Unsuitability of the Oxidation-Reduction Potential Measurement for the Quantification of Fecal Redox Status in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of ORP in Fecal Samples
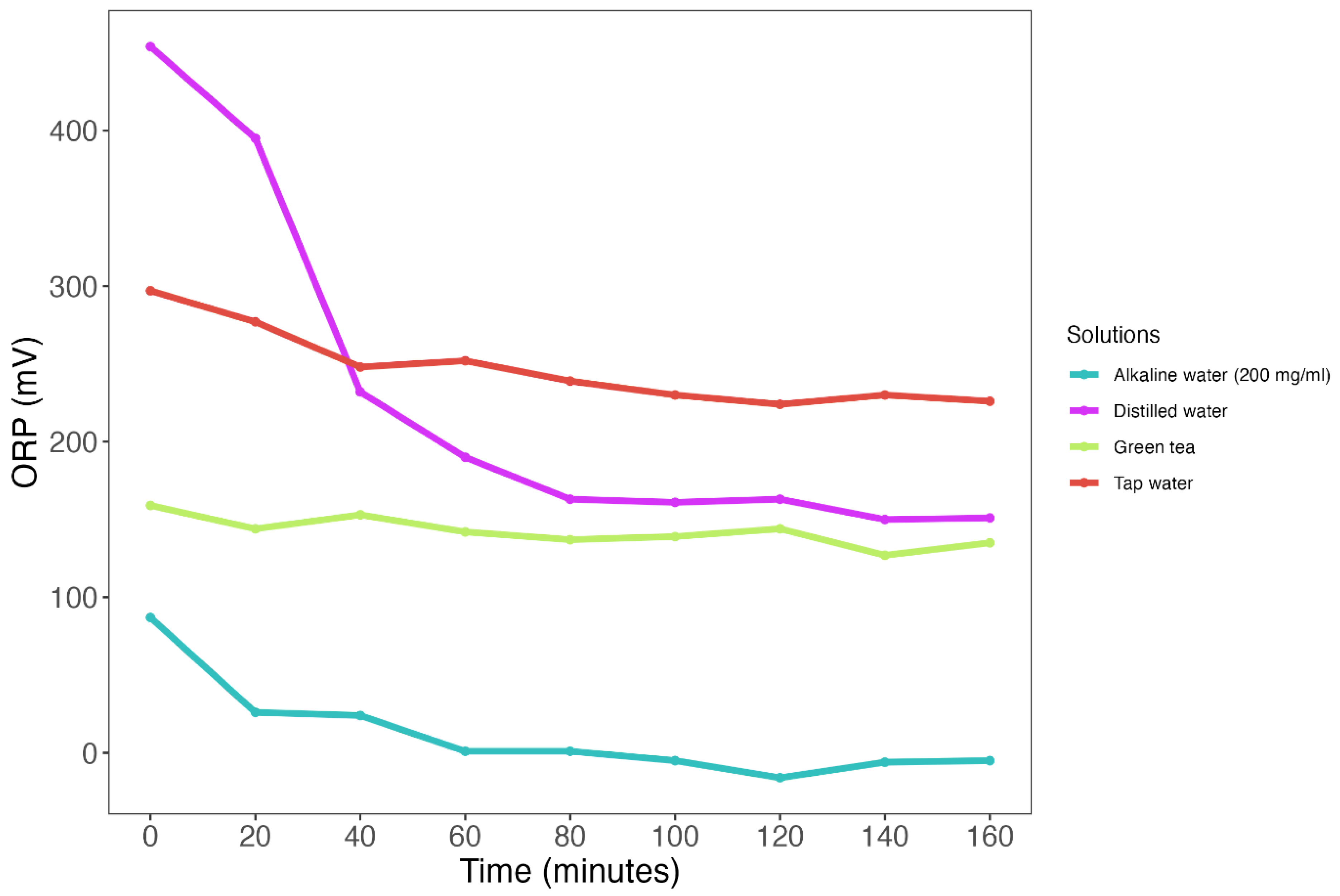
2.3. Stability of ORP Measurements across Various Solutions
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. ORP Measurements Show Poor Temporal Stability across Various Test Solutions
3.3. Both Patients and Controls Exhibit Low Redox Potential Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, C. Inflammatory bowel disease pathogenesis: Where are we? J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S1), 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Yoshikawa, T. Molecular fingerprints of neutrophil-dependent oxidative stress in inflammatory bowel disease. J. Gastroenterol. 2007, 42, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, Y.; Zhai, L. Impact of Nutritional and Environmental Factors on Inflammation, Oxidative Stress, and the Microbiome. Biomed. Res. Int. 2018, 2018, 5606845. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Kawahara, T.; Diebold, B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 2007, 43, 319–331. [Google Scholar] [CrossRef]
- Fang, J.; Seki, T.; Tsukamoto, T.; Haibo, Q.; Hongzhuan, Y.; Liao, L.; Nakamura, H.; Maeda, H. Protection from inflammatory bowel disease and colitis-associated carcinogenesis with 4-vinyl-2,6-dimethoxyphenol (canolol) involves suppression of oxidative stress and inflammatory cytokines. Carcinogenesis 2013, 34, 2833–2841. [Google Scholar] [CrossRef]
- Rezaie, A. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelish, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Gabriëls, R.Y.; Borst, M.H.; Bulthuis, M.L.C.; Faber, K.N.; Goor, H.; Dijkstra, G. Serum Free Thiols Are Superior to Fecal Calprotectin in Reflecting Endoscopic Disease Activity in Inflammatory Bowel Disease. Antioxidants 2019, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Pawar, P.; Gilda, S.; Sharma, S.; Jagtap, S.; Paradkar, A.; Mahadik, K.; Ranjekar, P.; Harsulkar, A. Rectal gel application of Withania somnifera root extract expounds anti-inflammatory and muco-restorative activity in TNBS-induced inflammatory bowel disease. BMC Complement. Altern. Med. 2011, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Achitei, D.; Ciobica, A.; Balan, G.; Gologan, E.; Stanciu, C.; Stefanescu, G. Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig. Dis. Sci. 2013, 58, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.L.; de Quandros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoglu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Raoult, D. Linking gut redox to human microbiome. Human. Microbiome J. 2018, 10, 27–32. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef]
- Krueger, K.M.; Vavrus, C.E.; Lofton, M.E.; McClure, R.P.; Gantzer, P.; Carey, C.C.; Schreiber, M.E. Iron and manganese fluxes across the sediment-water interface in a drinking water reservoir. Water Res. 2020, 182, 116003. [Google Scholar] [CrossRef]
- Liu, G.; He, T.; Liu, Y.; Chen, Z.; Li, L.; Huang, Q.; Xie, Z.; Xie, Y.; Wu, L.; Liu, J. Study on the purification effect of aeration-enhanced horizontal subsurface-flow constructed wetland on polluted urban river water. Environ. Sci. Pollut. Res. Int. 2019, 26, 12867–12880. [Google Scholar] [CrossRef]
- Grütte, F.K.; Horn, R.; Haenel, H. Ernährung und biochemisch-mikrobiologische Vorgänge im Enddarm yon Säuglingen. Z. Für Kinderheilkd. 1965, 93, 23–39. [Google Scholar]
- Bjugstad, K.B.; Lalama, J.; Rael, L.T.; Salottolo, K.; Dauber, I.; Bar-Or, D. Poor acute outcome in congestive heart failure is associated with increases in the plasma static oxidation-reduction potentials (sORP) in men but not in women. Redox Rep. 2017, 22, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.E.; Gore, E.; Henrichs, K.F.; Conley, G.; Dorsey, C.; Bjugstad, K.B.; Refaai, M.A.; Blumberg, N.; Cholette, J.M. Oxidation Reduction Potential (ORP) is Predictive of Complications Following Pediatric Cardiac Surgery. Pediatr. Cardiol. 2018, 39, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Pons-Kühnemann, J.; Fechner, A.; Funk, M.; Gromer, S.; Gross, H.J.; Grünert, A.; Schirmer, R.H. Effects of antioxidants on glutathione levels and clinical recovery from the malnutrition syndrome kwashiorkor—A pilot study. Redox Rep. 2005, 10, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Imhann, F.; Collij, V.; Jankipersadsing, S.A.; Gurry, T.; Mujagic, Z.; Kurilshikov, A.; Bonder, M.J.; Jiang, X.; Tigchelaar, E.F.; et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 2018, 10, eaap8914. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome—A pilot study. Gut Microbes 2021, 13, 1875774. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Van der Velde, K.J.; Barbieri, R.; Alberts, R.; Voskuil, M.D.; Vich Vila, A.; Collij, V.; Spekhorst, L.M.; Van der Sloot, K.W.J.; Peters, V.; et al. The 1000IBD project: Multi-omics data of 1000 inflammatory bowel disease patients; data release 1. BMC Gastroenterol. 2019, 19, 5. [Google Scholar]
- Tigchelaar, E.F.; Zhernakova, A.; Dekens, J.A.; Hermes, G.; Baranska, A.; Mujagic, Z.; Swertz, M.A.; Muñoz, A.M.; Deelen, P.; Cénit, M.C.; et al. Cohort profile: LifeLines DEEP, a prospective, general population cohort study in the northern Netherlands: Study design and baseline characteristics. BMJ Open 2015, 5, e006772. [Google Scholar] [CrossRef]
- PCE Instruments. Manual Redox Meter-228. Manual pH Meter PCE-228. PCE Instruments. Available online: https://www.industrial-needs.com/manual/man-ph-meter-pce-228-v1.0-2016-01-22-en.pdf (accessed on 1 March 2023).
- Dick, J.M. A Thermodynamic Model for Water Activity and Redox Potential in Evolution and Development. J. Mol. Evol. 2022, 90, 182–199. [Google Scholar] [CrossRef]
- Gaisawat, M.B.; Iskandar, M.M.; MacPherson, C.W.; Tompkins, T.A.; Kubow, S. Probiotic Supplementation is Associated with Increased Antioxidant Capacity and Copper Chelation in C. difficile-Infected Fecal Water. Nutrients 2019, 11, 2007. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
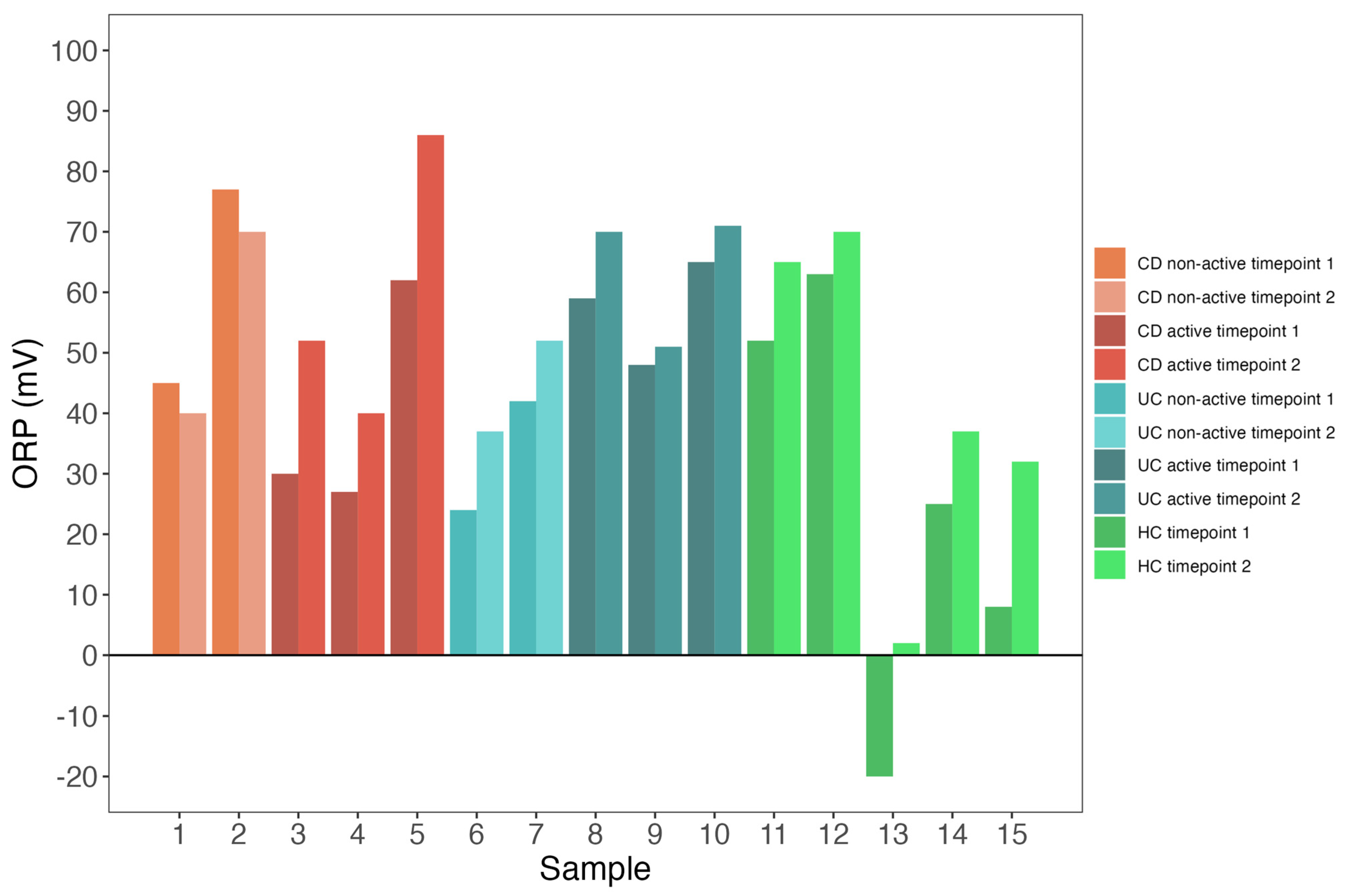

| Characteristic | CD, N = 5 1 | UC, N = 5 1 | HC, N = 5 1 | p-Value 2 |
|---|---|---|---|---|
| ORP Timepoint 1 | 45.0 (30.0, 62.0) | 48.0 (42.0, 59.0) | 25.0 (8.0, 52.0) | 0.47 |
| ORP Timepoint 2 | 52.0 (40.0, 70.0) | 52.0 (51.0, 70.0) | 37.0 (32.0, 65.0) | 0.36 |
| Sex | 1.00 | |||
| Female | 3 (60%) | 3 (60%) | 3 (60%) | |
| Male | 2 (40%) | 2 (40%) | 2 (40%) | |
| Age (years) | 52.0 (48.0, 53.0) | 44.0 (37.0, 45.0) | 60.0 (57.0, 65.0) | 0.05 |
| Body Mass Index (kg/m2) | 26.9 (25.9, 32.7) | 24.8 (23.7, 26.8) | - | 0.35 |
| Active disease | 3 (60%) | 3 (60%) | - | |
| HBI | 5 (2, 6) | - | - | |
| SCCAI | - | 4 (2, 5) | - | |
| Montreal Age | ||||
| A1 (<17 y) | 0 (0%) | 0 (0%) | - | |
| A2 (17–40 y) | 3 (60%) | 5 (100%) | - | |
| A3 (>40 y) | 2 (40%) | 0 (0%) | - | |
| Montreal Location | ||||
| L1 (Terminal ileum) | 1 (20%) | - | - | |
| L2 (Colon) | 0 (0%) | - | - | |
| L3 (Ileocolon) | 4 (80%) | - | - | |
| Montreal Behavior | ||||
| B1 (Non-stricturing, non-penetrating) | 3 (60%) | - | - | |
| B2 (Stricturing) | 1 (20%) | - | - | |
| B3 (Penetrating) | 1 (20%) | - | - | |
| Montreal Extension | ||||
| E1 (Proctitis) | - | 1 (20%) | - | |
| E2 (Left-sided UC) | - | 0 (0%) | - | |
| E3 (Pancolitis UC) | - | 4 (80%) | - | |
| Medication use | ||||
| Aminosalicylates, n (%) | 0 (0%) | 4 (80%) | - | |
| Steroids, n (%) | 1 (20%) | 1 (20%) | - | |
| Thiopurines, n (%) | 0 (0%) | 0 (0%) | - | |
| Anti-TNF, n (%) | 2 (40%) | 1 (20%) | - | |
| Surgical history, n (%) | ||||
| Ileocecal resection, n (%) | 2 (40%) | 0 (0%) | - | |
| Colectomy, n (%) | 1 (20%) | 0 (0%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geertsema, S.; Jansen, B.H.; van Goor, H.; Dijkstra, G.; Faber, K.N.; Bourgonje, A.R. Unsuitability of the Oxidation-Reduction Potential Measurement for the Quantification of Fecal Redox Status in Inflammatory Bowel Disease. Biomedicines 2023, 11, 3107. https://doi.org/10.3390/biomedicines11123107
Geertsema S, Jansen BH, van Goor H, Dijkstra G, Faber KN, Bourgonje AR. Unsuitability of the Oxidation-Reduction Potential Measurement for the Quantification of Fecal Redox Status in Inflammatory Bowel Disease. Biomedicines. 2023; 11(12):3107. https://doi.org/10.3390/biomedicines11123107
Chicago/Turabian StyleGeertsema, Sem, Bernadien H. Jansen, Harry van Goor, Gerard Dijkstra, Klaas Nico Faber, and Arno R. Bourgonje. 2023. "Unsuitability of the Oxidation-Reduction Potential Measurement for the Quantification of Fecal Redox Status in Inflammatory Bowel Disease" Biomedicines 11, no. 12: 3107. https://doi.org/10.3390/biomedicines11123107
APA StyleGeertsema, S., Jansen, B. H., van Goor, H., Dijkstra, G., Faber, K. N., & Bourgonje, A. R. (2023). Unsuitability of the Oxidation-Reduction Potential Measurement for the Quantification of Fecal Redox Status in Inflammatory Bowel Disease. Biomedicines, 11(12), 3107. https://doi.org/10.3390/biomedicines11123107







