Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
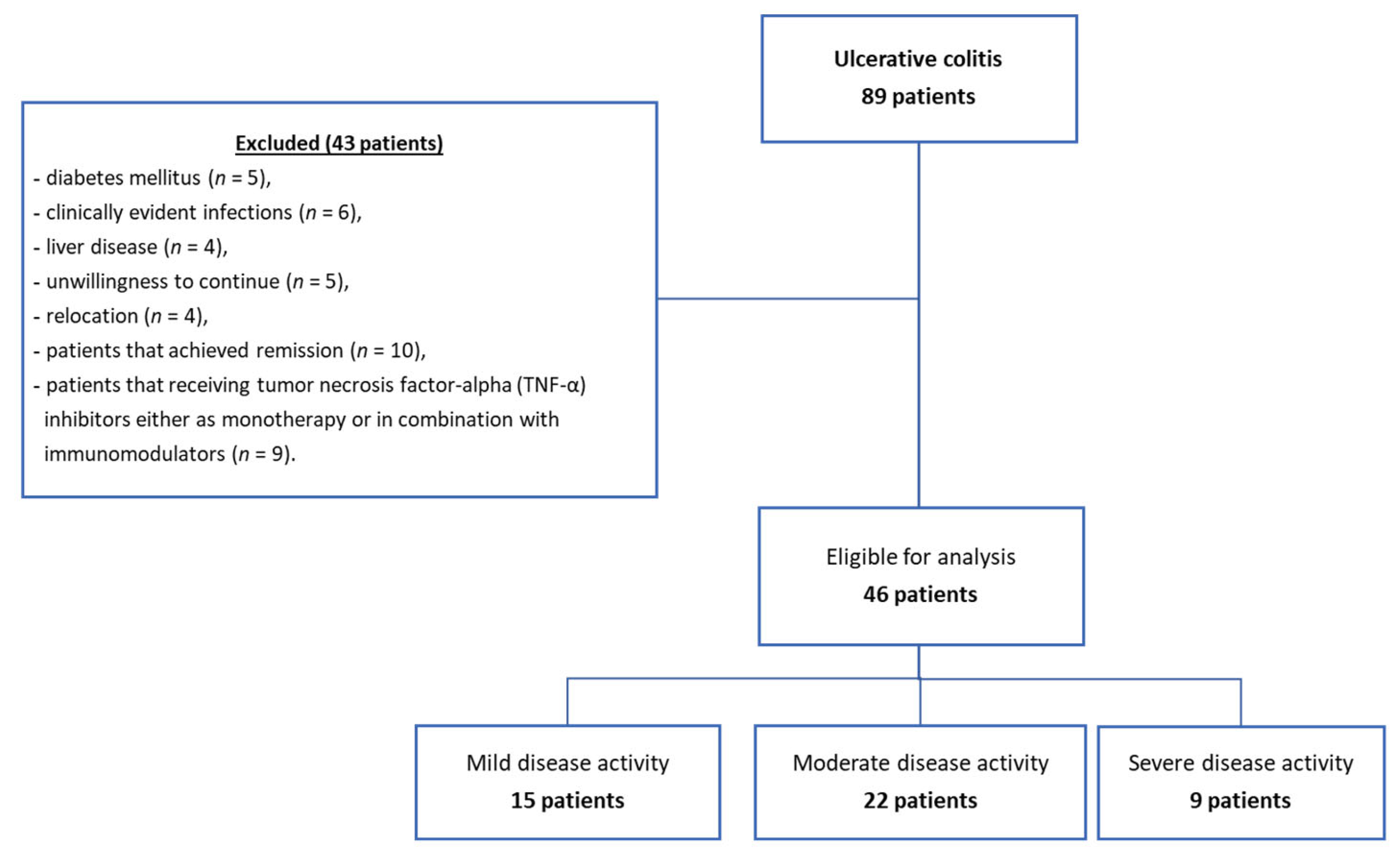
2.2. Patient Selection
2.3. Sample Collection
2.4. Immunological Assessment
2.5. Complete Blood Counting (CBC)
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Subjects
3.2. Concentrations of Serum HIF-1α and Disease Activity
3.3. Concentrations of Serum HIF-1α and Extent of Disease
3.4. The Inflammatory Status Biomarkers
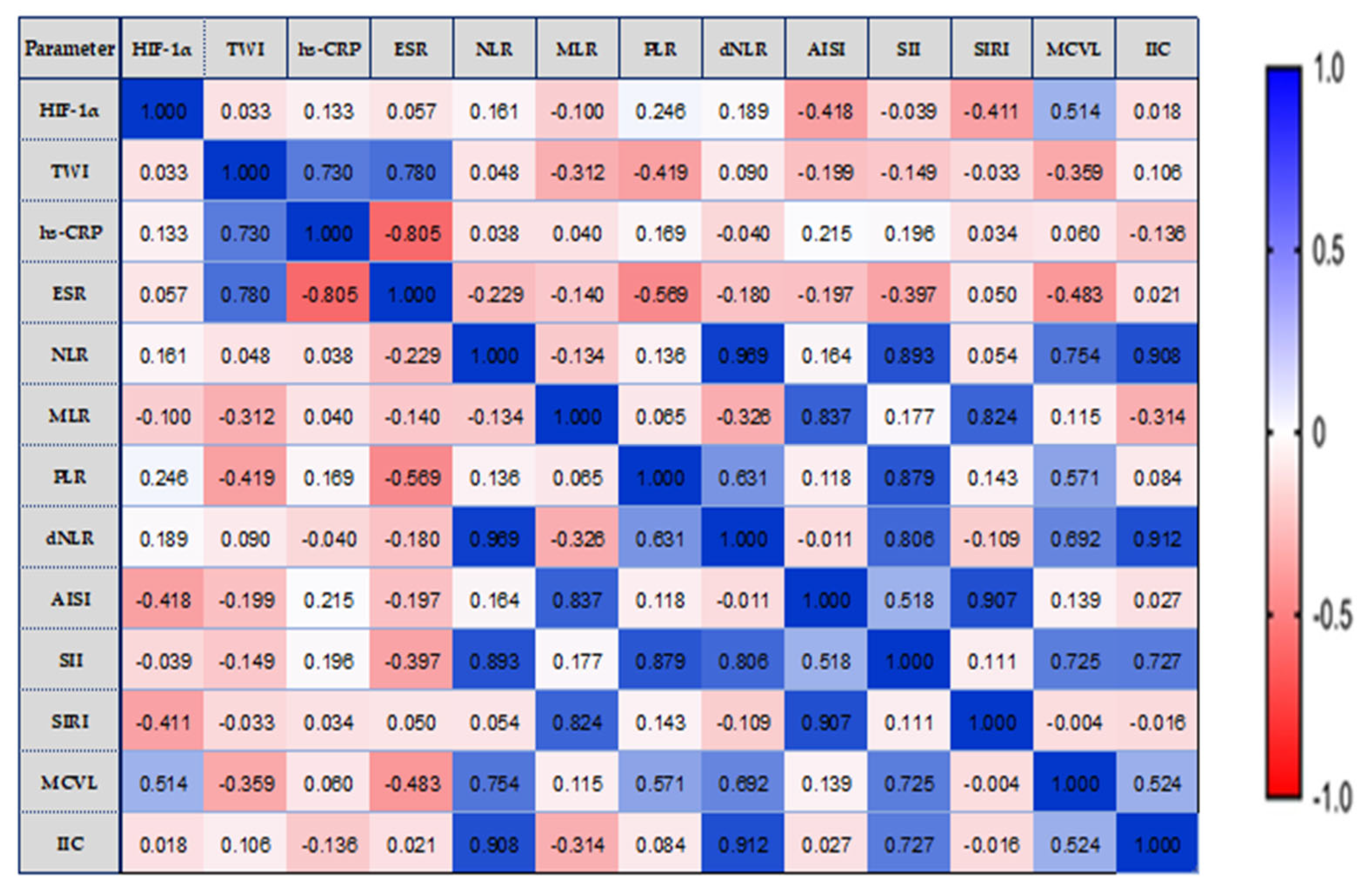
3.5. Correlations between Serum Concentrations of HIF-1α, TWI, and Inflammatory Status Biomarkers
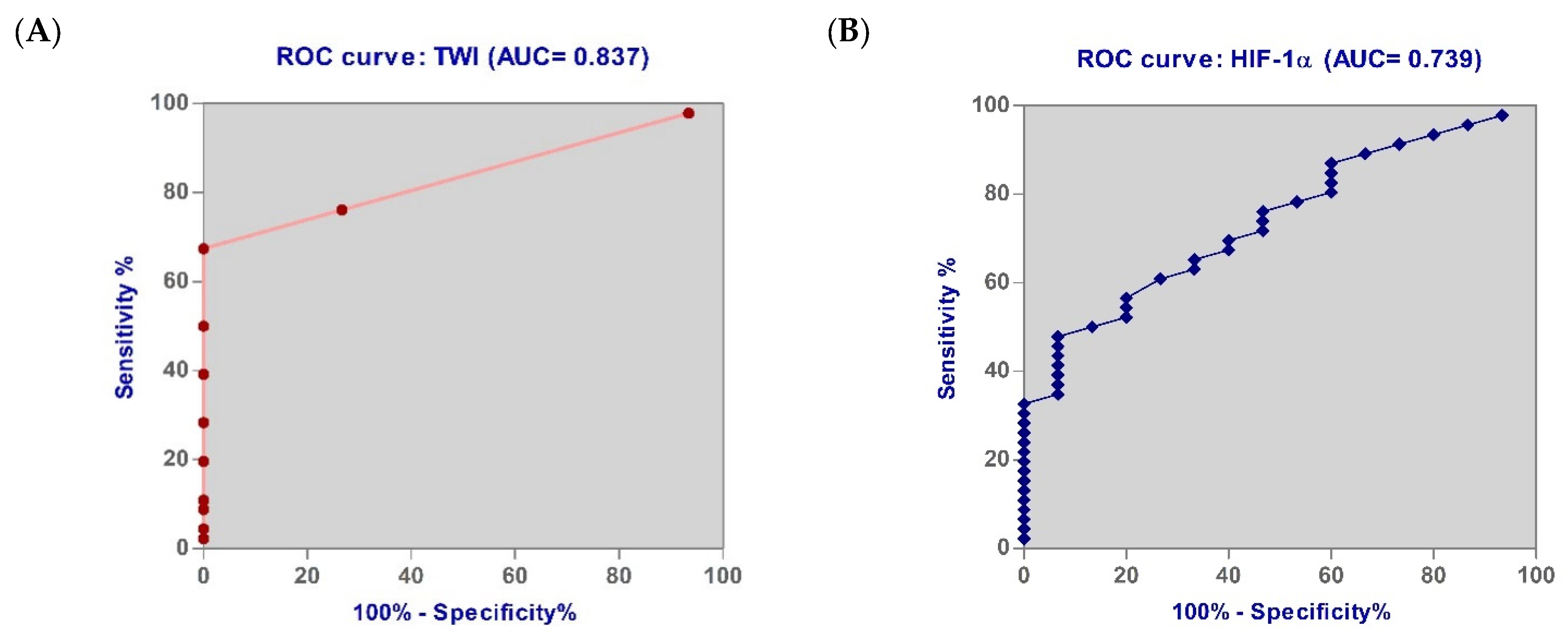
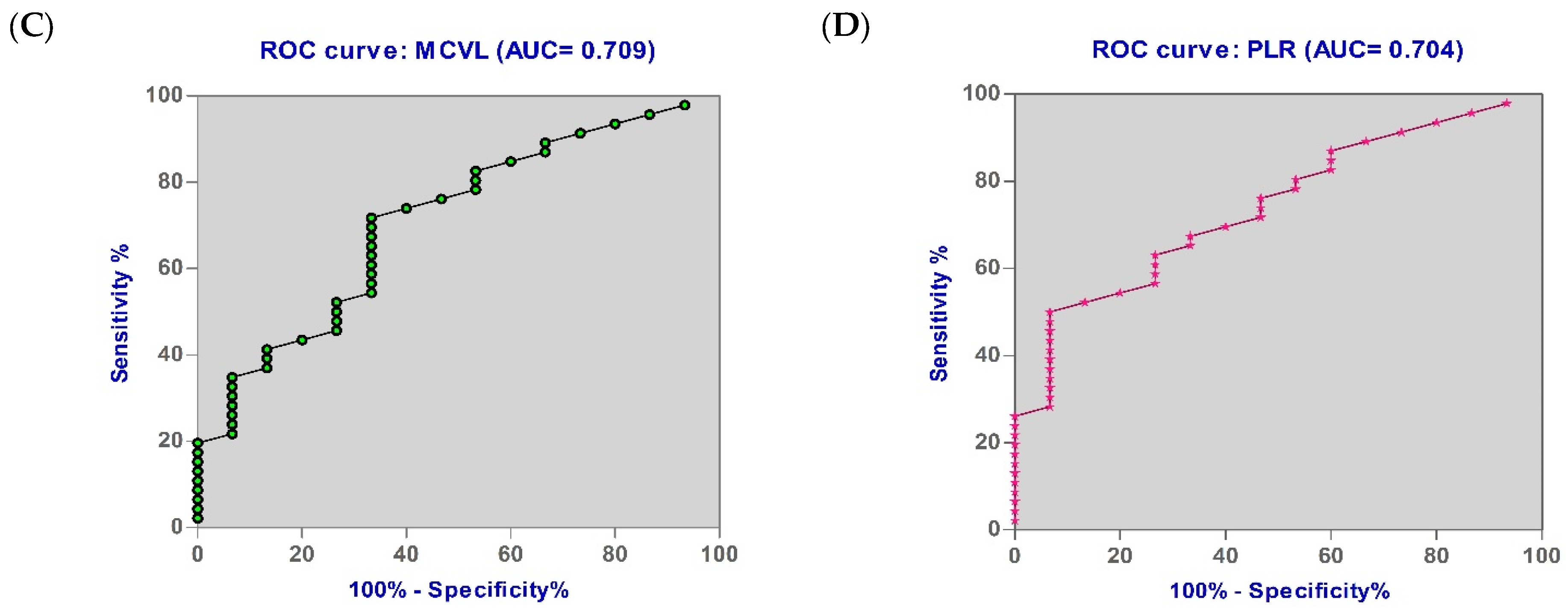
3.6. Diagnostic Accuracy of the Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartor, B.R. Mechanisms of Disease: Pathogenesis of Crohn’s Disease and Ulcerative Colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Bratton, D.L.; Colgan, S.P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 2014, 13, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 2014, 40, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Eltzschig, H.K. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 2012, 74, 153–175. [Google Scholar] [CrossRef]
- Bowser, J.L.; Lee, J.W.; Yuan, X.; Eltzschig, H.K. The hypoxia-adenosine link during inflammation. J. Appl. Physiol. 2017, 123, 1303–1320. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Rosenberger, P.; Schwab, J.M.; Mirakaj, V.; Masekowsky, E.; Mager, A.; Morote-Garcia, J.C.; Unertl, K.; Eltzschig, H.K. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009, 10, 195–202. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Kerber, E.L.; Padberg, C.; Koll, N.; Schuetzhold, V.; Fandrey, J.; Winning, S. The Importance of Hypoxia-Inducible Factors (HIF-1 and HIF-2) for the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2020, 21, 8551. [Google Scholar] [CrossRef]
- Bäcker, V.; Cheung, F.Y.; Siveke, J.T.; Fandrey, J.; Winning, S. Knockdown of myeloid cell hypoxia-inducible factor-1α ameliorates the acute pathology in DSS-induced colitis. PLoS ONE 2017, 12, e0190074. [Google Scholar] [CrossRef]
- Flück, K.; Breves, G.; Fandrey, J.; Winning, S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016, 9, 379–390. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokari, R.; Hozumi, H.; Kurihara, C.; Ueda, T.; Watanabe, C.; Tomita, K.; Nakamura, M.; Komoto, S.; Okada, Y.; et al. HIF-1 in T cells ameliorated dextran sodium sulfate-induced murine colitis. J. Leukoc. Biol. 2012, 91, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Karhausen, J.; Furuta, G.T.; Tomaszewski, J.E.; Johnson, R.S.; Colgan, S.P.; Haase, V.H. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig. 2004, 114, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dong, W. Role of hypoxia-inducible factor-1α in pathogenesis and disease evaluation of ulcerative colitis. Exp. Ther. Med. 2016, 11, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, B.; Hao, J.; Shi, X.; Ge, J.; Xu, H. Correlation of Hypoxia-inducible factor-1α and C-reactive protein with disease evaluation in patients with ulcerative colitis. Am. J. Transl. Res. 2020, 12, 7826–7835. [Google Scholar] [PubMed]
- Bowser, J.L.; Phan, L.H.; Eltzschig, H.K. The Hypoxia-Adenosine Link during Intestinal Inflammation. J. Immunol. 2018, 200, 897–907. [Google Scholar] [CrossRef]
- Van Welden, S.; Selfridge, A.C.; Hindryckx, P. Intestinal hypoxia and hypoxia induced signalling as therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 596–611. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Sivridis, E.; Maltezos, E.; Papazoglou, D.; Simopoulos, C.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Hypoxia inducible factor 1 alpha and 2 alpha over expression in inflammatory bowel disease. J. Clin. Pathol. 2003, 56, 209–213. [Google Scholar] [CrossRef]
- Lin, N.; Shay, J.E.; Xie, H.; Lee, D.S.; Skuli, N.; Tang, Q.; Zhou, Z.; Azzam, A.; Meng, H.; Wang, H.; et al. Myeloid Cell Hypoxia-Inducible Factors Promote Resolution of Inflammation in Experimental Colitis. Front. Immunol. 2018, 9, 2565. [Google Scholar] [CrossRef]
- Keely, S.; Campbell, E.L.; Baird, A.W.; Hansbro, P.M.; Shalwitz, R.A.; Kotsakis, A.; McNamee, E.N.; Eltzschig, H.K.; Kominsky, D.J.; Colgan, S.P. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 2014, 7, 114–123. [Google Scholar] [CrossRef]
- Ghobadi, H.; Mohammadshahi, J.; Javaheri, N.; Fouladi, N.; Mirzazadeh, Y.; Aslani, M.R. Role of leukocytes and systemic inflammation indexes (NLR, PLR, MLP, dNLR, NLPR, AISI, SIR-I, and SII) on admission predicts in-hospital mortality in nonelderly and elderly COVID-19 patients. Front. Med. 2022, 9, 916453. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Scano, V.; Cau, S.; Babudieri, S.; Perra, R.; Ruzzittu, G.; Zinellu, E.; Pirina, P.; Carru, C.; et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules 2020, 25, 5725. [Google Scholar] [CrossRef]
- Zhang, M.H.; Wang, H.; Wang, H.G.; Wen, X.; Yang, X.Z. Effective immune-inflammation index for ulcerative colitis and activity assessments. World J. Clin. Cases 2021, 9, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhuang, T.; Ping, Y.; Zhang, Y.; Wang, X.; Yu, P.; Duan, X. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin. Chim. Acta 2021, 517, 122–126. [Google Scholar] [CrossRef]
- Pakoz, Z.B.; Ustaoglu, M.; Vatansever, S.; Yuksel, E.S.; Topal, F. Serum Immune-Inflammation Index Assessment in the Patients with Ulcerative Colitis. Gastroenterol. Res. Pract. 2022, 2022, 9987214. [Google Scholar] [CrossRef]
- Lin, H.; Bai, Z.; Wu, Q.; Chu, G.; Zhang, Y.; Guo, X.; Qi, X. Inflammatory Indexes for Assessing the Severity and Disease Progression of Ulcerative Colitis: A Single-Center Retrospective Study. Front. Public Health 2022, 10, 851295. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yu, Q.; Ou, X.; Cao, D. Systemic inflammation response index (SIRI) as a predictor for predicting inflammatory bowel disease (IBD) severity. Biomed. Res. 2018, 29, 938–944. [Google Scholar] [CrossRef]
- Jeong, Y.; Jeon, S.R.; Kim, H.G.; Moon, J.R.; Lee, T.H.; Jang, J.Y.; Cho, J.H.; Park, J.S.; Park, H.; Lee, K.H.; et al. The role of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in ulcerative colitis. Intest. Res. 2021, 19, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Pang, X.; Ji, G.; Ma, X.; Li, J.; Chang, Y.; Ma, C. Application of the neutrophil to lymphocyte ratio in the diagnosis and activity determination of ulcerative colitis: A meta-analysis and systematic review. Medicine 2021, 100, e27551. [Google Scholar] [CrossRef]
- Langley, B.O.; Guedry, S.E.; Goldenberg, J.Z.; Hanes, D.A.; Beardsley, J.A.; Ryan, J.J. Inflammatory Bowel Disease and Neutrophil-Lymphocyte Ratio: A Systematic Scoping Review. J. Clin. Med. 2021, 10, 4219. [Google Scholar] [CrossRef] [PubMed]
- Okba, A.M.; Amin, M.M.; Abdelmoaty, A.S.; Ebada, H.E.; Kamel, A.H.; Allam, A.S.; Sobhy, O.M. Neutrophil/lymphocyte ratio and lymphocyte/monocyte ratio in ulcerative colitis as non-invasive biomarkers of disease activity and severity. Autoimmun. Highlights 2019, 10, 4. [Google Scholar] [CrossRef]
- Demir, A.K.; Demirtas, A.; Kaya, S.U.; Tastan, I.; Butun, I.; Sagcan, M.; Sahin, S.; Tasliyurt, T.; Yilmaz, A. The relationship between the neutrophile lymphocyte ratio and disease activity in patients with ulcerative colitis. Kaohsiung J. Med. Sci. 2015, 31, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Celikbilek, M.; Dogan, S.; Ozbakır, O.; Zararsız, G.; Kücük, H.; Gürsoy, S.; Yurci, A.; Güven, K.; Yücesoy, M. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J. Clin. Lab. Anal. 2013, 27, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Cherfane, C.E.; Gessel, L.; Cirillo, D.; Zimmerman, M.B.; Polyak, S. Monocytosis and a Low Lymphocyte to Monocyte Ratio are Effective Biomarkers of Ulcerative Colitis Disease Activity. Inflamm. Bowel Dis. 2015, 21, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, M.Y.; Ozin, Y.O.; Kaplan, M.; Ates, I.; Kalkan, I.H.; Kilic, Z.; Yuksel, M.; Kayacetin, E. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J. Med. Biochem. 2018, 37, 155–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Zhou, K.; Jiang, S. The predictive role of systemic inflammation response index (Siri) in the prognosis of stroke patients. Clin. Interv. Aging 2021, 16, 1997–2007. [Google Scholar] [CrossRef]
- Hu, B.; Yang, X.-R.; Xu, Y.; Sun, Y.-F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.-M.; Qiu, S.-J.; Zhou, J.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, J.Y.; Suh, C.H.; Kim, H.A. Systemic immune-inflammation index combined with ferritin can serve as a reliable assessment score for adult-onset Still’s disease. Clin. Rheumatol. 2021, 40, 661–668. [Google Scholar] [CrossRef]
- Liu, X.; Guan, G.; Cui, X.; Liu, Y.; Liu, Y.; Luo, F. Systemic Immune-Inflammation Index (SII) Can Be an Early Indicator for Predicting the Severity of Acute Pancreatitis: A Retrospective Study. Int. J. Gen. Med. 2021, 14, 9483–9489. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Satoh, T.; Yoshino, Y.; Kondo, S.; Kawakami, Y.; Katayama, T.; Sasaki, Y.; Takasu, A.; Kogure, T.; Hirota, M.; et al. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Noninvasive Predictors of the Therapeutic Outcomes of Systemic Corticosteroid Therapy in Ulcerative Colitis. Inflamm. Intest. Dis. 2021, 6, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hosomi, S.; Yamagami, H.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; Tanigawa, T.; et al. Neutrophil-to-Lymphocyte Ratio for Predicting Loss of Response to Infliximab in Ulcerative Colitis. PLoS ONE 2017, 12, e0169845. [Google Scholar] [CrossRef]
- Nishida, Y.; Hosomi, S.; Yamagami, H.; Sugita, N.; Itani, S.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; et al. Pretreatment neutrophil-to-lymphocyte ratio predicts clinical relapse of ulcerative colitis after tacrolimus induction. PLoS ONE 2019, 14, e0213505. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Rossari, F.; Barberio, B.; Demarzo, M.G.; Tapete, G.; Albano, E.; Baiano Svizzero, G.; Ceccarelli, L.; Mumolo, M.G.; Brombin, C.; et al. Novel prognostic biomarkers of mucosal healing in ulcerative colitis patients treated with anti-TNF: Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Inflamm. Bowel Dis. 2020, 26, 1579–1587. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Mendieta-Escalante, E.A. Diagnostic utility of the neutrophil-platelet ratio as a novel marker of activity in patients with Ulcerative Colitis. PLoS ONE 2020, 15, e0231988. [Google Scholar] [CrossRef]
- Cifci, S.; Ekmen, N. Prediction of Mucosal Health by NLR, CRP x NLR and MPV in Ulcerative Colitis: Can Their Availability Change According to Treatment Options? Cureus 2021, 13, e19942. [Google Scholar] [CrossRef]
- Argeny, S.; Stift, A.; Bergmann, M.; Mittlböck, M.; Maschke, S.; Yang, Y.; Chitsabesan, P.; Riss, S. Prognostic value of preoperative neutrophil-to-lymphocyte ratio in Crohn’s disease. Wien. Klin. Wochenschr. 2018, 130, 398–403. [Google Scholar] [CrossRef]
- Xu, M.; Cen, M.; Chen, X.; Chen, H.; Liu, X.; Cao, Q. Correlation between Serological Biomarkers and Disease Activity in Patients with Inflammatory Bowel Disease. Biomed. Res. Int. 2019, 2019, 6517549. [Google Scholar] [CrossRef]
- Radulescu, P.M.; Davitoiu, D.V.; Baleanu, V.D.; Padureanu, V.; Ramboiu, D.S.; Surlin, M.V.; Bratiloveanu, T.C.; Georgescu, E.F.; Streba, C.T.; Mercut, R.; et al. Has COVID-19 Modified the Weight of Known Systemic Inflammation Indexes and the New Ones (MCVL and IIC) in the Assessment as Predictive Factors of Complications and Mortality in Acute Pancreatitis? Diagnostics 2022, 12, 3118. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef]
- Truelove, S.C.; Witts, L.J. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br. Med. J. 1955, 2, 375–378. [Google Scholar] [CrossRef]
- Available online: https://www.nice.org.uk/guidance/ng130/chapter/Recommendations (accessed on 10 September 2023).
- Torun, S.; Tunc, B.D.; Suvak, B.; Yildiz, H.; Tas, A.; Sayilir, A.; Ozderin, Y.O.; Beyazit, Y.; Kayacetin, E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: A promising marker in predicting disease severity. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 491–497. [Google Scholar] [CrossRef]
- Vermeire, S.; Van Assche, G.; Rutgeerts, P. Laboratory markers in IBD: Useful, magic, or unnecessary toys? Gut 2006, 55, 426–431. [Google Scholar] [CrossRef]
- Ye, X.; Xuejing, H.; Xunlei, P. Value of ratio of neutrophils to lymphocytes in diagnosis and evaluation of ulcerative colitis. Jiangsu Med. J. 2014, 40, 2042–2044. [Google Scholar]
- Jingyuan, T.; Hesheng, L. Clinical value of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in the assessment of ulcerative colitis. Chin. J. Gastroenterol. Hepatol. 2019, 28, 291–295. [Google Scholar]
- Feng, W.; Liu, Y.; Zhu, L.; Xu, L.; Shen, H. Evaluation of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential markers for ulcerative colitis: A retrospective study. BMC Gastroenterol. 2022, 22, 485. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Takamori, S.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Oba, T.; Osoegawa, A.; Tagawa, T.; Takenoyama, M.; et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer 2020, 145, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, B.; Huang, X. Study on the evaluation value of serum HIF-1α on disease activity and mucosal healing in patients with ulcerative colitis. Dig. J. Integr. West. Med. 2022, 30, 844–848. [Google Scholar]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4807–4812. [Google Scholar] [CrossRef]
- Welcker, K.; Martin, A.; Kölle, P.; Siebeck, M.; Gross, M. Increased intestinal permeability in patients with inflammatory bowel disease. Eur. J. Med. Res. 2004, 9, 456–460. [Google Scholar]
- Postovalova, E.A.; Makarova, O.V.; Kosyreva, A.M.; Dzhalilova, D.S. Hypoxia is a key mechanism for regulating inflammation in ulcerative colitis. Russ. Open Med. J. 2020, 9, e0101. [Google Scholar] [CrossRef]
- Pastor Rojo, O.; López San Román, A.; Albéniz Arbizu, E.; de la Hera Martínez, A.; Ripoll Sevillano, E.; Albillos Martínez, A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.; Bo, S. Colonoscopic perforation in inflammatory bowel disease. Gastroenterol. Hepatol. 2013, 9, 573–583. [Google Scholar]
- Yoon, J.Y.; Park, S.J.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Schwarzenberg, S.J.; Sharp, H.; Greenwood, D.; Weisdorf-Schindele, S. Role of serology and routine laboratory tests in childhood inflammatory bowel disease. Inflamm. Bowel Dis. 2002, 8, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am. J. Gastroenterol. 2008, 103, 162–169. [Google Scholar] [CrossRef]
- Li, Y.; Nuij, V.J.; Baars, J.E.; Biermann, K.; Kuipers, E.J.; Peppelenbosch, M.P.; de Haar, C.; Janneke van der Woude, C. Increased suppressor of cytokine signaling-3 expression predicts mucosal relapse in ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 132–140. [Google Scholar] [CrossRef]
- Fidan, K.; Kocak, M.Z. Assessment of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ulcerative colitis: A retrospective study. EurasianJ Med. Oncol. 2017, 1, 224–227. [Google Scholar]




| Characteristics | UC (N = 46) | C (N = 23) |
|---|---|---|
| Age (year) (mean ± stdev) | 49.30 ± 18.00 | 54.30 ± 5.75 |
| Sex ratio (male/female) (%) | 25/21 (54.35%) | 13/10 (56.52%) |
| Residence, n (%) | ||
| Urban | 30 (65.22%) | 14 (60.87%) |
| Rural | 16 (34.78%) | 9 (39.13%) |
| Montreal classification of extent of ulcerative colitis, n (%) | ||
| E1—Ulcerative proctitis | 15 (32.61%) | - |
| E2—Left-sided UC (distal UC) | 18 (39.13%) | - |
| E3—Extensive UC (pancolitis) | 13 (28.26%) | - |
| Truelove and Witts index (TWI), n (mean ± stdev) | ||
| TWI score ≤ 5—mild activity | 15 (3.20 ± 0.56) | - |
| TWI score = 5–9 moderate activity | 22 (7.23 ± 1.15) | - |
| TWI score ≥ 9 severe activity | 9 (11.33 ± 1.50) | - |
| Parameter (Mean ± Stdev) | Disease Activity (TWI Severity Index) | p-Value from Kruskal-Wallis/ One-Way ANOVA | |||
|---|---|---|---|---|---|
| Mi (N = 15) | Mo (N = 22) | S (N = 9) | C (n = 23) | ||
| HIF-1α (ng/L) | 91.49 ± 42.21 | 157.20 ± 74.57 | 396.70 ± 86.51 | 51.90 ± 24.34 | p < 0.05 |
| Extent of UC (Montreal classification) | |||||
| E1 (N = 15) | E2 (N = 18) | E3 (N = 13) | C (n = 23) | ||
| 93.46 ± 36.45 | 211.52 ± 134.68 | 245.64 ± 141.46 | 51.90 ± 24.34 | ||
| Parameter (Mean ± Stdev) | Mi Group (N = 15) | Mo Group (N = 22) | S Group (N = 9) | p-Value | ||
|---|---|---|---|---|---|---|
| Mo vs. Mi | S vs. Mi | S vs. Mo | ||||
| hs-CRP (mg/dL) | 16.53 ± 5.89 | 29.27 ± 24.30 | 31.56 ± 12.44 | 0.0240 * | 0.0084 * | 0.6957 |
| ESR (mm/1st h) | 20.27 ± 7.65 | 51.59 ± 14.34 | 97.33 ± 19.14 | p < 0.0001 * | p < 0.0001 * | 0.0002 * |
| FIB (mg/dL) | 354.53 ± 51.79 | 438.86 ± 85.30 | 599.00 ± 110.01 | 0.0339 * | 0.0002 * | 0.0002 * |
| Haemoglobin (g/dL) | 13.11 ± 1.19 | 12.38 ± 11.71 | 9.30 ± 1.44 | 0.2075 | 0.0006 * | 0.0419 * |
| WBC (×103/μL) | 6.03 ± 0.98 | 8.45 ± 0.90 | 12.33 ± 1.18 | p < 0.0001 * | p < 0.0001 * | p < 0.0001 * |
| NEU (×103/μL) | 4.41 ± 0.84 | 5.89 ± 1.05 | 8.43 ± 1.55 | p < 0.0001 * | p < 0.0001 * | 0.0001 * |
| LYM (×103/μL) | 3.36 ± 1.09 | 2.19 ± 0.39 | 1.29 ± 0.31 | 0.0069 * | 0.0003 * | 0.0024 * |
| MON (×103/μL) | 0.33 ± 0.17 | 0.37 ± 0.14 | 0.54 ± 0.33 | 0.4909 | 0.1050 | 0.0484 * |
| PLT (×103/μL) | 2.15 ± 0.51 | 2.60 ± 1.07 | 3.36 ±0.78 | 0.3694 | 0.0495 * | 0.7901 |
| MCV (fL) | 85.81 ± 6.67 | 91.58 ± 4.49 | 102.81 ± 3.72 | 0.3968 | p < 0.0001 * | 0.0001 * |
| RDW (%) | 13.40 ± 0.73 | 14.25 ± 1.44 | 18.74 ± 2.54 | 0.0029 * | 0.0006 * | 0.0041 * |
| NLR | 2.55 ± 1.15 | 2.81 ± 0.86 | 3.58 ± 1.03 | 0.6641 | 0.0413 * | 0.0517 |
| MLR | 0.16 ± 0.10 | 0.17 ± 0.06 | 0.26 ± 0.14 | 0.2368 | 0.0489 * | 0.0520 |
| PLR | 66.01 ± 28.50 | 119.28 ± 46.50 | 274.84 ± 86.03 | 0.0124 * | 0.0002 * | 0.0278 * |
| dNLR | 2.19 ± 0.92 | 2.40 ± 0.69 | 2.84 ± 0.72 | 0.0888 | 0.1062 | 0.0644 |
| AISI | 2364.94 ± 1027.46 | 2664.84 ± 1421.79 | 3941.33 ± 2611.54 | 0.1193 | 0.0403 * | 0.0447 * |
| SII | 560.83 ± 312.94 | 696.89 ± 308.38 | 1192.70 ± 376.13 | 0.0572 | 0.0306 * | 0.0467 * |
| SIRI | 984.93 ± 418.07 | 1183.45 ± 795.83 | 1301.41 ± 735.55 | 0.1033 | 0.0270 * | 0.0656 |
| MCVL | 31.34 ± 10.98 | 42.91 ± 7.63 | 70.05 ± 16.44 | 0.0073 * | 0.0005 * | 0.0127 * |
| IIC | 3.70 ± 1.33 | 4.10 ± 1.13 | 4.78 ± 1.83 | 0.6066 | 0.0298 * | 0.0467 * |
| Parameter (Mean ± Stdev) | E1 (N = 15) | E2 (N = 18) | E3 (N = 13) | p-Value | ||
|---|---|---|---|---|---|---|
| E2 vs. E1 | E3 vs. E1 | E3 vs. E2 | ||||
| hs-CRP (mg/dL) | 23.17 ± 20.22 | 24.54 ± 22.92 | 29.33 ± 13.06 | 0.5350 | 0.0068 * | 0.0714 |
| ESR (mm/1st hr) | 32.13 ± 19.97 | 50.15 ± 25.24 | 63.22 ± 37.05 | 0.0204 * | 0.0120 * | 0.0923 |
| FIB (mg/dL) | 371.73 ± 59.03 | 471.78 ± 136.93 | 484.31 ± 109.22 | 0.0391 * | 0.0003 * | 0.1576 |
| Haemoglobin (g/dL) | 12.82 ± 1.59 | 12.50 ± 0.84 | 11.41 ± 2.47 | 0.5790 | 0.1989 | 0.3366 |
| WBC (×103/μL) | 7.56 ± 1.56 | 9.566 ± 2.98 | 9.69 ± 1.84 | 0.0176 * | 0.0040 * | 0.0712 |
| NEU (×103/μL) | 5.572 ± 1.47 | 6.7 ± 2.24 | 6.92 ± 1.62 | 0.0462 * | 0.0270 * | 0.2650 |
| LYM (×103/μL) | 2.40 ± 1.21 | 2.37 ± 0.82 | 1.70 ± 0.66 | 0.7160 | 0.0483 * | 0.0418 * |
| MON (×103/μL) | 0.35 ± 0.15 | 0.40 ± 0.13 | 0.42 ± 0.27 | 0.5279 | 0.3918 | 0.7186 |
| PLT (×103/μL) | 2.74 ± 0.79 | 2.89 ± 0.13 | 3.07 ± 0.65 | 0.7988 | 0.2218 | 0.8850 |
| MCV (fL) | 87.83 ± 4.55 | 89.62 ± 11.90 | 96.15 ± 8.90 | 0.3812 | 0.0466 * | 0.1281 |
| RDW (%) | 14.38 ± 1.35 | 15.37 ± 3.03 | 16.21 ± 3.21 | 0.6906 | 0.1279 | 0.3892 |
| NLR | 3.19 ± 1.10 | 3.21 ± 1.44 | 3.32 ± 1.06 | 0.5017 | 0.0437 * | 0.7177 |
| MLR | 0.17 ± 0.05 | 0.19 ± 0.10 | 0.21 ± 0.10 | 0.4907 | 0.0427 * | 0.9315 |
| PLR | 139.84 ± 82.16 | 140.46 ± 77.05 | 197.48 ± 91.16 | 0.3398 | 0.0168 * | 0.0264 * |
| dNLR | 2.65 ± 1.06 | 2.72 ± 0.93 | 2.76 ± 0.89 | 0.8102 | 0.6643 | 1.0000 |
| AISI | 3257.40 ± 1125.00 | 3493.60 ± 2533.50 | 3500.50 ± 2023.70 | 0.4836 | 0.8538 | 0.5508 |
| SII | 883.65 ± 481.33 | 930.10 ± 472.05 | 1034.00 ± 434.16 | 0.6997 | 0.0267 * | 0.5804 |
| SIRI | 1093.50 ± 489.63 | 1204.20 ± 427.51 | 1316.60 ± 865.57 | 0.0684 | 0.0456 * | 0.0942 |
| MCVL | 42.21 ± 14.84 | 47.79 ± 19.80 | 54.96 ± 18.88 | 0.5089 | 0.0353 * | 0.3028 |
| IIC | 4.20 ± 1.42 | 4.71 ± 2.33 | 4.75 ± 2.26 | 0.8707 | 0.0461 * | 0.8886 |
| Parameter | AUC | Cut off Values | Sensitivity % | Specificity % | p-Value |
|---|---|---|---|---|---|
| TWI | 0.837 | 6.50 | 100.09 | 67.39 | 0.049 |
| HIF-1α | 0.739 | 119.80 | 73.33 | 60.87 | 0.036 |
| MCVL | 0.709 | 45.63 | 73.33 | 58.70 | 0.025 |
| PLR | 0.704 | 129.40 | 73.33 | 54.35 | 0.047 |
| IIC | 0.610 | 2.965 | 66.67 | 56.52 | 0.078 |
| SIRI | 0.568 | 791.90 | 60.00 | 63.04 | 0.090 |
| SII | 0.592 | 900.30 | 60.00 | 52.17 | 0.078 |
| MLR | 0.568 | 0.170 | 60.00 | 54.35 | 0.089 |
| NLR | 0.527 | 2.97 | 53.33 | 50.00 | 0.083 |
| AISI | 0.509 | 2783 | 60.00 | 52.17 | 0.091 |
| dNLR | 0.506 | 2.485 | 60.00 | 50.00 | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poenariu, I.S.; Boldeanu, L.; Ungureanu, B.S.; Caragea, D.C.; Cristea, O.M.; Pădureanu, V.; Siloși, I.; Ungureanu, A.M.; Statie, R.-C.; Ciobanu, A.E.; et al. Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis. Biomedicines 2023, 11, 3137. https://doi.org/10.3390/biomedicines11123137
Poenariu IS, Boldeanu L, Ungureanu BS, Caragea DC, Cristea OM, Pădureanu V, Siloși I, Ungureanu AM, Statie R-C, Ciobanu AE, et al. Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis. Biomedicines. 2023; 11(12):3137. https://doi.org/10.3390/biomedicines11123137
Chicago/Turabian StylePoenariu, Ioan Sabin, Lidia Boldeanu, Bogdan Silviu Ungureanu, Daniel Cosmin Caragea, Oana Mariana Cristea, Vlad Pădureanu, Isabela Siloși, Anca Marinela Ungureanu, Răzvan-Cristian Statie, Alina Elena Ciobanu, and et al. 2023. "Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis" Biomedicines 11, no. 12: 3137. https://doi.org/10.3390/biomedicines11123137







