PSMA-Expression Is Highly Associated with Histological Subtypes of Renal Cell Carcinoma: Potential Implications for Theranostic Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Immunohistochemical Analyses
2.3. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Immunohistochemical PSMA Expression in Non-Neoplastic Kidney Tissue
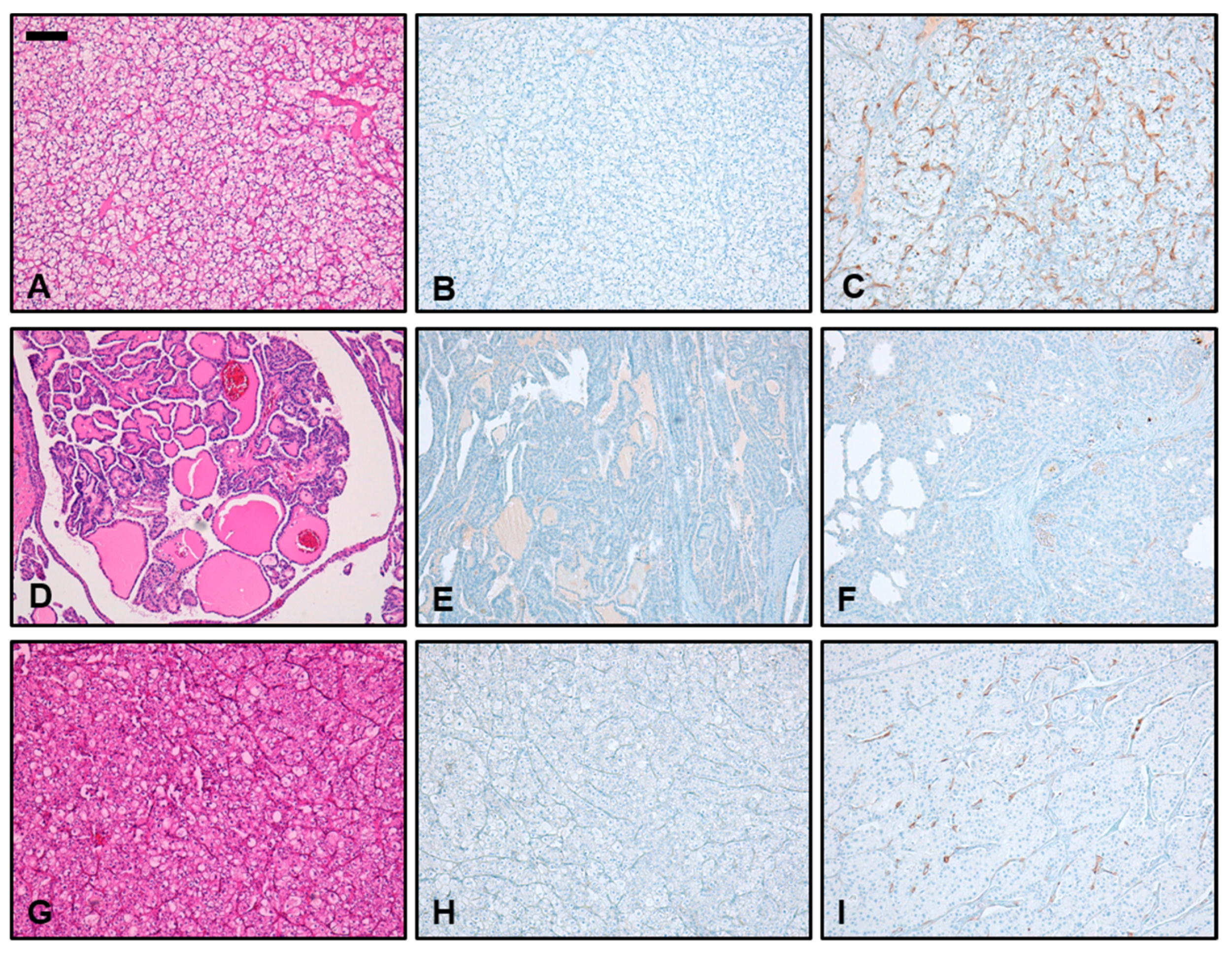
3.3. Immunohistochemical PSMA Expression in Different Subtypes of RCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernandez-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Skolarikos, A.; Alivizatos, G.; Laguna, P.; de la Rosette, J. A review on follow-up strategies for renal cell carcinoma after nephrectomy. Eur. Urol. 2007, 51, 1490–1501. [Google Scholar] [CrossRef]
- Ljungberg, B.; Campbell, S.C.; Choi, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Lam, T.B.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A. The 2021 Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Immune Checkpoint Inhibitor—Based Combination Therapies for Treatment-Naive Metastatic Clear-Cell Renal Cell Carcinoma Are Standard of Care; Elsevier: Amsterdam, The Netherlands, 2021; Volume 80, pp. 393–397. [Google Scholar]
- Sharma, R.; Kadife, E.; Myers, M.; Kannourakis, G.; Prithviraj, P.; Ahmed, N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 186. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Ziegelmuller, B.; Ljungberg, B.; Bensalah, K.; Bex, A.; Canfield, S.; Giles, R.H.; Hora, M.; Kuczyk, M.A.; Merseburger, A.S.; et al. Imaging in Suspected Renal-Cell Carcinoma: Systematic Review. Clin. Genitourin. Cancer 2019, 17, e345–e355. [Google Scholar] [CrossRef]
- Pozzessere, C.; Bassanelli, M.; Ceribelli, A.; Rasul, S.; Li, S.; Prior, J.O.; Cicone, F. Renal Cell Carcinoma: The Oncologist Asks, Can PSMA PET/CT Answer? Curr. Urol. Rep. 2019, 20, 68. [Google Scholar] [CrossRef]
- Tadayoni, A.; Paschall, A.K.; Malayeri, A.A. Assessing lymph node status in patients with kidney cancer. Transl. Androl. Urol. 2018, 7, 766–773. [Google Scholar] [CrossRef]
- Roussel, E.; Capitanio, U.; Kutikov, A.; Oosterwijk, E.; Pedrosa, I.; Rowe, S.P.; Gorin, M.A. Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review. Eur. Urol. 2022, 81, 476–488. [Google Scholar] [CrossRef]
- Urso, L.; Castello, A.; Rocca, G.C.; Lancia, F.; Panareo, S.; Cittanti, C.; Uccelli, L.; Florimonte, L.; Castellani, M.; Ippolito, C.; et al. Role of PSMA-ligands imaging in Renal Cell Carcinoma management: Current status and future perspectives. J. Cancer Res. Clin. Oncol. 2022, 148, 1299–1311. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Oflas, M.; Ozluk, Y.; Sanli, O.; Ozkan, Z.G.; Kuyumcu, S. 68Ga-PSMA Uptake Patterns of Clear Cell Renal Carcinoma Across Different Histopathological Subtypes. Clin. Nucl. Med. 2022, 47, e45–e46. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, C.; Sathekge, M.; de Spiegeleer, B.; De Jonghe, P.J.; Debruyne, P.R.; Borms, M.; Beels, L.; Maes, A. PSMA expression on neovasculature of solid tumors. Histol. Histopathol. 2020, 35, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Mittlmeier, L.M.; Unterrainer, M.; Rodler, S.; Todica, A.; Albert, N.; Burgard, C.; Cyran, C.; Kunz, W.; Ricke, J.; Bartenstein, P. 18F-PSMA-1007 PET/CT for response assessment in patients with metastatic renal cell carcinoma undergoing tyrosine kinase or checkpoint inhibitor therapy: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Gühne, F.; Seifert, P.; Theis, B.; Steinert, M.; Freesmeyer, M.; Drescher, R. PSMA-PET/CT in patients with recurrent clear cell renal cell carcinoma: Histopathological correlations of imaging findings. Diagnostics 2021, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Raveenthiran, S.; Esler, R.; Yaxley, J.; Kyle, S. The use of 68Ga-PET/CT PSMA in the staging of primary and suspected recurrent renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2280–2288. [Google Scholar] [CrossRef]
- Rhee, H.; Blazak, J.; Tham, C.M.; Ng, K.L.; Shepherd, B.; Lawson, M.; Preston, J.; Vela, I.; Thomas, P.; Wood, S. Pilot study: Use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 2016, 6, 76. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Buchbender, C.; Boos, J.; Giessing, M.; Ermert, J.; Antke, C.; Antoch, G.; Hautzel, H. Diagnostic potential of PET/CT using a 68Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: Initial experience. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 102–107. [Google Scholar] [CrossRef]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: Findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef]
- Al-Ahmadie, H.A.; Olgac, S.; Gregor, P.D.; Tickoo, S.K.; Fine, S.W.; Kondagunta, G.V.; Scher, H.I.; Morris, M.J.; Russo, P.; Motzer, R.J. Expression of prostate-specific membrane antigen in renal cortical tumors. Mod. Pathol. 2008, 21, 727–732. [Google Scholar] [CrossRef]
- Baccala, A.; Sercia, L.; Li, J.; Heston, W.; Zhou, M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology 2007, 70, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zschabitz, S.; Erlmeier, F.; Stohr, C.; Herrmann, E.; Polifka, I.; Agaimy, A.; Trojan, L.; Strobel, P.; Becker, F.; Wulfing, C.; et al. Expression of Prostate-specific Membrane Antigen (PSMA) in Papillary Renal Cell Carcinoma—Overview and Report on a Large Multicenter Cohort. J. Cancer 2022, 13, 1706–1712. [Google Scholar] [CrossRef]
- Spatz, S.; Tolkach, Y.; Jung, K.; Stephan, C.; Busch, J.; Ralla, B.; Rabien, A.; Feldmann, G.; Brossart, P.; Bundschuh, R.A.; et al. Comprehensive Evaluation of Prostate Specific Membrane Antigen Expression in the Vasculature of Renal Tumors: Implications for Imaging Studies and Prognostic Role. J. Urol. 2018, 199, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Chen, C.H. Potential New Therapeutic Approaches for Renal Cell Carcinoma. Semin. Nephrol. 2020, 40, 86–97. [Google Scholar] [CrossRef]
- Ferraro, D.A.; Ruschoff, J.H.; Muehlematter, U.J.; Kranzbuhler, B.; Muller, J.; Messerli, M.; Husmann, L.; Hermanns, T.; Eberli, D.; Rupp, N.J.; et al. Immunohistochemical PSMA expression patterns of primary prostate cancer tissue are associated with the detection rate of biochemical recurrence with 68Ga-PSMA-11-PET. Theranostics 2020, 10, 6082–6094. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, P.J.; Stricker, P.; Hruby, G.; Kneebone, A.; Ting, F.; Thompson, B.; Nguyen, Q.; Ho, B.; Emmett, L. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016, 117, 732–739. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M., Jr.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Tariq, A.; Kwok, M.; Pearce, A.; Rhee, H.; Kyle, S.; Marsh, P.; Raveenthiran, S.; Wong, D.; McBean, R.; Westera, J.; et al. The role of dual tracer PSMA and FDG PET/CT in renal cell carcinoma (RCC) compared to conventional imaging: A multi-institutional case series with intra-individual comparison. Urol. Oncol. 2022, 40, 66.e61–66.e69. [Google Scholar] [CrossRef] [PubMed]
- Salas Fragomeni, R.A.; Amir, T.; Sheikhbahaei, S.; Harvey, S.C.; Javadi, M.S.; Solnes, L.B.; Kiess, A.P.; Allaf, M.E.; Pomper, M.G.; Gorin, M.A.; et al. Imaging of Nonprostate Cancers Using PSMA-Targeted Radiotracers: Rationale, Current State of the Field, and a Call to Arms. J. Nucl. Med. 2018, 59, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.R.; Carducci, M.A.; Denmeade, S.R.; Markowski, M.C.; Pomper, M.G.; Pierorazio, P.M.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2019, 33, 617–623. [Google Scholar] [CrossRef]
- Yin, Y.; Campbell, S.P.; Markowski, M.C.; Pierorazio, P.M.; Pomper, M.G.; Allaf, M.E.; Rowe, S.P.; Gorin, M.A. Inconsistent Detection of Sites of Metastatic Non-Clear Cell Renal Cell Carcinoma with PSMA-Targeted [18F]DCFPyL PET/CT. Mol. Imaging Biol. 2019, 21, 567–573. [Google Scholar] [CrossRef]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar]
- Nguyen, D.P.; Xiong, P.L.; Liu, H.; Pan, S.; Leconet, W.; Navarro, V.; Guo, M.; Moy, J.; Kim, S.; Ramirez-Fort, M.K.; et al. Induction of PSMA and Internalization of an Anti-PSMA mAb in the Vascular Compartment. Mol. Cancer Res. 2016, 14, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Laimer, J.; Chaux, A.; Schafer, G.; Obrist, P.; Brunner, A.; Kronberger, I.E.; Laimer, K.; Gurel, B.; Koller, J.B.; et al. High expression of prostate-specific membrane antigen in the tumor-associated neo-vasculature is associated with worse prognosis in squamous cell carcinoma of the oral cavity. Mod. Pathol. 2012, 25, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Minner, S.; Wittmer, C.; Graefen, M.; Salomon, G.; Steuber, T.; Haese, A.; Huland, H.; Bokemeyer, C.; Yekebas, E.; Dierlamm, J.; et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate 2011, 71, 281–288. [Google Scholar] [CrossRef]
- Unger, C.; Bronsert, P.; Michalski, K.; Bicker, A.; Juhasz-Boss, I. Expression of Prostate Specific Membrane Antigen (PSMA) in Breast Cancer. Geburtshilfe Frauenheilkd. 2022, 82, 50–58. [Google Scholar] [CrossRef]
- Devlin, A.M.; Ling, E.H.; Peerson, J.M.; Fernando, S.; Clarke, R.; Smith, A.D.; Halsted, C.H. Glutamate carboxypeptidase II: A polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Hum. Mol. Genet. 2000, 9, 2837–2844. [Google Scholar] [CrossRef]
- Yao, V.; Berkman, C.E.; Choi, J.K.; O’Keefe, D.S.; Bacich, D.J. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate 2010, 70, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, R.; Jiang, W.G.; Cui, Y.X. MDM2 and PSMA Play Inhibitory Roles in Metastatic Breast Cancer Cells Through Regulation of Matrix Metalloproteinases. Anticancer Res. 2016, 36, 1143–1151. [Google Scholar] [PubMed]
- Conway, R.E.; Petrovic, N.; Li, Z.; Heston, W.; Wu, D.; Shapiro, L.H. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol. Cell. Biol. 2006, 26, 5310–5324. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Becker, A.; Eppard, E.; Kurpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef]
- Hartrampf, P.E.; Weinzierl, F.X.; Serfling, S.E.; Pomper, M.G.; Rowe, S.P.; Higuchi, T.; Seitz, A.K.; Kubler, H.; Buck, A.K.; Werner, R.A. Hematotoxicity and Nephrotoxicity in Prostate Cancer Patients Undergoing Radioligand Therapy with [177Lu]Lu-PSMA I&T. Cancers 2022, 14, 647. [Google Scholar] [CrossRef]
- Satapathy, S.; Sharma, A.; Sood, A.; Maheshwari, P.; Gill, H.J.S. Delayed Nephrotoxicity after 225Ac-PSMA-617 Radioligand Therapy. Clin. Nucl. Med. 2022, 47, e466–e467. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Garje, R.; Elhag, D.; Yasin, H.A.; Acharya, L.; Vaena, D.; Dahmoush, L. Comprehensive review of chromophobe renal cell carcinoma. Crit. Rev. Oncol. Hematol. 2021, 160, 103287. [Google Scholar] [CrossRef]
- Gorin, M.; Rowe, S. PSMA: A Potential Therapeutic Target in RCC. Nat. Rev. Urol. 2017, 14, 646–647. [Google Scholar] [CrossRef]
- Widel, M. Radionuclides in Radiation-Induced Bystander Effect; May It Share in Radionuclide Therapy? Neoplasma 2017, 64, 641–654. [Google Scholar] [CrossRef]
- Haberkorn, U.; Giesel, F.; Morgenstern, A.; Kratochwil, C. The Future of Radioligand Therapy: α, β, or Both? J. Nucl. Med. 2017, 58, 1017–1018. [Google Scholar] [CrossRef] [PubMed]



| n = 65 | ccRCC (n = 21) | pRCC (n = 24) | cpRCC (n = 20) | |||
|---|---|---|---|---|---|---|
| PSMA Low (n = 0) | PSMA Strong (n = 21) | PSMA Low (n = 22) | PSMA Strong (n = 2) | PSMA Low (n = 10) | PSMA Strong (n = 10) | |
| Median age (years, range) | -- | 71 (37–82) | 68 (47–82) | 78 (73–83) | 69 (38–90) | 61 (36–72) |
| Sex | ||||||
| Male, n (%) | -- | 15 (71) | 21 (95) | 2 (100) | 6 (60) | 7 (70) |
| Female, n (%) | -- | 6 (29) | 1 (5) | 0 (0) | 4 (40) | 3 (30) |
| T stage | ||||||
| T1, n (%) | -- | 16 (76) | 14 (64) | 1 (50) | 6 (60) | 7 (70) |
| T2, n (%) | -- | 1 (5) | 2 (9) | 1 (50) | 3 (30) | 2 (20) |
| T3, n (%) | -- | 4 (19) | 6 (27) | 0 (0) | 1 (10) | 1 (1) |
| T4, n (%) | -- | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Lymph node status | ||||||
| NX, n (%) | -- | 21 (100) | 20 (91) | 1 (50) | 10 (100) | 9 (90) |
| N0, n (%) | -- | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 1 (10) |
| N1, n (%) | -- | 0 (0) | 1 (5) | 1 (50) | 0 (0) | 0 (0) |
| Lymphovascular invasion | ||||||
| L0, n (%) | -- | 21 (100) | 21 (95) | 1 (50) | 10 (100) | 10 (100) |
| L1, n (%) | -- | 0 (0) | 1 (5) | 1 (50) | 0 (0) | 0 (0) |
| Vascular invasion | ||||||
| V0, n (%) | -- | 19 (90) | 21 (95) | 1 (50) | 10 (100) | 9 (90) |
| V1, n (%) | -- | 2 (10) | 1 (5) | 1 (50) | 0 (0) | 1 (10) |
| Perineural invasion | ||||||
| Pn0, n (%) | -- | 21 (100) | 22 (100) | 2 (100) | 10 (100) | 10 (100) |
| Pn1, n (%) | -- | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Distant metastasis | ||||||
| MX, n (%) | 1 (5) | 3 (14) | 1 (50) | 10 (100) | 1 (10) | |
| M0, n (%) | -- | 20 (95) | 18 (82) | 1 (50) | 0 (0) | 9 (90) |
| M1, n (%) | -- | 0 (0) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
| Residual status | ||||||
| RX, n (%) | -- | 2 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| R0, n (%) | -- | 19 (90) | 21 (95) | 2 (100) | 9 (90) | 10 (100) |
| R1, n (%) | -- | 0 (0) | 1 (5) | 0 (0) | 1 (10) | 0 (0) |
| Grading | ||||||
| G1 | -- | 5 (24) | 6 (27) | 1 (50) | NA | NA |
| G2 | -- | 11 (52) | 10 (45) | 0 (0) | NA | NA |
| G3 | -- | 5 (24) | 4 (18) | 1 (50) | NA | NA |
| G4 | -- | 0 (0) | 1 (5) | 0 (0) | NA | NA |
| Tumor Subtype | ccRCC (n = 21) | cpRCC (n = 20) | pRCC (n = 24) | |||
|---|---|---|---|---|---|---|
| Focal (%) | Diffuse (%) | Focal (%) | Diffuse (%) | Focal (%) | Diffuse (%) | |
| Staining Extent | 4 (19%) | 17 (81%) | 14 (70%) | 6 (30%) | 23 (91%) | 1 (4%) |
| Weak (%) | Strong (%) | Weak (%) | Strong (%) | Weak (%) | Strong (%) | |
| Staining Intensity | 0 (0%) | 21 (100%) | 10 (50%) | 10 (50%) | 22 (92%) | 2 (8%) |
| Mean Staining Intensity | 2.67 (±0.47) | 1.75 (±0.94) | 1.08 (±0.49) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, V.N.; Unterrainer, L.M.; Brendel, M.; Kunte, S.C.; Holzgreve, A.; Allmendinger, F.; Bartenstein, P.; Klauschen, F.; Unterrainer, M.; Staehler, M.; et al. PSMA-Expression Is Highly Associated with Histological Subtypes of Renal Cell Carcinoma: Potential Implications for Theranostic Approaches. Biomedicines 2023, 11, 3095. https://doi.org/10.3390/biomedicines11113095
Bui VN, Unterrainer LM, Brendel M, Kunte SC, Holzgreve A, Allmendinger F, Bartenstein P, Klauschen F, Unterrainer M, Staehler M, et al. PSMA-Expression Is Highly Associated with Histological Subtypes of Renal Cell Carcinoma: Potential Implications for Theranostic Approaches. Biomedicines. 2023; 11(11):3095. https://doi.org/10.3390/biomedicines11113095
Chicago/Turabian StyleBui, Vinh Ngoc, Lena M. Unterrainer, Matthias Brendel, Sophie C. Kunte, Adrien Holzgreve, Fabian Allmendinger, Peter Bartenstein, Frederick Klauschen, Marcus Unterrainer, Michael Staehler, and et al. 2023. "PSMA-Expression Is Highly Associated with Histological Subtypes of Renal Cell Carcinoma: Potential Implications for Theranostic Approaches" Biomedicines 11, no. 11: 3095. https://doi.org/10.3390/biomedicines11113095
APA StyleBui, V. N., Unterrainer, L. M., Brendel, M., Kunte, S. C., Holzgreve, A., Allmendinger, F., Bartenstein, P., Klauschen, F., Unterrainer, M., Staehler, M., & Ledderose, S. (2023). PSMA-Expression Is Highly Associated with Histological Subtypes of Renal Cell Carcinoma: Potential Implications for Theranostic Approaches. Biomedicines, 11(11), 3095. https://doi.org/10.3390/biomedicines11113095






