, %) | 1 (4.5%) | 1 (5.0%) | 2 (6.5%) | 6 (22.2%) * | 0.011 |
| A*/A* | A*/GG | GG/A* | GG/GG | p-Value | |
|---|---|---|---|---|---|
| Platelet count (×109/L) | |||||
| POD3 | 71.00 ± 6.22 | 57.95 ± 6.21 | 75.90 ± 5.56 | 50.29 ± 5.44 | 0.006 |
| POD7 | 95.23 ± 11.24 | 104.00 ± 14.52 | 96.77 ± 9.74 | 81.44 ± 9.69 | 0.547 |
| POD28 | 168.55 ± 14.89 | 172.85 ± 18.73 | 172.03 ± 14.86 | 149.81 ± 12.34 | 0.656 |
| Blood concentration of tacrolimus (ng/mL) | |||||
| POD3 | 3.22 ± 1.85 | 5.59 ± 3.35 | 4.53 ± 2.72 | 6.22 ± 2.93 | 0.003 |
| POD7 | 3.29 ± 1.74 | 5.80 ± 2.55 | 5.18 ± 2.19 | 6.24 ± 2.93 | 0.000 |
| POD28 | 9.02 ± 5.09 | 7.69 ± 2.96 | 7.70 ± 3.55 | 8.97 ± 3.77 | 0.455 |
| Tacrolimus dosage (mg/d) | |||||
| POD3 | 2.77 ± 0.69 | 2.80 ± 0.83 | 3.00 ± 1.06 | 2.48 ± 1.34 | 0.313 |
| POD7 | 3.68 ± 1.21 | 3.47 ± 1.16 | 4.16 ± 1.18 | 2.70 ± 1.46 | 0.000 |
| POD28 | 4.57 ± 1.54 | 3.83 ± 1.69 | 4.43 ± 1.41 | 3.48 ± 1.76 | 0.056 |
| Risk Factors | β | SE | Wald Value | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Spleen size (mm) | ||||||
| >180 vs. 120–150 | 1.77 | 1.55 | 1.302 | 5.89 | 0.28–123.74 | 0.254 |
| 150–180 vs. 120–150 | 1.24 | 1.46 | 0.712 | 3.46 | 0.194–61.57 | 0.399 |
| Blood concentration of tacrolimus (ng/mL) | ||||||
| >10 vs. <5 | 20.62 | 0.00 | 0.00 | 99.81 | 89.81–169.88 | 0.000 |
| 5–10 vs. <5 | 2.54 | 1.17 | 4.73 | 12.72 | 1.29–125.86 | 0.030 |
| CYP3A5 (donor/recipient) GG/GG | 0.477 | 1.05 | 0.21 | 1.61 | 0.21–12.49 | 0.648 |
| Use anticoagulation | 0.18 | 0.05 | 0.05 | 0.84 | 0.18–3.85 | 0.817 |
| Creatinine ≥ 1.0 mg/dL | 1.09 | 0.85 | 1.65 | 2.98 | 0.56–15.75 | 0.198 |
| Child–Pugh ≥ 7 | 0.58 | 0.78 | 0.66 | 0.56 | 0.14–2.28 | 0.418 |
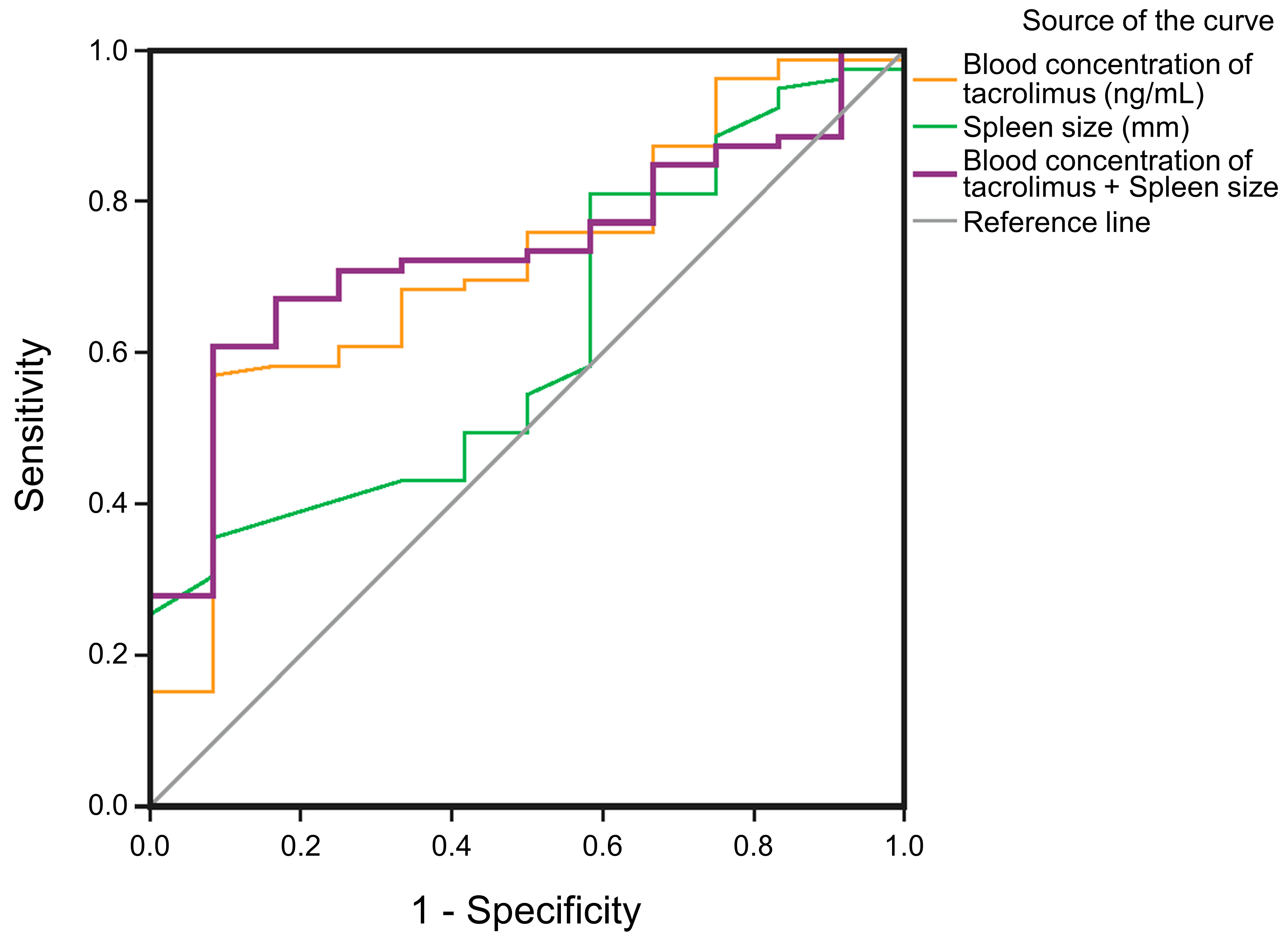
| Variable | Spleen Size (mm) | Blood Concentration of Tacrolimus (ng/mL) | Spleen Size + Blood Concentration of Tacrolimus |
|---|---|---|---|
| AUC | 0.617 | 0.719 | 0.735 |
| Cut-off value | 166.50 | 4.74 | 0.82 # |
| Sensitivity | 81.0% | 57.0% | 77.2% |
| Specificity | 41.7% | 91.7% | 41.7% |
| p-value | 0.195 | 0.015 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Chen, Q.; Liu, J.; Li, S.; Wang, H.; Tang, R.; Zhang, Z. Effects of CYP3A5 Genotypes on Thrombocytopenia in Liver Transplantation Patients Treated with Tacrolimus. Biomedicines 2023, 11, 3088. https://doi.org/10.3390/biomedicines11113088
Guo Z, Chen Q, Liu J, Li S, Wang H, Tang R, Zhang Z. Effects of CYP3A5 Genotypes on Thrombocytopenia in Liver Transplantation Patients Treated with Tacrolimus. Biomedicines. 2023; 11(11):3088. https://doi.org/10.3390/biomedicines11113088
Chicago/Turabian StyleGuo, Zhe, Qi Chen, Juan Liu, Shan Li, He Wang, Rui Tang, and Zhenyu Zhang. 2023. "Effects of CYP3A5 Genotypes on Thrombocytopenia in Liver Transplantation Patients Treated with Tacrolimus" Biomedicines 11, no. 11: 3088. https://doi.org/10.3390/biomedicines11113088
APA StyleGuo, Z., Chen, Q., Liu, J., Li, S., Wang, H., Tang, R., & Zhang, Z. (2023). Effects of CYP3A5 Genotypes on Thrombocytopenia in Liver Transplantation Patients Treated with Tacrolimus. Biomedicines, 11(11), 3088. https://doi.org/10.3390/biomedicines11113088







