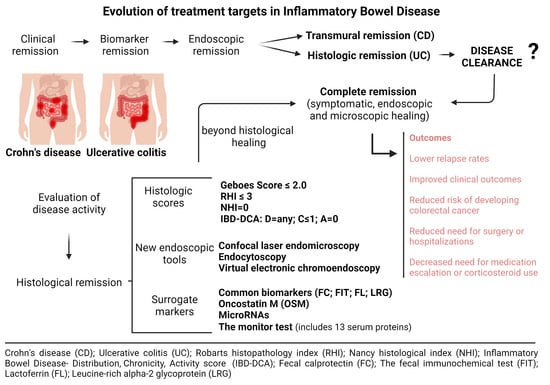
Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing
Abstract
:1. Introduction
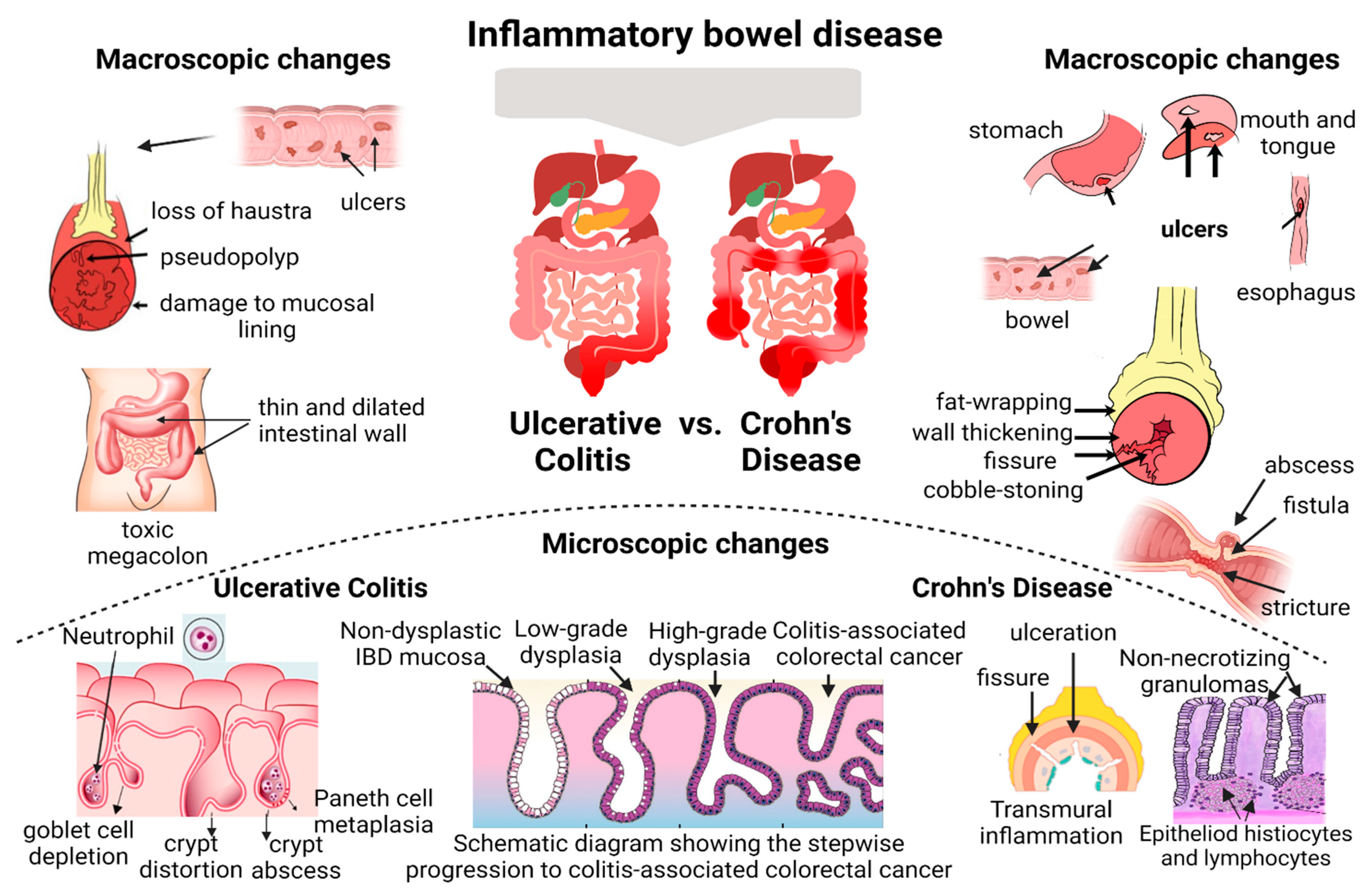
2. Histological Healing—Current Concept and Clinical Relevance
3. Currently Available Diagnostic Tools
3.1. Histological Healing Scoring Systems—Endoscopic Biopsies
| Author | Score/Index | Year of Publication | Comments | Items |
|---|---|---|---|---|
| Histological scoring systems in ulcerative colitis | ||||
| Geboes et al. [34]; Jauregui-Amezaga, A et al. [35] | Original and Simplified Geboes score | 2000; 2017 | The main limitation—both scores have not been fully validated Reproducible grading system Histological remission is defined as GS ≤ 6.0 GS ≤ 2.0 | Simplified Geboes score Grade 0: No inflammatory activity Grade 1: Basal plasma cells Grade 2A: Eosinophils in lamina propria Grade 2B: Neutrophils in lamina propria Grade 3: Neutrophils in epithelium Grade 4: Epithelial injury (in crypt and surface epithelium) |
| Gupta et al. [43] | Harpaz score | 2007 | Partially validated | Grade 0: no cryptitis Grade 1: cryptitis < 50% crypts Grade 2: cryptitis > 50% crypts Grade 3: ulcerations or erosions |
| Marchal-Bressenot et al. [38] | Nancy histological index (NHI) | 2015 | Validated and widely used in clinical practice Correlation between the Nancy index and the Geboes index is very good Histological remission defined as NHI = 0 | Grade 0: no histological significant disease Grade 1: chronic inflammatory infiltrate with no acute inflammatory infiltrate Grade 2: mildly active disease Grade 3: moderately active disease Grade 4: severely active disease |
| Mosli et al. [40] | Robarts histopathology index (RHI) | 2017 | New validated histopathological index Based on Geboes index and modified Riley index Histological remission defined as RHI ≤ 3 | Chronic inflammatory infiltrate 0 = no increase; 1 = mild but unequivocal increase; 2 = moderate increase; 3 = marked increase Lamina propria neutrophils 0 = none; 1 = mild but unequivocal increase; 2 = moderate increase; 3 = marked increase. Neutrophils in epithelium 0 = none; 1 = 50% crypts involved. Erosion or ulceration 0 = no erosion, ulceration, or granulation of tissue; 1 = recovering epithelium + adjacent inflammation; 2 = probable erosion focally stripped; 3 = unequivocal erosion; 4 = ulcer or granulation of tissue |
| Histological scoring systems in Crohn’s disease | ||||
| D’Haens et al. [42] | Global Histology Activity (GHAS) Score | 1998 | Not formally validated The only one used on a larger scale. GHAS score ≥ 10 indicates severe histological activity | Epithelial damage 0 = normal; 1 = focal; 2 = extensive architectural changes 0 = normal; 1 = moderate; 2 = severe mononuclear cells in lamina propria 0 = normal; 1 = moderate increase; 2 = severe increase Neutrophils in lamina propria 0 = normal; 1 = moderate increase; 2 = severe increase Neutrophils in epithelium 1 = surface epithelium; 2 = cryptitis; 3 = crypt abscess Erosion or ulceration 0 = no; 1 = yes Granuloma 0 = no; 1 = yes Number of segmental biopsy specimens affected 1 = < 1/3; 2 = 1/3–2/3; 3 = > 2/3 |
| Histological scoring systems in ulcerative colitis and Crohn’s disease | ||||
| Lang-Schwarz et al. [41] | IBD-DCA | 2021 | Common scoring available for UC and CD Validated by a large group of IBD specialists Provides reliable information on treatment response | Distribution 0 = normal; 1 = < 50% of tissue affected per same biopsy site; 2 = > 50% of tissue affected per same biopsy Chronicity 0 = normal; 1 = crypt distortion and/or mild lymphoplasmacytosis; 2 = marked lymphoplasmacytosis and/or basal plasmacytosis Activity 0 = normal; 1 = two or more neutrophils in lamina propria in one high-power field and/or any presence of intraepithelial neutrophils; 2 = crypt abscesses, erosions, ulcers |
3.2. New Endoscopic Tools
4. Surrogate Markers for Histological Healing
4.1. Common Biomarkers Predicting Histological Healing
4.2. Novel Biomarkers Predicting Histological Healing
5. Future Directions—Beyond Histological Healing in IBD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Si, X.; Yang, L.; Wang, H.; Sun, Y.; Liu, N. Association between intestinal microbiota and inflammatory bowel disease. Anim. Models Exp. Med. 2022, 5, 311–322. [Google Scholar] [CrossRef]
- Fabian, O.; Bajer, L. Histopathological assessment of the microscopic activity in inflammatory bowel diseases: What are we looking for? World J. Gastroenterol. 2022, 28, 5300–5312. [Google Scholar] [CrossRef]
- Chateau, T.; Feakins, R.; Marchal-Bressenot, A.; Magro, F.; Danese, S.; Peyrin-Biroulet, L. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am. J. Gastroenterol. 2020, 115, 179–189. [Google Scholar] [PubMed]
- Ma, C.; Sedano, R.; Almradi, A.; Vande Casteele, N.; Parker, C.E.; Guizzetti, L.; Schaeffer, D.F.; Riddell, R.H.; Pai, R.K.; Battat, R.; et al. An International Consensus to Standardize Integration of Histopathology in Ulcerative Colitis Clinical Trials. Gastroenterology 2021, 160, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Roda, G.; Peyrin-Biroulet, L. Evolving therapeutic goals in ulcerative colitis: Towards disease clearance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; D’haens, G.; Lee, W.J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2020, 14, 254–266. [Google Scholar] [CrossRef]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, L.; Riis, L.B. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig. Med. Res. 2021, 4, 3. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar]
- Agrawal, M.; Colombel, J.F. Treat-to-Target in Inflammatory Bowel Diseases, What Is the Target and How Do We Treat? Gastrointest. Endosc. Clin. N. Am. 2019, 29, 421–436. [Google Scholar] [CrossRef]
- Kim, K.O. Endoscopic activity in inflammatory bowel disease: Clinical significance and application in practice. Clin. Endosc. 2022, 55, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Atreya, R.; Neurath, M.F. Is histological healing a feasible endpoint in ulcerative colitis? Expert Rev. Gastroenterol. Hepatol. 2021, 15, 665–674. [Google Scholar] [CrossRef]
- Ponte, A.; Pinho, R.; Fernandes, S.; Rodrigues, A.; Alberto, L.; Silva, J.C.; Silva, J.; Rodrigues, J.; Sousa, M.; Silva, A.P.; et al. Impact of histological and endoscopic remissions on clinical recurrence and recurrence-free time in ulcerative colitis. Inflamm. Bowel Dis. 2017, 23, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Pai, R.K.; Hartman, D.J.; Rivers, C.R.; Regueiro, M.; Schwartz, M.; Binion, D.G.; Pai, R.K. Complete resolution of mucosal neutrophils associates with improved long-term clinical outcomes of patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2510–2517.e5. [Google Scholar] [CrossRef]
- Cushing, K.C.; Tan, W.; Alpers, D.H.; Deshpande, V.; Ananthakrishnan, A.N. Complete histologic normalisation is associated with reduced risk of relapse among patients with ulcerative colitis in complete endoscopic remission. Aliment. Pharmacol. Ther. 2020, 51, 347–355. [Google Scholar] [CrossRef]
- Kevans, D.; Kirsch, R.; Dargavel, C.; Kabakchiev, B.; Riddell, R.; Silverberg, M.S. Histological markers of clinical relapse in endoscopically quiescent ulcerative colitis. Inflamm. Bowel Dis. 2020, 26, 1722–1729. [Google Scholar] [CrossRef]
- Lobatón, T.; Bessissow, T.; Ruiz-Cerulla, A.; De Hertogh, G.; Bisschops, R.; Guardiola, J.; Van Assche, G.; Vermeire, S.; Ferrante, M. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: A prospective multicenter study. United Eur. Gastroenterol. J. 2018, 6, 765–772. [Google Scholar]
- Pai, R.K.; Jairath, V. What is the role of histopathology in the evaluation of disease activity in Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101601. [Google Scholar]
- Hu, A.B.; Tan, W.; Deshpande, V.; Ananthakrishnan, A.N. Ileal or Colonic Histologic Activity Is Not Associated with Clinical Relapse in Patients with Crohn’s Disease in Endoscopic Remission. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2021, 19, 1226–1233.e1. [Google Scholar] [CrossRef]
- Villanacci, V.; Baert, F.; Cornillie, F.; De Hertogh, G.; Panés, J. Challenges Faced by Cross-sectional Imaging and Histological Endpoints in Clinical Trials. J. Crohn’s Colitis 2017, 11 (Suppl. S2), S586–S592. [Google Scholar] [CrossRef]
- Park, S.; Abdi, T.; Gentry, M.; Laine, L. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients with Ulcerative Colitis: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2016, 111, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Narang, V.; Kaur, R.; Garg, B.; Mahajan, R.; Midha, V.; Sood, N.; Sood, A. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intest. Res. 2018, 16, 55–61. [Google Scholar] [CrossRef]
- Ozaki, R.; Kobayashi, T.; Okabayashi, S.; Nakano, M.; Morinaga, S.; Hara, A.; Ohbu, M.; Matsuoka, K.; Toyonaga, T.; Saito, E.; et al. Histological Risk Factors to Predict Clinical Relapse in Ulcerative Colitis with Endoscopically Normal Mucosa. J. Crohn’s Colitis 2018, 12, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Burger, D.C.; Delo, J.; Walsh, A.J.; Thomas, S.; von Herbay, A.; Buchel, O.C.; White, L.; Brain, O.; Keshav, S.; et al. Beyond endoscopic mucosal healing in UC: Histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016, 65, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bessissow, T.; Lemmens, B.; Ferrante, M.; Bisschops, R.; Van Steen, K.; Geboes, K.; Van Assche, G.; Vermeire, S.; Rutgeerts, P.; De Hertogh, G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am. J. Gastroenterol. 2012, 107, 1684–1692. [Google Scholar] [CrossRef]
- Calafat, M.; Lobatón, T.; Hernández-Gallego, A.; Mañosa, M.; Torres, P.; Cañete, F.; Cabré, E.; Ojanguren, I.; Domènech, E. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2017, 49, 1327–1331. [Google Scholar] [CrossRef]
- Christensen, B.; Erlich, J.; Gibson, P.R.; Turner, J.R.; Hart, J.; Rubin, D.T. Histologic Healing Is More Strongly Associated with Clinical Outcomes in Ileal Crohn’s Disease than Endoscopic Healing. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 2518–2525.e1. [Google Scholar] [CrossRef]
- Brennan, G.T.; Melton, S.D.; Spechler, S.J.; Feagins, L.A. Clinical Implications of Histologic Abnormalities in Ileocolonic Biopsies of Patients with Crohn’s Disease in Remission. J. Clin. Gastroenterol. 2017, 51, 43–48. [Google Scholar] [CrossRef]
- Flores, B.M.; O’Connor, A.; Moss, A.C. Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 1006–1111.e8. [Google Scholar]
- Pai, R.K.; Lauwers, G.Y.; Pai, R.K. Measuring Histologic Activity in Inflammatory Bowel Disease: Why and How. Adv. Anat. Pathol. 2022, 29, 37–47. [Google Scholar] [CrossRef]
- Truelove, S.C.; Richards, W.C. Biopsy studies in ulcerative colitis. Br. Med. J. 1956, 1, 1315–1318. [Google Scholar] [CrossRef]
- Novak, G.; Parker, C.E.; Pai, R.K.; MacDonald, J.K.; Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Jairath, V.; Khanna, R. Histologic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst. Rev. 2017, 7, CD012351. [Google Scholar] [CrossRef]
- Mosli, M.H.; Parker, C.E.; Nelson, S.A.; Baker, K.A.; MacDonald, J.K.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Levesque, B.G.; Jairath, V. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2017, 5, CD011256. [Google Scholar] [CrossRef]
- Geboes, K. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef]
- Jauregui-Amezaga, A.; Geerits, A.; Das, Y.; Lemmens, B.; Sagaert, X.; Bessissow, T.; Lobatón, T.; Ferrante, M.; Van Assche, G.; Bisschops, R.; et al. A Simplified Geboes Score for Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Vespa, E.; D’Amico, F.; Sollai, M.; Allocca, M.; Furfaro, F.; Zilli, A.; Dal Buono, A.; Gabbiadini, R.; Danese, S.; Fiorino, G. Histological Scores in Patients with Inflammatory Bowel Diseases: The State of the Art. J. Clin. Med. 2022, 11, 939. [Google Scholar] [CrossRef]
- Neri, B.; Mossa, M.; Scucchi, L.; Sena, G.; Palmieri, G.; Biancone, L. Histological scores in inflammatory bowel disease. J. Dig. Dis. 2021, 22, 9–22. [Google Scholar] [CrossRef]
- Marchal-Bressenot, A.; Salleron, J.; Boulagnon-Rombi, C.; Bastien, C.; Cahn, V.; Cadiot, G.; Diebold, M.D.; Danese, S.; Reinisch, W.; Schreiber, S.; et al. Development and validation of the Nancy histological index for UC. Gut 2017, 66, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Doherty, G.; Peyrin-Biroulet, L.; Svrcek, M.; Borralho, P.; Walsh, A.; Carneiro, F.; Rosini, F.; de Hertogh, G.; Biedermann, L.; et al. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J. Crohn’s Colitis 2020, 14, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Feagan, B.G.; Zou, G.; Sandborn, W.J.; D’Haens, G.; Khanna, R.; Shackelton, L.M.; Walker, C.W.; Nelson, S.; Vandervoort, M.K.; et al. Development and validation of a histological index for UC. Gut 2015, 66, 50–58. [Google Scholar] [CrossRef]
- Lang-Schwarz, C.; Angeloni, M.; Agaimy, A.; Atreya, R.; Becker, C.; Dregelies, T.; Danese, S.; Fléjou, J.F.; Gaßler, N.; Grabsch, H.I.; et al. Validation of the ‘Inflammatory Bowel Disease-Distribution, Chronicity, Activity [IBD-DCA] Score’ for Ulcerative Colitis and Crohn’s Disease. J. Crohn’s Colitis 2021, 15, 1621–1630. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Geboes, K.; Peeters, M.; Baert, F.; Penninckx, F.; Rutgeerts, P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998, 114, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic Inflammation Is a Risk Factor for Progression to Colorectal Neoplasia in Ulcerative Colitis: A Cohort Study. Gastroenterology 2007, 133, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.R.Z.U.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Nunes, P.B.; Cathomas, G.; et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Pouw, R.E.; Bisschops, R.; Gecse, K.B.; de Hertogh, G.; Iacucci, M.; Rutter, M.; Barret, M.; Biermann, K.; Czakó, L.; Hucl, T.; et al. Endoscopic tissue sampling—Part 2: Lower gastrointestinal tract. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1261–1273. [Google Scholar] [CrossRef]
- Gupta, A.; Yu, A.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Treat to target: The role of histologic healing in inflammatory bowel diseases, a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2020, 19, 1800–1813. [Google Scholar] [CrossRef]
- Magro, F.; Sabino, J.; Rosini, F.; Tripathi, M.; Borralho, P.; Baldin, P.; Danese, S.; Driessen, A.; Gordon, I.O.; Iacucci, M.; et al. ECCO Position on Harmonisation of Crohn’s Disease Mucosal Histopathology. J. Crohn’s Colitis 2022, 16, 876–883. [Google Scholar] [CrossRef]
- Alfarone, L.; Parigi, T.L.; Gabbiadini, R.; Dal Buono, A.; Spinelli, A.; Hassan, C.; Iacucci, M.; Repici, A.; Armuzzi, A. Technological advances in inflammatory bowel disease endoscopy and histology. Front. Med. 2022, 9, 1058875. [Google Scholar] [CrossRef]
- Rasmussen, D.N.; Karstensen, J.G.; Riis, L.B.; Brynskov, J.; Vilmann, P. Confocal Laser Endomicroscopy in Inflammatory Bowel Disease--A Systematic Review. J. Crohn’s Colitis 2015, 9, 1152–1159. [Google Scholar] [CrossRef]
- Buchner, A.M. Confocal Laser Endomicroscopy in the Evaluation of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1302–1312. [Google Scholar] [CrossRef]
- Rahmi, G.; Coron, E.; Perrod, G.; Levy, M.; Moreau, J.; Moussata, D.; Perez-Cuadrado-Robles, E.; Chupin, A.; Quénéhervé, L.; Bourreille, A.; et al. Probe-based Confocal Laser Endomicroscopy for In Vivo Assessment of Histological Healing in Ulcerative Colitis: Development and Validation of the ENHANCE Index. J. Crohn’s Colitis 2021, 15, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Karstensen, J.G.; Săftoiu, A.; Brynskov, J.; Hendel, J.; Ciocalteu, A.; Klausen, P.; Klausen, T.W.; Riis, L.B.; Vilmann, P. Confocal laser endomicroscopy in ulcerative colitis: A longitudinal study of endomicroscopic changes and response to medical therapy (with videos). Gastrointest. Endosc. 2016, 84, 279–286.e1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Xie, X.J.; Yu, T.; Gu, X.M.; Zuo, X.L.; Zhou, C.J.; Huang, W.Q.; Chen, H.; Li, Y.Q. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am. J. Gastroenterol. 2010, 105, 1391–1396. [Google Scholar] [CrossRef]
- Maione, F.; Giglio, M.C.; Luglio, G.; Rispo, A.; D’Armiento, M.; Manzo, B.; Cassese, G.; Schettino, P.; Gennarelli, N.; Siciliano, S.; et al. Confocal laser endomicroscopy in ulcerative colitis: Beyond endoscopic assessment of disease activity. Tech. Coloproctology 2017, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tontini, G.E.; Mudter, J.; Vieth, M.; Atreya, R.; Günther, C.; Zopf, Y.; Wildner, D.; Kiesslich, R.; Vecchi, M.; Neurath, M.F.; et al. Confocal laser endomicroscopy for the differential diagnosis of ulcerative colitis and Crohn’s disease: A pilot study. Endoscopy 2015, 47, 437–443. [Google Scholar] [CrossRef]
- Nardone, O.M.; Cannatelli, R.; Zardo, D.; Ghosh, S.; Iacucci, M. Can advanced endoscopic techniques for assessment of mucosal inflammation and healing approximate histology in inflammatory bowel disease? Ther. Adv. Gastroenterol. 2019, 12, 1756284819863015. [Google Scholar] [CrossRef]
- Bessho, R.; Kanai, T.; Hosoe, N.; Kobayashi, T.; Takayama, T.; Inoue, N.; Mukai, M.; Ogata, H.; Hibi, T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J. Gastroenterol. 2011, 46, 1197–1202. [Google Scholar] [CrossRef]
- Nakazato, Y.; Naganuma, M.; Sugimoto, S.; Bessho, R.; Arai, M.; Kiyohara, H.; Ono, K.; Nanki, K.; Mutaguchi, M.; Mizuno, S.; et al. Endocytoscopy can be used to assess histological healing in ulcerative colitis. Endoscopy 2017, 49, 560–563. [Google Scholar] [CrossRef]
- Vitali, F.; Morgenstern, N.; Eckstein, M.; Atreya, R.; Waldner, M.; Hartmann, A.; Neurath, M.F.; Rath, T. Endocytoscopy for assessing histologic inflammation in ulcerative colitis: Development and prospective validation of the ELECT (ErLangen Endocytoscopy in ColiTis) score (with videos). Gastrointest. Endosc. 2023, 97, 100–111.e1. [Google Scholar] [CrossRef]
- Maeda, Y.; Kudo, S.E.; Mori, Y.; Misawa, M.; Ogata, N.; Sasanuma, S.; Wakamura, K.; Oda, M.; Mori, K.; Ohtsuka, K. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest. Endosc. 2019, 89, 408–415. [Google Scholar] [CrossRef]
- Iacucci, M.; Jeffery, L.; Acharjee, A.; Nardone, O.M.; Zardo, D.; Smith, S.C.L.; Bazarova, A.; Cannatelli, R.; Shivaji, U.N.; Williams, J.; et al. Ultra-high Magnification Endocytoscopy and Molecular Markers for Defining Endoscopic and Histologic Remission in Ulcerative Colitis-An Exploratory Study to Define Deep Remission. Inflamm. Bowel Dis. 2021, 27, 1719–1730. [Google Scholar] [CrossRef]
- Nardone, O.M.; Cannatelli, R.; Ghosh, S.; Iacucci, M. New endoscopic tools in inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Daperno, M.; Lazarev, M.; Arsenascu, R.; Tontini, G.E.; Akinola, O.; Gui, X.S.; Villanacci, V.; Goetz, M.; Lowerison, M.; et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: The PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest. Endosc. 2017, 86, 1118–1127.e5. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Smith, S.C.L.; Bazarova, A.; Shivaji, U.N.; Bhandari, P.; Cannatelli, R.; Daperno, M.; Ferraz, J.; Goetz, M.; Gui, X.; et al. An International Multicenter Real-Life Prospective Study of Electronic Chromoendoscopy Score PICaSSO in Ulcerative Colitis. Gastroenterology 2021, 160, 1558–1569.e8. [Google Scholar] [CrossRef] [PubMed]
- Nardone, O.M.; Bazarova, A.; Bhandari, P.; Cannatelli, R.; Daperno, M.; Ferraz, J.; Goetz, M.; Gui, X.; Hayee, B.; De Hertogh, G.; et al. PICaSSO virtual electronic chromendoscopy accurately reflects combined endoscopic and histological assessment for prediction of clinical outcomes in ulcerative colitis. United Eur. Gastroenterol. J. 2022, 10, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Nardone, O.M.; Snir, Y.; Hodson, J.; Cannatelli, R.; Labarile, N.; Siau, K.; Hassan, C.; Yanai, H.; Dotan, I.; Ghosh, S.; et al. Advanced technology for assessment of endoscopic and histological activity in ulcerative colitis: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221092594. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Cannatelli, R.; Parigi, T.L.; Nardone, O.M.; Tontini, G.E.; Labarile, N.; Buda, A.; Rimondi, A.; Bazarova, A.; Bisschops, R.; et al. A virtual chromoendoscopy artificial intelligence system to detect endoscopic and histologic activity/remission and predict clinical outcomes in ulcerative colitis. Endoscopy 2023, 55, 332–341. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Colombel, J.F.; Sandborn, W.J.; Marshall, J.K.; Daperno, M.; Reinisch, W.; Dulai, P.S. Predicting endoscopic remission in Crohn’s disease by the modified multiplier SES-CD (MM-SES-CD). Gut 2022, 71, 1078–1087. [Google Scholar] [CrossRef]
- Vinsard, D.G.; Fetzer, J.; Raffals, L.H.; Agrawal, U.; Singh, J.; Patel, M.H.; Avvaru, H.K.; Kaur, M.P.; Lahori, S.; Sampath, S.; et al. Development of an artificial intelligence tool for detection of polypoid lesions in inflammatory bowel disease (IBD-CADE). Gastrointest. Endosc. 2022, 95, AB220–AB221. [Google Scholar] [CrossRef]
- Wagatsuma, K.; Yokoyama, Y.; Nakase, H. Role of Biomarkers in the Diagnosis and Treatment of Inflammatory Bowel Disease. Life 2021, 11, 1375. [Google Scholar] [CrossRef]
- Dragoni, G.; Innocenti, T.; Galli, A. Biomarkers of Inflammation in Inflammatory Bowel Disease: How Long before Abandoning Single-Marker Approaches? Dig. Dis. 2021, 39, 190–203. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, G.; Lin, J.; Li, L.; Zeng, Z.; Chen, M.; Zhang, S. Serum Biomarkers for Inflammatory Bowel Disease. Front. Med. 2020, 7, 123. [Google Scholar] [CrossRef]
- Dai, C.; Jiang, M.; Sun, M.J.; Cao, Q. Fecal immunochemical test for predicting mucosal healing in ulcerative colitis patients: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2018, 33, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.G.; Kim, H.W.; Park, S.B.; Kang, D.H.; Choi, C.W.; Kim, S.J.; Nam, H.S. Clinical implications of fecal calprotectin and fecal immunochemical test on mucosal status in patients with ulcerative colitis. Medicine 2019, 98, e17080. [Google Scholar] [CrossRef]
- Shi, H.Y.; Chan, F.K.L.; Chan, A.W.H.; Higashimori, A.; Kyaw, M.; Ching, J.Y.L.; Luk, A.K.C.; Wong, S.H.; Wu, J.C.Y.; Sung, J.J.Y.; et al. Accuracy of Faecal Immunochemical Test to Predict Endoscopic and Histological Healing in Ulcerative Colitis: A Prospective Study Based on Validated Histological Scores. J. Crohn’s Colitis 2017, 11, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Panchal, H.; Dubinsky, M.C. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1600–1604. [Google Scholar] [CrossRef]
- D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Review article: Faecal calprotectin and histologic remission in ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Kormilitzin, A.; Hinds, C.; Sexton, V.; Brain, O.; Keshav, S.; Uhlig, H.; Geddes, J.; Goodwin, G.; Peters, M.; et al. Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis-a Prospective Analysis. J. Crohn’s Colitis 2019, 13, 424–430. [Google Scholar] [CrossRef]
- Hart, L.; Chavannes, M.; Kherad, O.; Maedler, C.; Mourad, N.; Marcus, V.; Afif, W.; Bitton, A.; Lakatos, P.L.; Brassard, P.; et al. Faecal Calprotectin Predicts Endoscopic and Histological Activity in Clinically Quiescent Ulcerative Colitis. J. Crohn’s Colitis 2020, 14, 46–52. [Google Scholar] [CrossRef]
- Langhorst, J.; Kairey, L.; Oberle, A.; Boone, J.; Dobos, G.; Juette, H.; Tannapfel, A.; Rueffer, A. Assessing Histological Inflammatory Activity in Patients with Ulcerative Colitis: A Diagnostic Accuracy Study Testing Fecal Biomarkers Lactoferrin and Calprotectin. Crohn’s Colitis 360 2020, 2, otaa053. [Google Scholar] [CrossRef]
- Yasutomi, E.; Inokuchi, T.; Hiraoka, S.; Takei, K.; Igawa, S.; Yamamoto, S.; Ohmori, M.; Oka, S.; Yamasaki, Y.; Kinugasa, H.; et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci. Rep. 2021, 11, 11086. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.; Mohamed, H.; Abo Halima, A.; Elshaarawy, M.; Khedr, A. Changes in Serum Oncostatin M Levels during Treatment of Inflammatory Bowel Disease. Egypt. J. Hosp. Med. 2022, 89, 7217-7125. [Google Scholar] [CrossRef]
- Verdier, J.; Breunig, I.R.; Ohse, M.C.; Roubrocks, S.; Kleinfeld, S.; Roy, S.; Streetz, K.; Trautwein, C.; Roderburg, C.; Sellge, G. Faecal Micro-RNAs in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2020, 14, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Bubier, J.A.; Wiles, M.V.; Shultz, L.D.; Amiji, M.M.; Hosur, V. Role of MicroRNA in Inflammatory Bowel Disease: Clinical Evidence and the Development of Preclinical Animal Models. Cells 2021, 10, 2204. [Google Scholar] [CrossRef]
- Bertani, L.; Baglietto, L.; Antonioli, L.; Fornai, M.; Tapete, G.; Albano, E.; Ceccarelli, L.; Mumolo, M.G.; Pellegrini, C.; Lucenteforte, E.; et al. Assessment of serum cytokines predicts clinical and endoscopic outcomes to vedolizumab in ulcerative colitis patients. Br. J. Clin. Pharmacol. 2020, 86, 1296–1305. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Abreu, M.T.; Dubinsky, M.C. A Noninvasive Method to Assess Mucosal Healing in Patients* with Crohn’s Disease. Gastroenterol. Hepatol. 2018, 14 (Suppl. S2), 1–12. [Google Scholar]
- Schoepfer, A.M.; Vavricka, S.; Zahnd-Straumann, N.; Straumann, A.; Beglinger, C. Monitoring inflammatory bowel disease activity: Clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J. Crohn’s Colitis 2012, 6, 412–418. [Google Scholar] [CrossRef]
- Santiago, P.; Braga-Neto, M.B.; Loftus, E.V. Novel Therapies for Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 453–465. [Google Scholar]
- Abdulla, M.; Mohammed, N. A Review on Inflammatory Bowel Diseases: Recent Molecular Pathophysiology Advances. Biol. Targets Ther. 2022, 16, 129–140. [Google Scholar] [CrossRef]
- Ramos, L.; Teo-Loy, J.; Barreiro-de Acosta, M. Disease clearance in ulcerative colitis: Setting the therapeutic goals for future in the treatment of ulcerative colitis. Front. Med. 2023, 9, 1102420. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Fiorino, G.; Solitano, V.; Massarini, E.; Guillo, L.; Allocca, M.; Furfaro, F.; Zilli, A.; Bonovas, S.; Magro, F.; et al. Ulcerative colitis: Impact of early disease clearance on long-term outcomes—A multicenter cohort study. United Eur. Gastroenterol. J. 2022, 10, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S., Jr.; Colombel, J.F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Danese, S.; Schreiber, S.; Loftus, E., Jr.; Colombel, J.F.; Peyrin-Biroulet, L.; Agboton, C.; Lindner, D.; Lirio, R.; Sands, B. P271 evolving targets in ulcerative colitis: Defining disease clearance in the VARSITY study. J. Crohn’s Colitis 2021, 15 (Suppl. S1), S305. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Determination of the Optimal Treatment Target in Ulcerative Colitis (VERDICT). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04259138 (accessed on 2 March 2023).
- Wilkens, R.; Novak, K.L.; Maaser, C.; Panaccione, R.; Kucharzik, T. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: Current status and future perspectives. Ther. Adv. Gastroenterol. 2021, 14, 17562848211006672. [Google Scholar] [CrossRef]
- Noor, N.M.; Sousa, P.; Paul, S.; Roblin, X. Early Diagnosis, Early Stratification, and Early Intervention to Deliver Precision Medicine in IBD. Inflamm. Bowel Dis. 2022, 28, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Albader, F.; Golovics, P.A.; Gonczi, L.; Bessissow, T.; Afif, W.; Lakatos, P.L. Therapeutic drug monitoring in inflammatory bowel disease: The dawn of reactive monitoring. World J. Gastroenterol. 2021, 27, 6231–6247. [Google Scholar] [CrossRef]
- Bojarski, C.; Waldner, M.; Rath, T.; Schürmann, S.; Neurath, M.F.; Atreya, R.; Siegmund, B. Innovative Diagnostic Endoscopy in Inflammatory Bowel Diseases: From High-Definition to Molecular Endoscopy. Front. Med. 2021, 8, 655404. [Google Scholar] [CrossRef]
- Marafini, I.; Monteleone, G. Precision Medicine in Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 12, 653924. [Google Scholar] [CrossRef]
| Study | Type of Study | Disease | N Patients | Endoscopic Activity | Histological Index | Outcome |
|---|---|---|---|---|---|---|
| Park et al. [21] | Systematic review and meta-analysis | UC | 1360 patients | Endoscopic remission | Truelove and Richards index; Riley index; Geboes score. Histological remission- present in 964 patients (71%). | 52% relative risk reduction in relapse/exacerbation for UC patients with histologic remission compared to histologic activity. |
| Narang et al. [22] | Prospective observational study | UC | 76 patients in clinical remission for at least 6 months. 46 patients with endoscopic remission included (Mayo score ≤ 1; 46/76, 60.5%), 1 year of follow-up. | Endoscopic remission | Geboes score; Histological remission in 67.3% (31/46) of patients, while 32.7% (15/46) with histologically active disease. | 87.1% (27/31) of patients with histological remission remained asymptomatic, while 12.9% (4/31) had relapsed. Among histologically active patients, 46.6% (7/15) sustained clinical remission, while 53.3% (8/15) had relapsed. (87.1% vs. 46.6%, p = 0.006). |
| Ozaki et al. [23] | Prospective study | UC | 194 patients, 20 months of follow-up. | Endoscopic remission | NHI was significantly higher in MES 1 than in MES 0 [1.11 ± 0.09 vs. 0.41 ± 0.07, p < 0.0001]. | 67 patients relapsed during the follow-up period; risk of relapse (HR- 2.18 [1.16–5.82]; p = 0.03). |
| Bryant et al. [24] | Prospective study | UC | 91 patients, 6 years of follow-up. | Endoscopic remission | 24% of patients had persistent inflammation. | Histological remission predicted lower rates of corticosteroid use and acute severe colitis requiring hospitalization during follow-up (HR 0.42 (0.2 to 0.9), p = 0.02; HR 0.21 (0.1 to 0.7), p = 0.02, respectively). |
| Bessissow et al. [25] | Cohort study | UC | 75 patients, 12 months of follow-up | Endoscopic remission | Geboes score ≥3.1 in 40% and basal plasmacytosis in 21% of patients. | The presence of basal plasmacytosis, predictive of CR; OR = 5.13 (95% CI: 1.32–19.99), p = 0.019. |
| Calafat et al. [26] | Retrospective observational study | UC | 113 patients underwent dysplasia surveillance colonoscopy between January 2005 and October 2015; follow-up of 12 months was included. The median time of follow-up—2.5 years. | Endoscopic remission | 62 patients (57%) presented NQHA, 33 (30%) presented CHA, and 22 (20%) presented AHA. Basal plasmacytosis- present in 9 patients (8%), six of them in association with AHA (5%). | 9 patients (8%) relapsed within the first year of follow-up and 37 patients (33%) relapsed during the whole follow-up period. The presence of AHA is a risk factor for clinical relapse. |
| Christensen et al. [27] | Retrospective study | CD | 101 patients, follow-up for a median of 21 months. | 63% of patients with endoscopic remission. | 55% of patients achieved histologic remission. | CR occurred in 42% (n = 42) of patients Histologic healing was associated with a decreased risk of CR (HR- 2.05; 95% CI, 1.07–3.94; p = 0.031). |
| Brennan et al. [28] | Retrospective cohort study | CD | 62 patients, follow-up for at least 6 months. A total of 103 patients with CD underwent elective colonoscopies during clinical remission. | 55 patients (53%) in endoscopic healing, 48 patients (47%) with active disease. | A semiqualitative score (0 to 3) was assigned for the histologic characteristics in each of the biopsy samples. | At 12 months, the rate of relapse was 25.5% in patients with histologic activity, compared with only 2.4% of patients without histologic activity at baseline. The presence of histological activity was associated with higher flare rates (p < 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jucan, A.E.; Gavrilescu, O.; Dranga, M.; Popa, I.V.; Mihai, I.-R.; Mihai, V.-C.; Stefanescu, G.; Drug, V.L.; Prelipcean, C.C.; Vulpoi, R.-A.; et al. Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing. Biomedicines 2023, 11, 3090. https://doi.org/10.3390/biomedicines11113090
Jucan AE, Gavrilescu O, Dranga M, Popa IV, Mihai I-R, Mihai V-C, Stefanescu G, Drug VL, Prelipcean CC, Vulpoi R-A, et al. Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing. Biomedicines. 2023; 11(11):3090. https://doi.org/10.3390/biomedicines11113090
Chicago/Turabian StyleJucan, Alina Ecaterina, Otilia Gavrilescu, Mihaela Dranga, Iolanda Valentina Popa, Ioana-Ruxandra Mihai, Vasile-Claudiu Mihai, Gabriela Stefanescu, Vasile Liviu Drug, Cristina Cijevschi Prelipcean, Radu-Alexandru Vulpoi, and et al. 2023. "Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing" Biomedicines 11, no. 11: 3090. https://doi.org/10.3390/biomedicines11113090
APA StyleJucan, A. E., Gavrilescu, O., Dranga, M., Popa, I. V., Mihai, I.-R., Mihai, V.-C., Stefanescu, G., Drug, V. L., Prelipcean, C. C., Vulpoi, R.-A., Barboi, O.-B., Ciortescu, I., & Mihai, C. (2023). Evaluation of Disease Activity in Inflammatory Bowel Disease: Diagnostic Tools in the Assessment of Histological Healing. Biomedicines, 11(11), 3090. https://doi.org/10.3390/biomedicines11113090