Inflammation in Prostate Cancer: Exploring the Promising Role of Phenolic Compounds as an Innovative Therapeutic Approach
Abstract
:1. Introduction
2. Epidemiology of Prostate Cancer
3. Diagnosis of Prostate Cancer
4. Pathophysiology of Prostate Cancer
5. Current Therapeutic Strategies Used in Prostate Cancer
6. Inflammation and Prostate Cancer
Origins of Inflammation
7. Role of Leukocytes in Prostate Cancer
7.1. Neutrophils
7.2. Basophils
7.3. Eosinophils
7.4. Mast Cells
7.5. Macrophages
7.6. T Cells
7.7. B Cells
7.8. Overall Remarks
8. Polyphenol Compounds in Prostate Cancer
Polyphenol-Gold Based Nanoparticles
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Packer, J.R.; Maitland, N.J. The Molecular and Cellular Origin of Human Prostate Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1238–1260. [Google Scholar] [CrossRef] [PubMed]
- Maitland, N.J.; Collins, A.T. Inflammation as the Primary Aetiological Agent of Human Prostate Cancer: A Stem Cell Connection? J. Cell Biochem. 2008, 105, 931–939. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Trudeau, K.; Rousseau, M.-C.; Barul, C.; Csizmadi, I.; Parent, M.-É. Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada. Nutrients 2020, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Godtman, R.A.; Kollberg, K.S.; Pihl, C.-G.; Månsson, M.; Hugosson, J. The Association between Age, Prostate Cancer Risk, and Higher Gleason Score in a Long-Term Screening Program: Results from the Göteborg-1 Prostate Cancer Screening Trial. Eur. Urol. 2022, 82, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Faria, I.; Aires, F.; Monteiro, A.; Pinto, G.; Sales, M.G.; Correa-Duarte, M.A.; Guerreiro, S.G.; Fernandes, R. Application of Gold Nanoparticles as Radiosensitizer for Metastatic Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 4122. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Modulation of Signaling Pathways in Prostate Cancer by Green Tea Polyphenols. Biochem. Pharmacol. 2013, 85, 667–672. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 283. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Giona, S. The Epidemiology of Prostate Cancer. In Prostate Cancer; Exon Publications: Brisbane City, Australia, 2021; pp. 1–16. [Google Scholar]
- Lillard, J.W.; Moses, K.A.; Mahal, B.A.; George, D.J. Racial Disparities in Black Men with Prostate Cancer: A Literature Review. Cancer 2022, 128, 3787–3795. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; De La Roza, G. The Worldwide Epidemiology of Prostate Cancer: Perspectives from Autopsy Studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar] [PubMed]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as Influenced by Race, Ethnicity, and Geographic Location. Am. J. Mens. Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Purification of a Human Prostate Specific Antigen. Investig. Urol. 1979, 17, 159–163. [Google Scholar]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.J.; Petros, J.A.; Andriole, G.L. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-Yr Follow-up of the European Randomized Study of Screening for Prostate Cancer (Figure Presented). Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- NCCN Guidelines for Patients, Early-Stage Prostate Cancer. Available online: https://www.nccn.org/patients/guidelines/content/PDF/prostate-early-patient.pdf (accessed on 30 October 2023).
- Logozzi, M.; Angelini, D.F.; Iessi, E.; Mizzoni, D.; Di Raimo, R.; Federici, C.; Lugini, L.; Borsellino, G.; Gentilucci, A.; Pierella, F.; et al. Increased PSA Expression on Prostate Cancer Exosomes in in Vitro Condition and in Cancer Patients. Cancer Lett. 2017, 403, 318–329. [Google Scholar] [CrossRef]
- Humphrey, P.A. Histopathology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030411. [Google Scholar] [CrossRef]
- Amin, M.B.; Omar, T.; Hussain, A.; Aron, M.; Brimo, F. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Gradind System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar]
- Kim, C.-H.; Bhattacharjee, S.; Prakash, D.; Kang, S.; Cho, N.-H.; Kim, H.-C.; Choi, H.-K.; Prakash, S.; Kang, D.; Cho, S.; et al. Artificial Intelligence Techniques for Prostate Cancer Detection through Dual-Channel Tissue Feature Engineering. Cancers 2021, 13, 1524. [Google Scholar] [CrossRef]
- Kweldam, C.F.; van Leenders, G.J.; van der Kwast, T. Grading of Prostate Cancer: A Work in Progress. Histopathology 2019, 74, 146–160. [Google Scholar] [CrossRef]
- Inamura, K. Prostatic Cancers: Understanding Their Molecular Pathology and the 2016 WHO Classification. Oncotarget 2018, 9, 14723–14737. [Google Scholar] [CrossRef] [PubMed]
- Avenel, C.; Tolf, A.; Dragomir, A.; Carlbom, I.B. Glandular Segmentation of Prostate Cancer: An Illustration of How the Choice of Histopathological Stain Is One Key to Success for Computational Pathology. Front. Bioeng. Biotechnol. 2019, 7, 125. [Google Scholar] [CrossRef]
- Dong, B.; Miao, J.; Wang, Y.; Luo, W.; Ji, Z.; Lai, H.; Zhang, M.; Cheng, X.; Wang, J.; Fang, Y.; et al. Single-Cell Analysis Supports a Luminal-Neuroendocrine Transdifferentiation in Human Prostate Cancer. Commun. Biol. 2020, 3, 778. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.H.; Malewska, A.; Joseph, D.B.; Malladi, V.S.; Lee, J.; Torrealba, J.; Mauck, R.J.; Gahan, J.C.; Raj, G.V.; Roehrborn, C.G.; et al. A Cellular Anatomy of the Normal Adult Human Prostate and Prostatic Urethra. Cell Rep. 2018, 25, 3530–3542.e5. [Google Scholar] [CrossRef] [PubMed]
- Grisanzio, C.; Signoretti, S. P63 in Prostate Biology and Pathology. J. Cell Biochem. 2008, 103, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Putzi, M.J.; De Marzo, A.M. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology 2000, 56, 828–832. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative Inflammatory Atrophy of the Prostate Implications for Prostatic Carcinogenesis. Am. J. Pathol. 1999, 155, 1985–1992. [Google Scholar] [CrossRef]
- Bethel, C.R.; Faith, D.; Li, X.; Guan, B.; Hicks, J.L.; Lan, F.; Jenkins, R.B.; Bieberich, C.J.; De Marzo, A.M. Decreased NKX3.1 Protein Expression in Focal Prostatic Atrophy, Prostatic Intraepithelial Neoplasia, and Adenocarcinoma: Association with Gleason Score and Chromosome 8p Deletion. Cancer Res. 2006, 66, 10683–10690. [Google Scholar] [CrossRef]
- Wang, W. Inflammation and Prostatic Carcinogenesis: A Morphological Study of the Human Prostate. Ph.D. Thesis, Department of Urology, Institute of Clinical Sciences, Sahlgrenska University Hospital, The Sahlgrenska Academy at Göteborg University, Gothenburg, Sweden, 2007. [Google Scholar]
- Shen, M.M.; Abate-Shen, C. Molecular Genetics of Prostate Cancer: New Prospects for Old Challenges. Genes. Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Qian, J. High-Grade Prostatic Intraepithelial Neoplasia. Mod. Pathol. 2004, 17, 360–379. [Google Scholar] [CrossRef]
- Sakr, W.A.; Haas, G.P.; Cassin, B.F.; Pontes, J.E.; Crissman, J.D. The Frequency of Carcinoma and Intraepithelial Neoplasia of the Prostate in Young Male Patients. J. Urol. 1993, 150, 379–385. [Google Scholar] [CrossRef]
- Kallakury, B.V.; Brien, T.P.; Lowry, C.V.; Muraca, P.J.; Fisher, H.A.; Kaufman, R.P., Jr.; Ross, J.S. Telomerase activity in human benign prostate tissue and prostatic adenocarcinomas. Diagn. Mol. Pathol. 1997, 6, 192–198. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting Chromosomes against Genome Instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wymenga, L.F.A.; Wisman, G.B.A.; Veenstra, R.; Ruiters, M.H.J.; Mensink, H.J.A. Telomerase Activity in Needle Biopsies from Prostate Cancer and Benign Prostates. Eur. J. Clin. Investig. 2000, 30, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- Park, K.; Dalton, J.T.; Narayanan, R.; Barbieri, C.E.; Hancock, M.L.; Bostwick, D.G.; Steiner, M.S.; Rubin, M.A. TMPRSS2:ERG Gene Fusion Predicts Subsequent Detection of Prostate Cancer in Patients with High-Grade Prostatic Intraepithelial Neoplasia. J. Clin. Oncol. 2014, 32, 206–211. [Google Scholar] [CrossRef]
- Geng, C.; He, B.; Xu, L.; Barbieri, C.E.; Eedunuri, V.K.; Chew, S.A.; Zimmermann, M.; Bond, R.; Shou, J.; Li, C.; et al. Prostate Cancer-Associated Mutations in Speckle-Type POZ Protein (SPOP) Regulate Steroid Receptor Coactivator 3 Protein Turnover. Proc. Natl. Acad. Sci. USA 2013, 110, 6997–7002. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Bruno, T.C.; Meeker, A.K.; De Marzo, A.M.; Isaacs, W.B.; Drake, C.G. Human Prostate-Infiltrating CD8+ T Lymphocytes Are Oligoclonal and PD-1+. Prostate 2009, 69, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, N.; Takayama, H.; Nakayama, M.; Nakai, Y.; Kawashima, A.; Mukai, M.; Nagahara, A.; Aozasa, K.; Tsujimura, A. Infiltration of Tumour-Associated Macrophages in Prostate Biopsy Specimens Is Predictive of Disease Progression after Hormonal Therapy for Prostate Cancer. BJU Int. 2011, 107, 1918–1922. [Google Scholar] [CrossRef]
- Woo, J.R.; Liss, M.A.; Muldong, M.T.; Palazzi, K.; Strasner, A.; Ammirante, M.; Varki, N.; Shabaik, A.; Howell, S.; Kane, C.J.; et al. Tumor Infiltrating B-Cells Are Increased in Prostate Cancer Tissue. J. Transl. Med. 2014, 12, 30. [Google Scholar] [CrossRef]
- Birnie, R.; Bryce, S.D.; Roome, C.; Dussupt, V.; Droop, A.; Lang, S.H.; Berry, P.A.; Hyde, C.F.; Lewis, J.L.; Stower, M.J.; et al. Gene Expression Profiling of Human Prostate Cancer Stem Cells Reveals a Pro-Inflammatory Phenotype and the Importance of Extracellular Matrix Interactions. Genome Biol. 2008, 9, R83. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Allory, Y.; Beukers, W.; Sagrera, A.; Flández, M.; Marqués, M.; Márquez, M.; Van Der Keur, K.A.; Dyrskjot, L.; Lurkin, I.; Vermeij, M.; et al. Telomerase Reverse Transcriptase Promoter Mutations in Bladder Cancer: High Frequency across Stages, Detection in Urine, and Lack of Association with Outcome. Eur. Urol. 2014, 65, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yeow, W.S.; Ertel, A.; Coleman, I.; Clegg, N.; Thangavel, C.; Morrissey, C.; Zhang, X.; Comstock, C.E.S.; Witkiewicz, A.K.; et al. The Retinoblastoma Tumor Suppressor Controls Androgen Signaling and Human Prostate Cancer Progression. J. Clin. Investig. 2010, 120, 4478–4492. [Google Scholar] [CrossRef]
- Luzzago, S.; Suardi, N.; Dell’Oglio, P.; Cardone, G.; Gandaglia, G.; Esposito, A.; De Cobelli, F.; Cristel, G.; Kinzikeeva, E.; Freschi, M.; et al. Multiparametric MRI Represents an Added Value but Not a Substitute of Follow-up Biopsies in Patients on Active Surveillance for Low-Risk Prostate Cancer. Eur. Urol. Suppl. 2017, 16, e1395–e1396. [Google Scholar] [CrossRef]
- Keyes, M.; Crook, J.; Morton, G.; Vigneault, E.; Usmani, N.; Morris, W.J. Treatment Options for Localized Prostate Cancer. Can. Fam. Physician 2013, 59, 1269–1274. [Google Scholar]
- Kipriyanov, E.A.; Karnaukh, P.A.; Vazhenin, I.A.; Vazhenin, A.V. Radical prostatectomy and robotic radiosurgery as treatment options for localized prostate cancer. Sib. J. Oncol. 2020, 19, 50–56. [Google Scholar] [CrossRef]
- Hoey, C.; Ray, J.; Jeon, J.; Huang, X.; Taeb, S.; Ylanko, J.; Andrews, D.W.; Boutros, P.C.; Liu, S.K. MiRNA-106a and Prostate Cancer Radioresistance: A Novel Role for LITAF in ATM Regulation. Mol. Oncol. 2018, 12, 1324–1341. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Wallner, K.; Lee, H.; Wasserman, S.; Dattoli, M. Low risk of urinary incontinence following prostate brachytherapy in patients with a prior transurethral prostate resection. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 565–569. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, C.; Nadiminty, N.; Lou, W.; Tummala, R.; Evans, C.P.; Gao, A.C. Inhibition of Abcb1 Expression Overcomes Acquired Docetaxel Resistance in Prostate Cancer. Mol. Cancer Ther. 2013, 12, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A. Cabazitaxel: A Novel Taxane for Metastatic Castration-Resistant Prostate Cancer-Current Implications and Future Prospects. J. Pharmacol. Pharmacother. 2013, 4, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Roth, B.J.; Dahm, P.; Engstrom, C.; Freedland, S.J.; Hussain, M.; Lin, D.W.; Lowrance, W.T.; Murad, M.H.; Oh, W.K.; et al. Castration-Resistant Prostate Cancer: AUA Guideline. J. Urol. 2013, 190, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Zraik, I.M.; Heß-Busch, Y. Management von Nebenwirkungen Der Chemotherapie Und Deren Langzeitfolgen. Urologe 2021, 60, 862–871. [Google Scholar] [CrossRef]
- Heidenreich, A.; Aus, G.; Bolla, M.; Joniau, S.; Matveev, V.B.; Schmid, H.P.; Zattoni, F. EAU Guidelines on Prostate Cancer. Eur. Urol. 2008, 53, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Seidenfeld, J.; Samson, D.J.; Hasselblad, V.; Aronson, N.; Albertsen, P.C.; Bennett, C.L.; Wilt, T.J. Single-Therapy Androgen Suppression in Men with Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2000, 132, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Belldegrun, A. Novel Therapeutic Strategies for Castration Resistant Prostate Cancer: Inhibition of Persistent Androgen Production and Androgen Receptor Mediated Signaling. J. Urol. 2011, 185, 787–794. [Google Scholar] [CrossRef]
- Wang, I.; Song, L.; Wang, B.Y.; Rezazadeh Kalebasty, A.; Uchio, E.; Zi, X. Prostate Cancer Immunotherapy: A Review of Recent Advancements with Novel Treatment Methods and Efficacy. Am. J. Clin. Exp. Urol. 2022, 10, 210–233. [Google Scholar] [PubMed]
- Perera, M.P.J.; Thomas, P.B.; Risbridger, G.P.; Taylor, R.; Azad, A.; Hofman, M.S.; Williams, E.D.; Vela, I. Chimeric Antigen Receptor T-Cell Therapy in Metastatic Castrate-Resistant Prostate Cancer. Cancers 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Muralidhar, A.; McNeel, D.G. Vaccines as Treatments for Prostate Cancer. Nat. Rev. Urol. 2023, 20, 544–559. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in Prostate Carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Houlahan, K.E.; Ramanand, S.G.; Lee, S.; Baek, G.; Yang, Y.; Chen, Y.; Strand, D.W.; Zhang, M.Q.; Boutros, P.C.; et al. Prostate Cancer Transcriptomic Regulation by the Interplay of Germline Risk Alleles, Somatic Mutations, and 3D Genomic Architecture. Cancer Discov. 2022, 12, 2838–2855. [Google Scholar] [CrossRef]
- Sfanos, K.S.; de Marzo, A.M. Prostate Cancer and Inflammation: The Evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Costanzo-Garvey, D.L.; Keeley, T.; Case, A.J.; Watson, G.F.; Alsamraae, M.; Yu, Y.; Su, K.; Heim, C.E.; Kielian, T.; Morrissey, C.; et al. Neutrophils Are Mediators of Metastatic Prostate Cancer Progression in Bone. Cancer Immunol. Immunother. 2020, 69, 1113–1130. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Roya, N.; Fatemeh, T.; Faramarz, M.A.; Milad, S.G.; Mohammad-Javad, S.; Najmeh, S.V.; Yousef, M.; Nader, B. Frequency of IL-10+CD19+ B Cells in Patients with Prostate Cancer Compared to Patients with Benign Prostatic Hyperplasia. Afr. Health Sci. 2020, 20, 1264–1272. [Google Scholar] [CrossRef]
- Deola, S.; Panelli, M.C.; Maric, D.; Selleri, S.; Dmitrieva, N.I.; Voss, C.Y.; Klein, H.; Stroncek, D.; Wang, E.; Marincola, F.M. Helper B Cells Promote Cytotoxic T Cell Survival and Proliferation Independently of Antigen Presentation through CD27/CD70 Interactions. J. Immunol. 2008, 180, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, L.; Yeh, S.; Cui, Y.; Li, X.; Chang, H.-C.; Jin, J.; Chang, C. Infiltrating T Cells Promote Prostate Cancer Metastasis via Modulation of FGF11→miRNA-541→androgen Receptor (AR)→MMP9 Signaling. Mol. Oncol. 2015, 9, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Jin, S.; Yang, Y.; Sheng, J.; He, Q.; Song, Y.; Yu, W.; Hu, S.; Jin, J. Infiltrating CD4+ T Cells Attenuate Chemotherapy Sensitivity in Prostate Cancer via CCL5 Signaling. Prostate 2019, 79, 1018–1031. [Google Scholar] [CrossRef]
- Kiniwa, Y.; Miyahara, Y.; Wang, H.Y.; Peng, W.; Peng, G.; Wheeler, T.M.; Thompson, T.C.; Old, L.J.; Wang, R.-F. CD8+ Foxp3+ Regulatory T Cells Mediate Immunosuppression in Prostate Cancer. Clin. Cancer Res. 2007, 13, 6947–6958. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Wang, H.W.; Gadea, B.B.; Shree, T.; Hunter, K.E.; Garfall, A.L.; Berman, T.; Joyce, J.A. IL-4 Induces Cathepsin Protease Activity in Tumor-Associated Macrophages to Promote Cancer Growth and Invasion. Genes Dev. 2010, 24, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.O.; Andrén, O.; Davidsson, S. M2 Macrophages and Regulatory T Cells in Lethal Prostate Cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Gao, W.Q. The Evolving Role of Immune Cells in Prostate Cancer. Cancer Lett. 2022, 525, 9–21. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willettt, W.C. The Causes and Prevention of Cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef]
- Doat, S.; Cénée, S.; Trétarre, B.; Rebillard, X.; Lamy, P.J.; Bringer, J.P.; Iborra, F.; Murez, T.; Sanchez, M.; Menegaux, F. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Prostate Cancer Risk: Results from the EPICAP Study. Cancer Med. 2017, 6, 2461–2470. [Google Scholar] [CrossRef]
- Salinas, C.A.; Kwon, E.M.; Fitzgerald, L.M.; Feng, Z.; Nelson, P.S.; Ostrander, E.A.; Peters, U.; Stanford, J.L. Use of Aspirin and Other Nonsteroidal Antiinflammatory Medications in Relation to Prostate Cancer Risk. Am. J. Epidemiol. 2010, 172, 578–590. [Google Scholar] [CrossRef]
- Longo, J.; Freedland, S.J.; Penn, L.Z.; Hamilton, R.J. Statins and Prostate Cancer—Hype or Hope? The Biological Perspective. Prostate Cancer Prostatic Dis. 2022, 25, 650–656. [Google Scholar] [CrossRef]
- Boudreau, D.M.; Yu, O.; Johnson, J. Statin Use and Cancer Risk: A Comprehensive Review. Expert. Opin. Drug Saf. 2010, 9, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, S.; Zenilman, J.M.; Ghanem, K.G.; Jadack, R.A.; Sokoll, L.J.; Elliott, D.J.; Nelson, W.G.; De Marzo, A.M.; Cole, S.R.; Isaacs, W.B.; et al. Sexually Transmitted Infections and Prostatic Inflammation/Cell Damage as Measured by Serum Prostate Specific Antigen Concentration. J. Urol. 2006, 175, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Moghoofei, M.; Keshavarz, M.; Ghorbani, S.; Babaei, F.; Nahand, J.S.; Tavakoli, A.; Mortazavi, H.S.; Marjani, A.; Mostafaei, S.; Monavari, S.H. Association between Human Papillomavirus Infection and Prostate Cancer: A Global Systematic Review and Meta-Analysis. Asia Pac. J. Clin. Oncol. 2019, 15, e59–e67. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Nelson, W.G.; De Marzo, A.M. The Dietary Charred Meat Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo [4,5-b] Pyridine Acts as Both a Tumor Initiator and Promoter in the Rat Ventral Prostate. Cancer Res. 2007, 67, 1378–1384. [Google Scholar] [CrossRef]
- Kirby, R.S.; Lowe, D.; Bultitude, M.I.; Shuttleworth, K.E.D. Intra-Prostatic Urinary Reflux: An Aetiological Factor in Abacterial Prostatitis. Br. J. Urol. 1982, 54, 729–731. [Google Scholar] [CrossRef]
- Aul, P.; Ichtenstein, L.; Olm, I.V.H.; Erkasalo, I.K.V.; Nastasia, A.; Liadou, I.; Aakko, J.; Aprio, K.; Arkku, M.; Oskenvuo, K.; et al. Environmental and heritable factors in the causation of cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland A BSTRACT Background the Contribution of Hereditary Factors. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar]
- Haenszel, W.; Kurihara, M. Studies of Japanese Migrants. I. Mortality from Cancer and Other Diseases among Japanese in the United States. J. Natl. Cancer Inst. 1968, 40, 43–68. [Google Scholar] [PubMed]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Giovannucci, E.; Wilson, K.M.; Aspelund, T.; Tryggvadottir, L.; Sigurdardottir, L.G.; Harris, T.B.; Launer, L.J.; et al. Body Fat Distribution on Computed Tomography Imaging and Prostate Cancer Risk and Mortality in the AGES-Reykjavik Study. Cancer 2019, 125, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chen, W.C.; Huang, C.P.; Hsu, C.Y.; Chang, Y.H. The Association of Prostate Cancer and Urinary Tract Infections: A New Perspective of Prostate Cancer Pathogenesis. Medicina 2023, 59, 483. [Google Scholar] [CrossRef] [PubMed]
- Takata, R.; Takahashi, A.; Fujita, M.; Momozawa, Y.; Saunders, E.J.; Yamada, H.; Maejima, K.; Nakano, K.; Nishida, Y.; Hishida, A.; et al. 12 New Susceptibility Loci for Prostate Cancer Identified by Genome-Wide Association Study in Japanese Population. Nat. Commun. 2019, 10, 4422. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H.; Damber, L.; Damber, J.-E. Studies of Genetic Factors in Prostate Cancer in a Twin Population. J. Urol. 1994, 152, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Akhoundova, D.; Feng, F.Y.; Pritchard, C.C.; Rubin, M.A. Molecular Genetics of Prostate Cancer and Role of Genomic Testing. Surg Pathol Clin. 2022, 15, 617–628. [Google Scholar] [CrossRef]
- Cihan, Y.B.; Arslan, A.; Ergul, M.A. Subtypes of White Blood Cells in Patients with Prostate Cancer or Benign Prostatic Hyperplasia and Healthy Individuals. Asian Pac. J. Cancer Prev. 2013, 14, 4779–4783. [Google Scholar] [CrossRef]
- Watts, E.L.; Perez-Cornago, A.; Kothari, J.; Allen, N.E.; Travis, R.C.; Key, T.J. Hematologic Markers and Prostate Cancer Risk: A Prospective Analysis in UK Biobank. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1615–1626. [Google Scholar] [CrossRef]
- Song, J.; Wang, W.; Yuan, Y.; Ban, Y.; Su, J.; Yuan, D.; Chen, W.; Zhu, J. Identification of Immune-Based Prostate Cancer Subtypes Using MRNA Expression. Biosci. Rep. 2021, 41, BSR20201533. [Google Scholar] [CrossRef]
- Adorno Febles, V.R.; Hao, Y.; Ahsan, A.; Wu, J.; Qian, Y.; Zhong, H.; Loeb, S.; Makarov, D.V.; Lepor, H.; Wysock, J.; et al. Single-Cell Analysis of Localized Prostate Cancer Patients Links High Gleason Score with an Immunosuppressive Profile. Prostate 2023, 83, 840–849. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-Infiltrating Neutrophils Constitute the Major In Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Taguchi, A.; Kawana, K.; Adachi, K.; Kawata, A.; Ogishima, J.; Nakamura, H.; Fujimoto, A.; Sato, M.; Inoue, T.; et al. Modification of the Tumor Microenvironment in KRAS or C-MYC-Induced Ovarian Cancer-Associated Peritonitis. PLoS ONE 2016, 11, e0160330. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cumpian, A.M.; Caetano, M.S.; Ochoa, C.E.; De La Garza, M.M.; Lapid, D.J.; Mirabolfathinejad, S.G.; Dickey, B.F.; Zhou, Q.; Moghaddam, S.J. Promoting Effect of Neutrophils on Lung Tumorigenesis Is Mediated by CXCR2 and Neutrophil Elastase. Mol. Cancer 2013, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Campregher, C.; Luciani, M.G.; Gasche, C. Activated Neutrophils Induce an HMSH2-Dependent G2/M Checkpoint Arrest and Replication Errors at a (CA)13-Repeat in Colon Epithelial Cells. Gut 2008, 57, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Tibullo, D.; Giallongo, C.; La Cava, P.; Chiarenza, A.; Motta, G.; Caruso, A.L.; Villari, L.; et al. The Prognostic Value of the Myeloid-Mediated Immunosuppression Marker Arginase-1 in Classic Hodgkin Lymphoma. Oncotarget 2016, 7, 67333–67346. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Strøbech, J.E.; Horton, E.R.; Jackstadt, R.; Laitala, A.; Bravo, M.C.; Maltese, G.; Jensen, A.R.D.; Reuten, R.; Rafaeva, M.; et al. Suppression of Tumor-Associated Neutrophils by Lorlatinib Attenuates Pancreatic Cancer Growth and Improves Treatment with Immune Checkpoint Blockade. Nat. Commun. 2021, 12, 3414. [Google Scholar] [CrossRef]
- Rodriguez, Y.I.; Campos, L.E.; Castro, M.G.; Bannoud, N.; Blidner, A.G.; Filippa, V.P.; Croci, D.O.; Rabinovich, G.A.; Alvarez, S.E. Tumor Necrosis Factor Receptor-1 (P55) Deficiency Attenuates Tumor Growth and Intratumoral Angiogenesis and Stimulates CD8+ T Cell Function in Melanoma. Cells 2020, 9, 2469. [Google Scholar] [CrossRef]
- Morimoto-Kamata, R.; Yui, S. Insulin-like Growth Factor-1 Signaling Is Responsible for Cathepsin G-Induced Aggregation of Breast Cancer MCF-7 Cells. Cancer Sci. 2017, 108, 1574–1583. [Google Scholar] [CrossRef]
- De Monte, L.; Wörmann, S.; Brunetto, E.; Heltai, S.; Magliacane, G.; Reni, M.; Paganoni, A.M.; Recalde, H.; Mondino, A.; Falconi, M.; et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016, 76, 1792–1803. [Google Scholar] [CrossRef]
- Baba, T.; Tanabe, Y.; Yoshikawa, S.; Yamanishi, Y.; Morishita, S.; Komatsu, N.; Karasuyama, H.; Hirao, A.; Mukaida, N. MIP-1a/CCL3-Expressing Basophil-Lineage Cells Drive the Leukemic Hematopoiesis of Chronic Myeloid Leukemia in Mice. Blood 2016, 127, 2607–2617. [Google Scholar] [CrossRef]
- Sektioglu, I.M.; Carretero, R.; Bulbuc, N.; Bald, T.; Tüting, T.; Rudensky, A.Y.; Hämmerling, G.J. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017, 77, 291–302. [Google Scholar] [CrossRef]
- de Paulis, A.; Prevete, N.; Fiorentino, I.; Rossi, F.W.; Staibano, S.; Montuori, N.; Ragno, P.; Longobardi, A.; Liccardo, B.; Genovese, A.; et al. Expression and Functions of the Vascular Endothelial Growth Factors and Their Receptors in Human Basophils. J. Immunol. 2006, 177, 7322–7331. [Google Scholar] [CrossRef] [PubMed]
- Cerny-Reiterer, S.; Ghanim, V.; Hoermann, G.; Aichberger, K.J.; Herrmann, H.; Muellauer, L.; Repa, A.; Sillaber, C.; Walls, A.F.; Mayerhofer, M.; et al. Identification of Basophils as a Major Source of Hepatocyte Growth Factor in Chronic Myeloid Leukemia: A Novel Mechanism of BCR-ABL1-Independent Disease Progression. Neoplasia 2012, 14, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Staiano, R.I.; Granata, F.; Detoraki, A.; Necchi, V.; Ricci, V.; Triggiani, M.; De Paulis, A.; Marone, G.; Genovese, A. Expression and Function of Angiopoietins and Their Tie Receptors in Human Basophils and Mast Cells. J. Biol. Regul. Homeost. Agents 2013, 27, 827–839. [Google Scholar]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting Edge: Th17 and Regulatory T Cell Dynamics and the Regulation by IL-2 in the Tumor Microenvironment. J. Immunol. 2007, 178, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum Levels of IL-6 and TNF-α Correlate with Clinicopathological Features and Patient Survival in Patients with Prostate Cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Gardner, T.A.; Higano, C.S.; Kantoff, P.W.; Small, E.J.; Wener, M.H.; Sims, R.B.; DeVries, T.; Sheikh, N.A.; Dreicer, R. A Transient Increase in Eosinophils Is Associated with Prolonged Survival in Men with Metastatic Castration-Resistant Prostate Cancer Who Receive Sipuleucel-T. Cancer Immunol. Res. 2014, 2, 988–999. [Google Scholar] [CrossRef]
- Mumberg, D.; Monach, P.A.; Wanderling, S.; Philip, M.; Toledano, A.Y.; Schreiber, R.D.; Schreiber, H.; Rowley, J.D. CD4 T Cells Eliminate MHC Class II-Negative Cancer Cells in Vivo by Indirect Effects of IFN-gamma. Proc. Natl. Acad. Sci. USA 1999, 96, 8633–8638. [Google Scholar] [CrossRef]
- Kawamata, H.; Kameyama, S.; Oyasu’, R. In Vitro and In Vivo Acceleration of the Neoplastic Phenotype of a Low-Tumorigenicity Rat Bladder Carcinoma Cell Line by Transfected Transforming Growth Factor-a. Mol. Carcinog. 1994, 9, 210–219. [Google Scholar] [CrossRef]
- Salven, P.; Ruotsalainen, T.; Mattson, K.; Joensuu, H. High pre-treatment serum level of vascular endothelial growth factor (vegf) is associated with poor outcome in small-cell lung cancer. Int. J. Cancer 1998, 79, 144–146. [Google Scholar] [CrossRef]
- Park, B.K.; Zhang, H.; Zeng, Q.; Dai, J.; Keller, E.T.; Giordano, T.; Gu, K.; Shah, V.; Pei, L.; Zarbo, R.J.; et al. NF-ΚB in Breast Cancer Cells Promotes Osteolytic Bone Metastasis by Inducing Osteoclastogenesis via GM-CSF. Nat. Med. 2007, 13, 62–69. [Google Scholar] [CrossRef]
- Taipale, J.; Lohi, J.; Saarinen, J.; Kovanen, P.T.; Keski-Oja, J. Human Mast Cell Chymase and Leukocyte Elastase Release Latent Transforming Growth Factor-PI from the Extracellular Matrix of Cultured Human Epithelial and Endothelial Cells. J. Biol. Chem. 1995, 270, 4689–4696. [Google Scholar] [CrossRef]
- Johnson, C.; Huynh, V.; Hargrove, L.; Kennedy, L.; Graf-Eaton, A.; Owens, J.; Trzeciakowski, J.P.; Hodges, K.; Demorrow, S.; Han, Y.; et al. Inhibition of Mast Cell-Derived Histamine Decreases Human Cholangiocarcinoma Growth and Differentiation via c-Kit/Stem Cell Factor-Dependent Signaling. Am. J. Pathol. 2016, 186, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, J.; Ghouse, S.M.; Lehmann, C.H.K.; Hoppe, A.; Schubert, N.; Nedospasov, S.A.; Dudziak, D.; Dudeck, A. Mast-Cell-Derived TNF Amplifies CD8+ Dendritic Cell Functionality and CD8+ T Cell Priming. Cell Rep. 2015, 13, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Litmanovich, A.; Khazim, K.; Cohen, I. The Role of Interleukin-1 in the Pathogenesis of Cancer and Its Potential as a Therapeutic Target in Clinical Practice. Oncol. Ther. 2018, 6, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.M.; Tamma, R.; Ruggieri, S.; Nico, B.; Toma, A.; Ribatti, D. Mast Cells in Breast Cancer Angiogenesis. Crit. Rev. Oncol. Hematol. 2017, 115, 23–26. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a Novel Angiogenic Factor Stored in Mast Cell Granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function: A New Vision of an Old Cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Murata, T.; Aritake, K.; Matsumoto, S.; Kamauchi, S.; Nakagawa, T.; Hori, M.; Momotani, E.; Urade, Y.; Ozaki, H. Prostagladin D 2 Is a Mast Cell-Derived Antiangiogenic Factor in Lung Carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 19802–19807. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of Snail by NF-ΚB Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef]
- Fu, X.T.; Dai, Z.; Song, K.; Zhang, Z.J.; Zhou, Z.J.; Zhou, S.L.; Zhao, Y.M.; Xiao, Y.S.; Sun, Q.M.; Ding, Z.; et al. Macrophage-Secreted IL-8 Induces Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells by Activating the JAK2/STAT3/Snail Pathway. Int. J. Oncol. 2015, 46, 587–596. [Google Scholar] [CrossRef]
- Kawata, M.; Koinuma, D.; Ogami, T.; Umezawa, K.; Iwata, C.; Watabe, T.; Miyazono, K. TGF-β-Induced Epithelial-Mesenchymal Transition of A549 Lung Adenocarcinoma Cells Is Enhanced by pro-Inflammatory Cytokines Derived from RAW 264.7 Macrophage Cells. J. Biochem. 2012, 151, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, X.; Zhang, Y.; Shen, Y.; Qian, Y. Macrophage-Derived MMP-9 and MMP-2 Are Closely Related to the Rupture of the Fibrous Capsule of Hepatocellular Carcinoma Leading to Tumor Invasion. Biol. Proced. Online 2023, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Wang, Q.; Zhang, X. Tumor-Recruited M2 Macrophages Promote Gastric and Breast Cancer Metastasis via M2 Macrophage-Secreted CHI3L1 Protein. J. Hematol. Oncol. 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Österreicher, C.H.; Hung, K.E.; et al. Adenoma-Linked Barrier Defects and Microbial Products Drive IL-23/IL-17-Mediated Tumour Growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhou, Y.; Bu, H.; Lv, T.; Shi, Y.; Yang, J. Deletion of Interleukin-6 in Monocytes/Macrophages Suppresses the Initiation of Hepatocellular Carcinoma in Mice. J. Exp. Clin. Cancer Res. 2016, 35, 131. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.J.L.; Paulis, Y.W.J.; Nowak-Sliwinska, P.; Deumelandt, K.L.; Hosaka, K.; Soetekouw, P.M.M.B.; Cimpean, A.M.; Raica, M.; Pauwels, P.; van den Oord, J.J.; et al. Targeting PDGF-Mediated Recruitment of Pericytes Blocks Vascular Mimicry and Tumor Growth. J. Pathol. 2018, 246, 447–458. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Ge, D.; Cunningham, D.M.; Huang, F.; Ma, L.; Burris, T.P.; You, Z. Targeting Th17-IL-17 Pathway in Prevention of Micro-Invasive Prostate Cancer in a Mouse Model. Prostate 2017, 77, 888–899. [Google Scholar] [CrossRef]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef]
- Osawa, Y.; Nagaki, M.; Banno, Y.; Brenner, D.A.; Asano, T.; Nozawa, Y.; Moriwaki, H.; Nakashima, S. Tumor Necrosis Factor Alpha-Induced Interleukin-8 Production via NF-ΚB and Phosphatidylinositol 3-Kinase/Akt Pathways Inhibits Cell Apoptosis in Human Hepatocytes. Infect. Immun. 2002, 70, 6294–6301. [Google Scholar] [CrossRef]
- SMith, P.; Hobisch, A.; Lin, D.; Culig, Z.; Keller, E. Interleukin-6 and Prostate Cancer Progression. Cytokine Growth Factor Rev. 2001, 12, 33–40. [Google Scholar] [CrossRef]
- Ammirante, M.; Luo, J.L.; Grivennikov, S.; Nedospasov, S.; Karin, M. B-Cell-Derived Lymphotoxin Promotes Castration-Resistant Prostate Cancer. Nature 2010, 464, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.V.; Prasad, D.V.R.; Banerjee, P.P.; Joshi, B.N.; Kumar, A.; Mishra, G.C. B Cells Activated by Lipopolysaccharide, But Not by Anti-Ig and Anti-CD40 Antibody, Induce Anergy in CD8+ T Cells: Role of TGF-Β1. J. Immunol. 2003, 170, 5897–5911. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, W.; Harris, D.P.; Sprague, F.; Mousseau, B.; Makris, M.; Kusser, K.; Honjo, T.; Mohrs, K.; Mohrs, M.; Randall, T.; et al. Cytokine-Producing Effector B Cells Regulate Type 2 Immunity to H. Polygyrus. Immunity 2009, 30, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.A.; Brown, S.; Mastroeni, P.; Gray, D. TLR and B Cell Receptor Signals to B Cells Differentially Program Primary and Memory Th1 Responses to Salmonella Enterica. J. Immunol. 2010, 185, 2783–2789. [Google Scholar] [CrossRef]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B Cell-Derived GABA Elicits IL-10+ Macrophages to Limit Anti-Tumour Immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef]
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernández-Calvo, G.; Khoyratty, T.E.; van Grinsven, E.; González-Hernández, S.; Nicolás-Ávila, J.Á.; et al. Co-Option of Neutrophil Fates by Tissue Environments. Cell 2020, 183, 1282–1297.e18. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M.C. Priming of the Neutrophil Respiratory Burst: Role in Host Defense and Inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the Understanding and Treatment of Sepsis-Induced Immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Fujita, K.; Imamura, R.; Tanigawa, G.; Nakagawa, M.; Hayashi, T.; Kishimoto, N.; Hosomi, M.; Yamaguchi, S. Low Serum Neutrophil Count Predicts a Positive Prostate Biopsy. Prostate Cancer Prostatic Dis. 2012, 15, 386–390. [Google Scholar] [CrossRef]
- Fujita, K.; Hosomi, M.; Nakagawa, M.; Tanigawa, G.; Imamura, R.; Uemura, M.; Nakai, Y.; Takayama, H.; Yamaguchi, S.; Nonomura, N. White Blood Cell Count Is Positively Associated with Benign Prostatic Hyperplasia. Int. J. Urol. 2014, 21, 308–312. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Alsamraae, M.; Costanzo-Garvey, D.; Teply, B.A.; Boyle, S.; Sommerville, G.; Herbert, Z.T.; Morrissey, C.; Dafferner, A.J.; Abdalla, M.Y.; Fallet, R.W.; et al. Androgen receptor inhibition suppresses anti-tumor neutrophil response against bone metastatic prostate cancer via regulation of TβRI expression. Cancer Letters 2023, 28, 216468. [Google Scholar] [CrossRef]
- Cohen, M.; Giladi, A.; Gorki, A.D.; Solodkin, D.G.; Zada, M.; Hladik, A.; Miklosi, A.; Salame, T.M.; Halpern, K.B.; David, E.; et al. Lung Single-Cell Signaling Interaction Map Reveals Basophil Role in Macrophage Imprinting. Cell 2018, 175, 1031–1044.e18. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Smith, K.E.; Wan, L.; Brown, J.R.; Russler, G.; Yantorni, L.; Caulfield, S.; Lafollette, J.; Moore, M.; Kucuk, O.; et al. Baseline Basophil and Basophil-to-Lymphocyte Status Is Associated with Clinical Outcomes in Metastatic Hormone Sensitive Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2022, 40, e9–e271. [Google Scholar] [CrossRef] [PubMed]
- Pellefigues, C.; Mehta, P.; Chappell, S.; Yumnam, B.; Old, S.; Camberis, M.; Le Gros, G. Diverse Innate Stimuli Activate Basophils through Pathways Involving Syk and IκB Kinases. Proc. Natl. Acad. Sci. USA 2021, 118, 2019524118. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Stephen-Victor, E.; Karnam, A.; Das, M.; Gilardin, L.; Maddur, M.S.; Wymann, S.; Vonarburg, C.; Chevailler, A.; Dimitrov, J.D.; et al. Intravenous Immunoglobulin Induces IL-4 in Human Basophils by Signaling through Surface-Bound IgE. J. Allergy Clin. Immunol. 2019, 144, 524–535.e8. [Google Scholar] [CrossRef]
- He, X.; Cao, Y.; Gu, Y.; Fang, H.; Wang, J.; Liu, X.; Lv, K.; Yu, K.; Fei, Y.; Lin, C.; et al. Clinical Outcomes and Immune Metrics in Intratumoral Basophil-Enriched Gastric Cancer Patients. Ann. Surg. Oncol. 2021, 28, 6439–6450. [Google Scholar] [CrossRef]
- Falkencrone, S.; Poulsen, L.K.; Bindslev-Jensen, C.; Woetmann, A.; Odum, N.; Poulsen, B.C.; Blom, L.; Jensen, B.M.; Gibbs, B.F.; Yasinska, I.M.; et al. IgE-Mediated Basophil Tumour Necrosis Factor Alpha Induces Matrix Metalloproteinase-9 from Monocytes. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 614–620. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating Basophil Count as a Prognostic Marker of Tumor Aggressiveness and Survival Outcomes in Colorectal Cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef]
- Fulkerson, P.C. Transcription Factors in Eosinophil Development and as Therapeutic Targets. Front. Med. 2017, 4, 115. [Google Scholar] [CrossRef]
- Hogan, S.P.; Rosenberg, H.F.; Moqbel, R.; Phipps, S.; Foster, P.S.; Lacy, P.; Kay, A.B.; Rothenberg, M.E. Eosinophils: Biological Properties and Role in Health and Disease. Clin. Exp. Allergy 2008, 38, 709–750. [Google Scholar] [CrossRef]
- Ponath, P.D.; Qin, S.; Postyl, T.W.; Wang, J.; Wu, L.; Gerardyl, N.P.; Newman, W.; Gerard, C.; Mackay, C.R. Molecular Cloning and Characterization of a Human Eotaxin Receptor Expressed Selectively on Eosinophils. J. Exp. Med. 1996, 183, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Sakkal, S.; Miller, S.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016, 23, 650–666. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Bagnasco, D.; Borriello, F.; Heffler, E.; Canonica, G.W. Interleukin-5 Pathway Inhibition in the Treatment of Eosinophilic Respiratory Disorders: Evidence and Unmet Needs. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 186–200. [Google Scholar] [CrossRef]
- Caruso, R.A.; Parisi, A.; Quattrocchi, E.; Scardigno, M.; Branca, G.; Parisi, C.; Lucianò, R.; Paparo, D.; Fedele, F. Ultrastructural Descriptions of Heterotypic Aggregation between Eosinophils and Tumor Cells in Human Gastric Carcinomas. Ultrastruct. Pathol. 2011, 35, 145–149. [Google Scholar] [CrossRef]
- Rodríguez Bustos, H.; Cortés Chau, F.; Cortés Pino, F.; Aguirre, P.; Bravo, G.; Gallegos Méndez, I.; Arriaza Onel, C.; Cuellar Godoy, C.; Aguayo González, F.; Espinoza Navarro, O. Morphological Changes of the Cellularity in the Prostatic Gland from Patients with Confirmed Cancer: Gleason Level and Presence of Eosinophils and Mast Cells: Cellular Bioindicators. Int. J. Morphol. 2020, 38, 882–887. [Google Scholar] [CrossRef]
- Furbert-Harris, P.M.; Parish-Gause, D.; Hunter, K.A.; Vaughn, T.R.; Howland, C.; Okomo-Awich, J.; Forrest, K.; Laniyan, I.; Abdelnaby, A.; Oredipe, O.A. Activated Eosinophils Upregulate the Metastasis Suppressor Molecule E-Cadherin on Prostate Tumor Cells. Cell Mol. Biol. 2003, 49, 1009–1016. [Google Scholar]
- Furbert-Harris, P.; Parish-Gause, D.; Laniyan, I.; Hunter, K.A.; Okomo-Awich, J.; Vaughn, T.R.; Forrest, K.C.; Howland, C.; Abdelnaby, A.; Oredipe, O.A. Inhibition of Prostate Cancer Cell Growth by Activated Eosinophils. Prostate 2003, 57, 165–175. [Google Scholar] [CrossRef]
- Moon, T.C.; St Laurent, C.D.; Morris, K.E.; Marcet, C.; Yoshimura, T.; Sekar, Y.; Befus, A.D. Advances in Mast Cell Biology: New Understanding of Heterogeneity and Function. Mucosal Immunol. 2010, 3, 111–128. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental Origin and Functional Specialization of Mast Cell Subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.F.; Barrett, N.A.; Austen, K.F.; Kim, E.Y.; Brenner, M.B.; Shaw, L.; Yu, B.; Goldrath, A.; Mostafavi, S.; Regev, A.; et al. Expression Profiling of Constitutive Mast Cells Reveals a Unique Identity within the Immune System. Nat. Immunol. 2016, 17, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikström, P.; et al. Mast Cells Are Novel Independent Prognostic Markers in Prostate Cancer and Represent a Target for Therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef]
- Zadvornyi, T.; Lukianova, N.; Borikun, T.; Tymoshenko, A.; Mushii, O.; Voronina, O.; Vitruk, I.; Stakhovskyi, E.; Chekhun, V. Mast Cells as a Tumor Microenvironment Factor Associated with the Aggressiveness of Prostate Cancer. Neoplasma 2022, 69, 1490–1498. [Google Scholar] [CrossRef]
- Ma, Z.; Yue, L.; Xu, Z.; Zeng, S.; Ma, Y.; Li, Z.; Li, W.; Wang, D. The Effect of Mast Cells on the Biological Characteristics of Prostate Cancer Cells. Cent. Eur. J. Immunol. 2018, 43, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Dang, Q.; Xie, H.; Yang, Z.; He, D.; Liang, L.; Song, W.; Yeh, S.; Chang, C. Infiltrating Mast Cells Enhance Prostate Cancer Invasion via Altering LncRNA-HOTAIR/PRC2-Androgen Receptor (AR)-MMP9 Signals and Increased Stem/Progenitor Cell Population. Oncotarget 2015, 6, 14179–14190. [Google Scholar] [CrossRef]
- Globa, T.; Şptefrţi, L.; Ceauşu, R.A.; Gaje, P.; Cimpean, A.M.; Raica, M. Mast Cell Phenotype in Benign and Malignant Tumors of the Prostate. Pol. J. Pathol. 2014, 65, 147–153. [Google Scholar] [CrossRef]
- Sullivan, H.H.; Heaphy, C.M.; Kulac, I.; Cuka, N.; Lu, J.; Barber, J.R.; de Marzo, A.M.; Lotan, T.L.; Joshu, C.E.; Sfanos, K.S. High Extratumoral Mast Cell Counts Are Associated with a Higher Risk of Adverse Prostate Cancer Outcomes. Cancer Epidemiol. Biomark. Prev. 2020, 29, 668–675. [Google Scholar] [CrossRef]
- Nonomura, N.; Takayama, H.; Nishimura, K.; Oka, D.; Nakai, Y.; Shiba, M.; Tsujimura, A.; Nakayama, M.; Aozasa, K.; Okuyama, A. Decreased Number of Mast Cells Infiltrating into Needle Biopsy Specimens Leads to a Better Prognosis of Prostate Cancer. Br. J. Cancer 2007, 97, 952–956. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage Plasticity and Interaction with Lymphocyte Subsets: Cancer as a Paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, K.; Laoui, D.; Gysemans, C.; Baeten, M.; Stangé, G.; Den Van Bossche, J.; Mack, M.; Pipeleers, D.; In’t Veld, P.; De Baetselier, P.; et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived from Ly6C(High) Monocytes. Cancer Res. 2010, 70, 5728–5739. [Google Scholar] [CrossRef]
- Laoui, D.; Movahedi, K.; van Overmeire, E.; van den Bossche, J.; Schouppe, E.; Mommer, C.; Nikolaou, A.; Morias, Y.; de Baetselier, P.; van Ginderachter, J.A. Tumor-Associated Macrophages in Breast Cancer: Distinct Subsets, Distinct Functions. Int. J. Dev. Biol. 2011, 55, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Sceneay, J.; Smyth, M.J.; Möller, A. The Pre-Metastatic Niche: Finding Common Ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef]
- Dai, J.; Lu, Y.; Roca, H.; Keller, J.M.; Zhang, J.; McCauley, L.K.; Keller, E.T. Immune Mediators in the Tumor Microenvironment of Prostate Cancer. Chin. J. Cancer 2017, 36, 29. [Google Scholar] [CrossRef]
- Cenerenti, M.; Saillard, M.; Romero, P.; Jandus, C. The Era of Cytotoxic CD4 T Cells. Front. Immunol. 2022, 13, 867189. [Google Scholar] [CrossRef]
- Miller, A.M.; Lundberg, K.; Özenci, V.; Banham, A.H.; Hellström, M.; Egevad, L.; Pisa, P. CD4+CD25high T Cells Are Enriched in the Tumor and Peripheral Blood of Prostate Cancer Patients. J. Immunol. 2006, 177, 7398–7405. [Google Scholar] [CrossRef]
- Barkin, J.; Rodriguez-Suarez, R.; Betito, K. Association between Natural Killer Cell Activity and Prostate Cancer: A Pilot Study. Can. J. Urol. 2017, 24, 8708–8713. [Google Scholar]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of Tumor-Infiltrating T-Cell Density with Molecular Subtype, Racial Ancestry and Clinical Outcomes in Prostate Cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin. Cancer Res. 2008, 14, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Lebien, T.W.; Thomas, T.F. B Lymphocytes: How They Develop and Function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Cui, C.; Wang, J.; Fagerberg, E.; Chen, P.M.; Connolly, K.A.; Damo, M.; Cheung, J.F.; Mao, T.; Askari, A.S.; Chen, S.; et al. Neoantigen-Driven B Cell and CD4 T Follicular Helper Cell Collaboration Promotes Anti-Tumor CD8 T Cell Responses. Cell 2021, 184, 6101–6118.e13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Y.; Hu, Z.; Zhao, M.; Li, M.; Bi, G.; Zheng, Y.; Liang, J.; Lu, T.; Jiang, W.; et al. Landscape and Dynamics of Single Tumor and Immune Cells in Early and Advanced-stage Lung Adenocarcinoma. Clin. Transl. Med. 2021, 11, e350. [Google Scholar] [CrossRef]
- Shao, Y.; Lo, C.M.; Ling, C.C.; Liu, X.B.; Ng, K.T.P.; Chu, A.C.Y.; Ma, Y.Y.; Li, C.X.; Fan, S.T.; Man, K. Regulatory B Cells Accelerate Hepatocellular Carcinoma Progression via CD40/CD154 Signaling Pathway. Cancer Lett. 2014, 355, 264–272. [Google Scholar] [CrossRef]
- Murakami, Y.; Saito, H.; Shimizu, S.; Kono, Y.; Shishido, Y.; Miyatani, K.; Matsunaga, T.; Fukumoto, Y.; Ashida, K.; Sakabe, T.; et al. Increased Regulatory B Cells Are Involved in Immune Evasion in Patients with Gastric Cancer. Sci. Rep. 2019, 9, 13083. [Google Scholar] [CrossRef]
- Carter, N.A.; Rosser, E.C.; Mauri, C. Interleukin-10 Produced by B Cells Is Crucial for the Suppression of Th17/Th1 Responses, Induction of T Regulatory Type 1 Cells and Reduction of Collagen-Induced Arthritis. Arthritis Res. Ther. 2012, 14, R32. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Roch, T.; Lampropoulou, V.; O’Connor, R.A.; Stervbo, U.; Hilgenberg, E.; Ries, S.; Dang, V.D.; Jaimes, Y.; Daridon, C.; et al. IL-35-Producing B Cells Are Critical Regulators of Immunity during Autoimmune and Infectious Diseases. Nature 2014, 507, 366–370. [Google Scholar] [CrossRef]
- Tian, J.; Zekzer, D.; Hanssen, L.; Lu, Y.; Olcott, A.; Kaufman, D.L. Lipopolysaccharide-Activated B Cells Down-Regulate Th1 Immunity and Prevent Autoimmune Diabetes in Nonobese Diabetic Mice. J. Immunol. 2001, 167, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 Is Required for Tumor Invasiveness and Angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and Prostate Cancer: The Role of Interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, Y.; Ricote, M.; García-Tuñón, I.; Rodriguez-Berriguete, G.; Touffehi, M.; Rais, N.B.; Fraile, B.; Paniagua, R.; Oueslati, R.; Royuela, M. Pro-Inflammatory Cytokines and Prostate-Specific Antigen in Hyperplasia and Human Prostate Cancer. Cancer Detect. Prev. 2008, 32, 23–32. [Google Scholar] [CrossRef]
- Cheng, J.; Li, L.; Liu, Y.; Wang, Z.; Zhu, X.; Bai, X. Interleukin-1α Induces Immunosuppression by Mesenchymal Stem Cells Promoting the Growth of Prostate Cancer Cells. Mol. Med. Rep. 2012, 6, 955–960. [Google Scholar] [CrossRef]
- Adler, H.L.; McCURDY, M.A.; Kattan, M.W.; Timme, T.L.; Scardino, P.T.; Thompson, T.C. Elevated levels of circulating interleukin-6 and transforming growth factor-p1 in patients with metastatic prostatic carcinoma. J. Urol. 1999, 161, 182–187. [Google Scholar] [CrossRef]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum Interleukin 6 as a Prognostic Factor in Patients with Prostate Cancer. Clin. Cancer Res. 2000, 6, 2702–2706. [Google Scholar]
- Sugimoto, Y.; Hirota, M.; Yoshikawa, K.; Sumitomo, M.; Nakamura, K.; Ueda, R.; Niwa, R.; Suzawa, T.; Yamasaki, M.; Shitara, K.; et al. The Therapeutic Potential of a Novel PSMA Antibody and Its IL-2 Conjugate in Prostate Cancer. Anticancer Res. 2014, 34, 89–97. [Google Scholar]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.A.; Kim, J.H.; Oh, K.; Kim, G.; Seo, M.W.; Shin, Y.K.; Sim, J.H.; Shin, H.M.; Seo, B.Y.; Lee, D.S.; et al. Interleukin-7 Contributes to the Invasiveness of Prostate Cancer Cells by Promoting Epithelial–Mesenchymal Transition. Sci. Rep. 2019, 9, 6917. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Han, P.; Li, X.; Zeng, H.; Zhu, Y.; Wei, Q. Evaluation of Interleukin-8 in Expressed Prostatic Secretion as a Reliable Biomarker of Inflammation in Benign Prostatic Hyperplasia. Urology 2009, 74, 340–344. [Google Scholar] [CrossRef]
- Culig, Z. Response to Androgens and Androgen Receptor Antagonists in the Presence of Cytokines in Prostate Cancer. Cancers 2021, 13, 2944. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.E.; Newman, M.E.; Paikl, D.; Stix, U.; Memaran-Dagda, N.; Lee, C.; Marberger, M.J. Expression and Function of Pro-Inflammatory Interleukin IL-17 and IL-17 Receptor in Normal, Benign Hyperplastic, and Malignant Prostate. Prostate 2003, 56, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory Cytokines IL-17 and TNF-α up-Regulate PD-L1 Expression in Human Prostate and Colon Cancer Cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, M.G.; Cho, K.; Park, B.J.; Chae, K.S.; Byun, D.S.; Ryu, B.K.; Park, Y.K.; Chi, S.G. Transforming Growth Factor-Β1 Activates Interleukin-6 Expression in Prostate Cancer Cells through the Synergistic Collaboration of the Smad2, P38-NF-ΚB, JNK, and Ras Signaling Pathways. Oncogene 2003, 22, 4314–4332. [Google Scholar] [CrossRef]
- Wang, H.; Fang, R.; Wang, X.F.; Zhang, F.; Chen, D.Y.; Zhou, B.; Wang, H.S.; Cai, S.H.; Du, J. Stabilization of Snail through AKT/GSK-3β Signaling Pathway Is Required for TNF-α-Induced Epithelial-Mesenchymal Transition in Prostate Cancer PC3 Cells. Eur. J. Pharmacol. 2013, 714, 48–55. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Chen, X.H.; Liu, Z.C.; Zhang, G.; Wei, W.; Wang, X.X.; Wang, H.; Ke, H.P.; Zhang, F.; Wang, H.S.; Cai, S.H.; et al. TGF-β and EGF Induced HLA-I Downregulation Is Associated with Epithelial-Mesenchymal Transition (EMT) through Upregulation of Snail in Prostate Cancer Cells. Mol. Immunol. 2015, 65, 34–42. [Google Scholar] [CrossRef]
- Zhang, F.; Lee, J.; Lu, S.; Pettaway, C.A.; Dong, Z. Blockade of Transforming Growth Factor-B Signaling Suppresses Progression of Androgen-Independent Human Prostate Cancer in Nude Mice. Clin. Cancer Res. 2005, 11, 4512–4520. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Steiner, G.E.; Handisurya, A.; Stix, U.; Haitel, A.; Knerer, B.; Gessl, A.; Lee, C.; Marberger, M. Increased Expression of Lymphocyte-Derived Cytokines in Benign Hyperplastic Prostate Tissue, Identification of the Producing Cell Types, and Effect of Differentially Expressed Cytokines on Stromal Cell Proliferation. Prostate 2002, 52, 43–58. [Google Scholar] [CrossRef]
- Tuxhorn, J.A.; McAlhany, S.J.; Yang, F.; Dang, T.D.; Rowley, D.R. Inhibition of Transforming Growth Factor-Beta Activity Decreases Angiogenesis in a Human Prostate Cancer-Reactive Stroma Xenograft Model. Cancer Res. 2002, 62, 6021–6025. [Google Scholar]
- Gillessen, S.; Naumov, Y.N.; Nieuwenhuis, E.E.S.; Exley, M.A.; Lee, F.S.; Mach, N.; Luster, A.D.; Blumberg, R.S.; Taniguchi, M.; Balk, S.P.; et al. CD1d-Restricted T Cells Regulate Dendritic Cell Function and Antitumor Immunity in a Granulocyte-Macrophage Colony-Stimulating Factor-Dependent Fashion. Proc. Natl. Acad. Sci. USA 2003, 100, 8874–8879. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhang, X.; Shi, X.; Wei, L.; Zheng, D.; Li, H.; Gao, J.; Li, J.; Hu, Z. Norcantharidin Enhances Antitumor Immunity of GM-CSF Prostate Cancer Cells Vaccine by Inducing Apoptosis of Regulatory T Cells. Cancer Sci. 2018, 109, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Kiu, H.; Nicholson, S.E. Biology and Significance of the JAK/STAT Signalling Pathways. Growth Factors 2012, 30, 88–106. [Google Scholar] [CrossRef]
- Li, W.X. Canonical and Non-Canonical JAK-STAT Signaling. Trends Cell Biol. 2008, 18, 545–551. [Google Scholar] [CrossRef]
- Liu, X.; He, Z.; Li, C.H.; Huang, G.; Ding, C.; Liu, H. Correlation Analysis of JAK-STAT Pathway Components on Prognosis of Patients with Prostate Cancer. Pathol. Oncol. Res. 2012, 18, 17–23. [Google Scholar] [CrossRef]
- Gao, B.; Shen, X.; Kunos, G.; Meng, Q.; Goldberg, I.D.; Rosen, E.M.; Fan, S. Constitutive Activation of JAK-STAT3 Signaling by BRCA1 in Human Prostate Cancer Cells. FEBS Lett. 2001, 488, 179–184. [Google Scholar] [CrossRef]
- Zhu, M.L.; Kyprianou, N. Androgen Receptor and Growth Factor Signaling Cross-Talk in Prostate Cancer Cells. Endocr. Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef]
- Bishop, J.L.; Thaper, D.; Zoubeidi, A. The Multifaceted Roles of STAT3 Signaling in the Progression of Prostate Cancer. Cancers 2014, 6, 829–859. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hodge, D.R.; Wang, L.; Yang, X.; Zhang, X.; Farrar, W.L. Co-Operative Functions between Nuclear Factors NFκB and CCAT/Enhancer-Binding Protein-β (C/EBP-β) Regulate the IL-6 Promoter in Autocrine Human Prostate Cancer Cells. Prostate 2004, 61, 354–370. [Google Scholar] [CrossRef]
- Jin, R.; Yi, Y.; Yull, F.E.; Blackwell, T.S.; Clark, P.E.; Koyama, T.; Smith, J.A.; Matusik, R.J. Nf-Kb Gene Signature Predicts Prostate Cancer Progression. Cancer Res. 2014, 74, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Ramamurthy, V.P.; Njar, V.C.O. Dissecting Major Signaling Pathways in Prostate Cancer Development and Progression: Mechanisms and Novel Therapeutic Targets. J. Steroid Biochem. Mol. Biol. 2017, 166, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.R.; Kyprianou, N. Growth Factor Signalling in Prostatic Growth: Significance in Tumour Development and Therapeutic Targeting. Br. J. Pharmacol. 2006, 147, 144–152. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β Signaling in Tumor Suppression and Cancer Progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Peraldo-Neia, C.; Migliardi, G.; Mello-Grand, M.; Montemurro, F.; Segir, R.; Pignochino, Y.; Cavalloni, G.; Torchio, B.; Mosso, L.; Chiorino, G.; et al. Epidermal Growth Factor Receptor (EGFR) Mutation Analysis, Gene Expression Profiling and EGFR Protein Expression in Primary Prostate Cancer. BMC Cancer 2011, 11, 31. [Google Scholar] [CrossRef]
- Fresno Vara, J.Á.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. P13K/Akt Signalling Pathway and Cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Z.; Jiang, B.H.; Shi, X. Role of PI3K/AKT/MTOR Signaling in the Cell Cycle Progression of Human Prostate Cancer. Biochem. Biophys. Res. Commun. 2003, 310, 1124–1132. [Google Scholar] [CrossRef]
- Steiner, H.; Godoy-Tundidor, S.; Rogatsch, H.; Berger, A.P.; Fuchs, D.; Comuzzi, B.; Bartsch, G.; Hobisch, A.; Culig, Z. Accelerated in Vivo Growth of Prostate Tumors That Up-Regulate Interleukin-6 Is Associated with Reduced Retinoblastoma Protein Expression and Activation of the Mitogen-Activated Protein Kinase Pathway. Am. J. Pathol. 2003, 162, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Mbatia, H.W.; Ramalingam, S.; Ramamurthy, V.P.; Martin, M.S.; Kwegyir-Afful, A.K.; Njar, V.C.O. Novel C-4 Heteroaryl 13- Cis -Retinamide Mnk/AR Degrading Agents Inhibit Cell Proliferation and Migration and Induce Apoptosis in Human Breast and Prostate Cancer Cells and Suppress Growth of MDA-MB-231 Human Breast and CWR22Rv1 Human Prostate Tumor Xenografts in Mice. J. Med. Chem. 2015, 58, 1900–1914. [Google Scholar] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Zhang, Z.; Chen, X.; Jia, Y.; Wang, B.; Kong, T. Curcumin Inhibits the Survival and Metastasis of Prostate Cancer Cells via the Notch-1 Signaling Pathway. APMIS 2017, 125, 134–140. [Google Scholar] [CrossRef]
- Pan, L.; Sha, J.; Lin, W.; Wang, Y.; Bian, T. Curcumin Inhibits Prostate Cancer Progression by Regulating the MiR-30a-5p/PCLAF Axis. Exp. Ther. Med. 2021, 22, 969. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin Sensitizes TRAIL-Resistant Xenografts: Molecular Mechanisms of Apoptosis, Metastasis and Angiogenesis. Mol. Cancer 2008, 7, 16. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Ding, N.; Van Doren, J.; Wei, X.-C.; Du, Z.-Y.; Conney, A.H.; Zhang, K.; Zheng, X. Effects of Curcumin Analogues for Inhibiting Human Prostate Cancer Cells and the Growth of Human PC-3 Prostate Xenografts in Immunodeficient Mice. Biol. Pharm. Bull. 2014, 36, 1029–1034. [Google Scholar] [CrossRef]
- Luo, C.; Li, Y.; Zhou, B.; Yang, L.; Li, H.; Feng, Z.; Li, Y.; Long, J.; Liu, J. A Monocarbonyl Analogue of Curcumin, 1,5-Bis(3-Hydroxyphenyl)-1,4- Pentadiene-3-One (Ca 37), Exhibits Potent Growth Suppressive Activity and Enhances the Inhibitory Effect of Curcumin on Human Prostate Cancer Cells. Apoptosis 2014, 19, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic Acid (6-Pentadecylsalicylic Acid) Inhibits Tumor Angiogenesis by Targeting Src/FAK/Rho GTpases Signaling Pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, B.; He, L.; Tang, Y.; Jiang, Z.; Yin, G.; Wang, J.; Jiang, X. Anacardic Acid (6-Pentadecylsalicylic Acid) Induces Apoptosis of Prostate Cancer Cells through Inhibition of Androgen Receptor and Activation of P53 Signaling. Chin. J. Cancer Res. 2012, 24, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Tolba, M.F.; Esmat, A.; Al-Abd, A.M.; Azab, S.S.; Khalifa, A.E.; Mosli, H.A.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic Acid Phenethyl Ester Synergistically Enhances Docetaxel and Paclitaxel Cytotoxicity in Prostate Cancer Cells. IUBMB Life 2013, 65, 716–729. [Google Scholar] [CrossRef]
- Lin, H.P.; Jiang, S.S.; Chuu, C.P. Caffeic Acid Phenethyl Ester Causes P21 Cip1 Induction, Akt Signaling Reduction, and Growth Inhibition in PC-3 Human Prostate Cancer Cells. PLoS ONE 2012, 7, e31286. [Google Scholar]
- Vanella, L.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Cardile, V.; Kim, D.H.; Abraham, N.G.; Sorrenti, V. Apoptotic Markers in a Prostate Cancer Cell Line: Effect of Ellagic Acid. Oncol. Rep. 2013, 30, 2804–2810. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Chewonarin, T.; Ogawa, K.; Suzuki, S.; Asamoto, M.; Takahashi, S.; Shirai, T.; Limtrakul, P. Ellagic Acid Inhibits Migration and Invasion by Prostate Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2013, 14, 2859–2863. [Google Scholar] [CrossRef]
- Veluri, R.; Singh, R.P.; Liu, Z.; Thompson, J.A.; Agarwal, R.; Agarwal, C. Fractionation of Grape Seed Extract and Identification of Gallic Acid as One of the Major Active Constituents Causing Growth Inhibition and Apoptotic Death of DU145 Human Prostate Carcinoma Cells. Carcinogenesis 2006, 27, 1445–1453. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic Acid, an Active Constituent of Grape Seed Extract, Exhibits Anti-Proliferative, pro-Apoptotic and Anti-Tumorigenic Effects against Prostate Carcinoma Xenograft Growth in Nude Mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol Reduces Prostate Cancer Growth and Metastasis by Inhibiting the Akt/MicroRNA-21 Pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef]
- Wang, T.T.Y.; Hudson, T.S.; Wang, T.C.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; et al. Differential Effects of Resveratrol on Androgen-Responsive LNCaP Human Prostate Cancer Cells in Vitro and in Vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef]
- Kumar, S.; Eroglu, E.; Stokes Iii, J.A.; Scissum-Gunn, K.; Saldanha, S.N.; Singh, U.P.; Manne, U.; Ponnazhagan, S.; Mishra, M.K. Resveratrol Induces Mitochondria-Mediated, Caspase-Independent Apoptosis in Murine Prostate Cancer Cells. Oncotarget 2017, 8, 20895. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol Enhances Prostate Cancer Cell Response to Ionizing Radiation. Modulation of the AMPK, Akt and MTOR Pathways. Oncotarget 2017, 8, 20895–20908. [Google Scholar] [CrossRef]
- Kwon, G.T.; Jung, J.I.; Song, H.R.; Woo, E.Y.; Jun, J.G.; Kim, J.K.; Her, S.; Park, J.H.Y. Piceatannol Inhibits Migration and Invasion of Prostate Cancer Cells: Possible Mediation by Decreased Interleukin-6 Signaling. J. Nutr. Biochem. 2012, 23, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lim, D.Y.; Cho, H.J.; Seon, M.R.; Kim, J.K.; Lee, B.Y.; Park, J.H.Y. Piceatannol, a Natural Stilbene from Grapes, Induces G1 Cell Cycle Arrest in Androgen-Insensitive DU145 Human Prostate Cancer Cells via the Inhibition of CDK Activity. Cancer Lett. 2009, 285, 166–173. [Google Scholar] [CrossRef]
- Jayasooriya, R.G.P.T.; Lee, Y.G.; Kang, C.H.; Lee, K.T.; Choi, Y.H.; Park, S.Y.; Hwang, J.K.; Kim, G.Y. Piceatannol Inhibits MMP-9-Dependent Invasion of Tumor Necrosis Factor-α-Stimulated DU145 Cells by Suppressing the Akt-Mediated Nuclear Factor-ΚB Pathway. Oncol. Lett. 2012, 5, 341–347. [Google Scholar] [CrossRef]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-Isothiocyanate Conjugate Suppresses Growth of Prostate Cancer Cells Irrespective of Androgen Receptor Status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.C.H.; Tsai, Y.C.; Lin, J.N.; Fan, L.L.; Pan, M.H.; Ho, C.T.; Wu, J.Y.; Way, T. Der Activation of AMPK by Pterostilbene Suppresses Lipogenesis and Cell-Cycle Progression in P53 Positive and Negative Human Prostate Cancer Cells. J. Agric. Food Chem. 2012, 60, 6399–6407. [Google Scholar] [CrossRef]
- Hagen, R.M.; Chedea, V.S.; Mintoff, C.P.; Bowler, E.; Morse, H.R.; Ladomery, M.R. Epigallocatechin-3-Gallate Promotes Apoptosis and Expression of the Caspase 9a Splice Variant in PC3 Prostate Cancer Cells. Int. J. Oncol. 2013, 43, 194–200. [Google Scholar] [CrossRef]
- Mukherjee, S.; Siddiqui, M.A.; Dayal, S.; Ayoub, Y.Z.; Malathi, K. Epigallocatechin-3-Gallate Suppresses Proinflammatory Cytokines and Chemokines Induced by Toll-like Receptor 9 Agonists in Prostate Cancer Cells. J. Inflamm. Res. 2014, 7, 89–101. [Google Scholar]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a Novel Dietary Flavonoid, Causes Apoptosis and Cell Cycle Arrest in Human Prostate Cancer LNCaP Cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef]
- Khan, N.; Asim, M.; Afaq, F.; Zaid, M.A.; Mukhtar, H. A Novel Dietary Flavonoid Fisetin Inhibits Androgen Receptor Signaling and Tumor Growth in Athymic Nude Mice. Cancer Res. 2008, 68, 8555–8563. [Google Scholar] [CrossRef]
- Suh, Y.; Afaq, F.; Khan, N.; Johnson, J.J.; Khusro, F.H.; Mukhtar, H. Fisetin Induces Autophagic Cell Death through Suppression of MTOR Signaling Pathway in Prostate Cancer Cells. Carcinogenesis 2010, 31, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive Effect of Quercetin, a Natural Dietary Flavonoid on Prostate Cancer in Invivo Model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin Inhibits Prostate Cancer by Attenuating Cell Survival and Inhibiting Anti-Apoptotic Pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef]
- Xing, N.; Chen, Y.; Mitchell, S.H. Quercetin Inhibits the Expression and Function of the Androgen Receptor in LNCaP Prostate Cancer Cells inhibited by quercetin in a dose-dependent manner. Carcinogenesis 2001, 22, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yen, C.Y.; Wu, R.S.C.; Yang, J.S.; Lu, H.F.; Lu, K.W.; Lo, C.; Chen, H.Y.; Tang, N.Y.; Wu, C.C.; et al. The Roles of Endoplasmic Reticulum Stress and Mitochondrial Apoptotic Signaling Pathway in Quercetin-Mediated Cell Death of Human Prostate Cancer PC-3 Cells. Environ. Toxicol. 2014, 29, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Xu, M.; et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/MTOR/P70S6K Signaling Pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant Flavone Apigenin Inhibits HDAC and Remodels Chromatin to Induce Growth Arrest and Apoptosis in Human Prostate Cancer Cells: In Vitro and in Vivo Study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, B.S.; Chun, S.Y.; Park, Y.K.; Kang, K.S.; Kwon, T.G. Apoptotic Effects of Genistein, Biochanin-A and Apigenin on LNCaP and PC-3 Cells by P21 through Transcriptional Inhibition of Polo-like Kinase-1. J. Korean Med. Sci. 2011, 26, 1489–1494. [Google Scholar] [CrossRef]
- Shukla, S.; Fu, P.; Gupta, S. Apigenin Induces Apoptosis by Targeting Inhibitor of Apoptosis Proteins and Ku70-Bax Interaction in Prostate Cancer. Apoptosis 2014, 19, 883–894. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Gupta, S. Apigenin Attenuates Insulin-like Growth Factor-I Signaling in an Autochthonous Mouse Prostate Cancer Model. Pharm. Res. 2012, 29, 1506–1517. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; MacLennan, G.T.; Gupta, S. Apigenin Inhibits Prostate Cancer Progression in TRAMP Mice via Targeting PI3K/Akt/FoxO Pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef]
- Matchett, M.D.; MacKinnon, S.L.; Sweeney, M.I.; Gottschall-Pass, K.T.; Hurta, R.A.R. Inhibition of Matrix Metalloproteinase Activity in DU145 Human Prostate Cancer Cells by Flavonoids from Lowbush Blueberry (Vaccinium angustifolium): Possible Roles for Protein Kinase C and Mitogen-Activated Protein-Kinase- Mediated Events. J. Nutr. Biochem. 2006, 17, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.M.; Erdman, J.W.; Lila, M.A. Differential Effects of Blueberry Proanthocyanidins on Androgen Sensitive and Insensitive Human Prostate Cancer Cell Lines. Cancer Lett. 2006, 231, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Neuwirt, H.; Arias, M.C.; Puhr, M.; Hobisch, A.; Culig, Z. Oligomeric Proanthocyanidin Complexes (OPC)Exert Anti-Proliferative and pro-Apoptotic Effects on Prostate Cancer Cells. Prostate 2008, 68, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-C.; Bennett, D.; Lee, Y.-S.; Wu, E.; Wu, J. In Silico and Biochemical Analyses Identify Quinone Reductase 2 as a Target of Piceatannol. Curr. Med. Chem. 2013, 20, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Adhami, V.M.; Mukhtar, H. Review: Green Tea Polyphenols in Chemoprevention of Prostate Cancer: Preclinical and Clinical Studies. Nutr. Cancer 2009, 61, 836–841. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Huston, M.; Debella, M.; Dibella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Lerma-García, M.; Ávila, M.; Fco Simó-Alfonso, E.; Ríos, Á.; Zougagh, M. Synthesis of Gold Nanoparticles Using Phenolic Acids and Its Application in Catalysis. J. Mater. Environ. Sci. 2014, 5, 1919–1926. [Google Scholar]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-Loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef] [PubMed]
- Saralkar, P.; Dash, A.K. Alginate Nanoparticles Containing Curcumin and Resveratrol: Preparation, Characterization, and In Vitro Evaluation Against DU145 Prostate Cancer Cell Line. AAPS PharmSciTech 2017, 18, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Bolat, Z.B.; Islek, Z.; Sahin, F.; Ucisik, M.H. Delivery of Curcumin within Emulsome Nanoparticles Enhances the Anti-Cancer Activity in Androgen-Dependent Prostate Cancer Cell. Mol. Biol. Rep. 2023, 50, 2531–2543. [Google Scholar] [CrossRef] [PubMed]
- Adahoun, M.A.; Al-Akhras, M.-A.H.; Jaafar, M.S.; Bououdina, M. Enhanced Anti-Cancer and Antimicrobial Activities of Curcumin Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Lillard, J.W.; Singh, R. Reversal of Drug Resistance by Planetary Ball Milled (PBM) Nanoparticle Loaded with Resveratrol and Docetaxel in Prostate Cancer. Cancer Lett. 2018, 427, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, Evaluation, Pharmacokinetic and Biodistribution Estimation of Resveratrol-Loaded Solid Lipid Nanoparticles for Prostate Cancer Targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-Loaded PLGA Nanoparticles Mediated Programmed Cell Death in Prostate Cancer Cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with Green Tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Kumar Nune, S.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin Receptor Specific Therapeutic Gold Nanoparticles (198 AuNP-EGCg) Show Efficacy in Treating Prostate Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A.N. Epigallocatechin Gallate-Loaded Polysaccharide Nanoparticles for Prostate Cancer Chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral Administration of Naturally Occurring Chitosan-Based Nanoformulated Green Tea Polyphenol EGCG Effectively Inhibits Prostate Cancer Cell Growth in a Xenograft Model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Chavva, S.; Deshmukh, S.; Kanchanapally, R.; Tyagi, N.; Coym, J.; Singh, A.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]

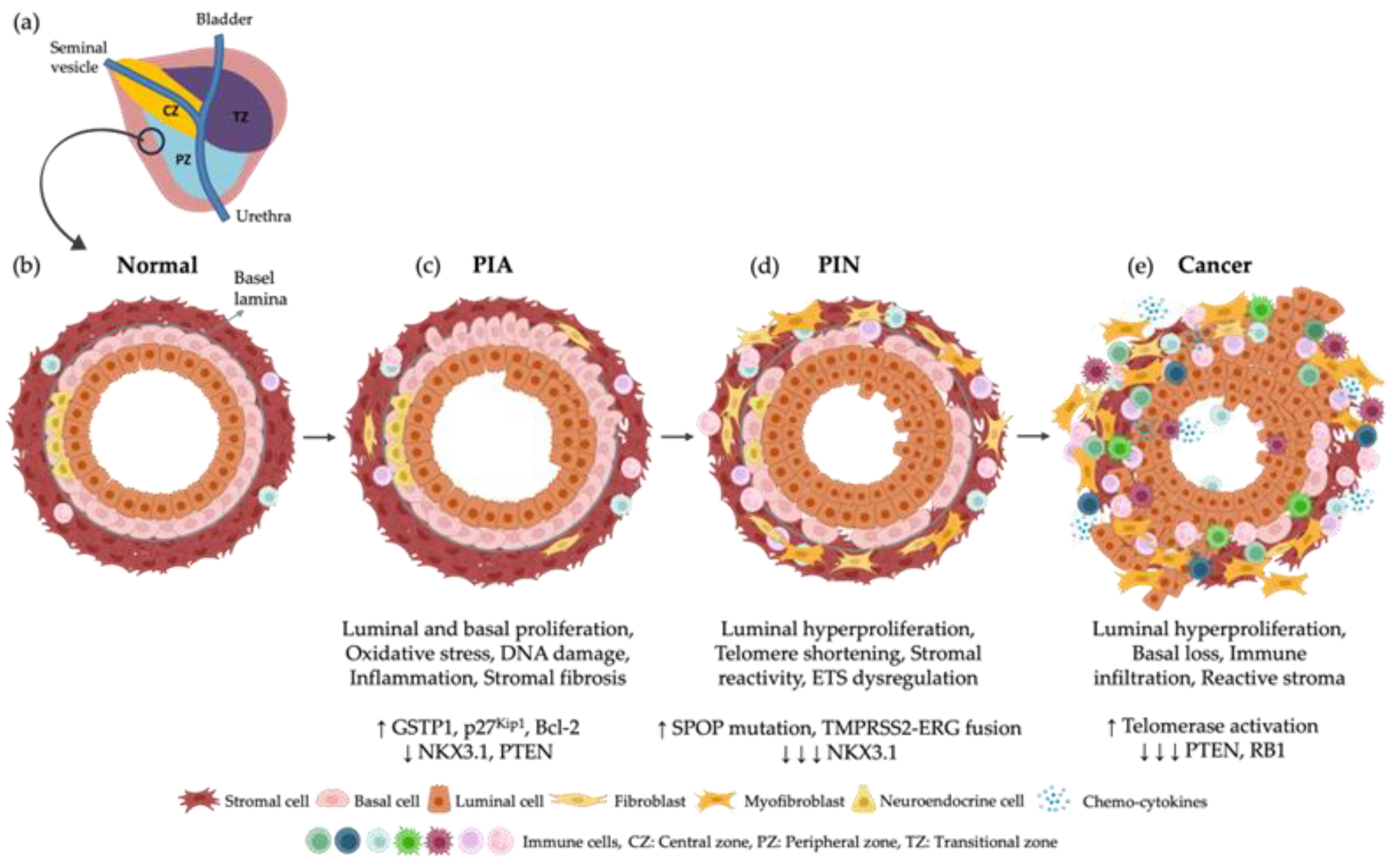
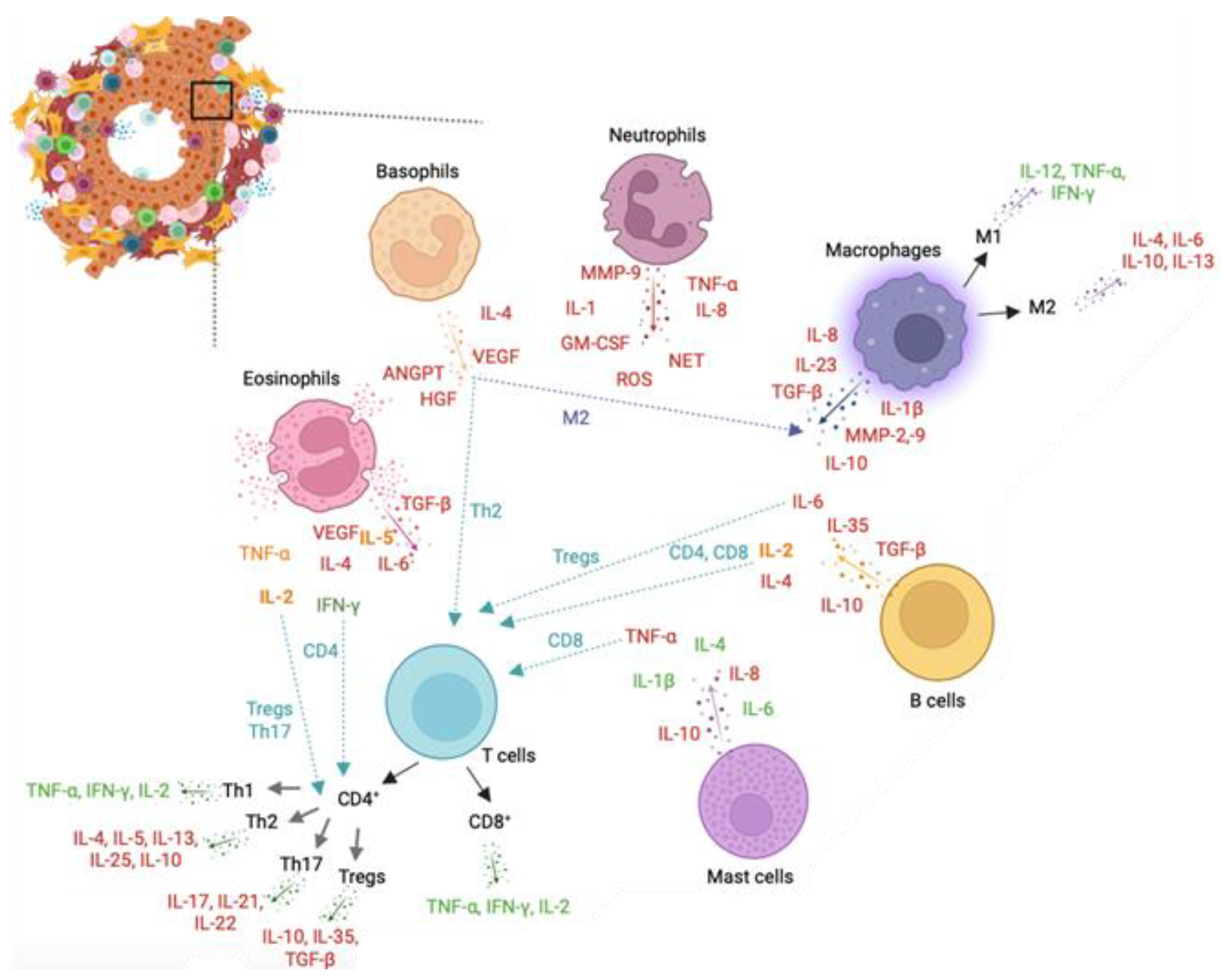

| Gleason Pattern | Gleason Sore | Grade Group | Risk | Histological Definition | Histological Image | Prognosis |
|---|---|---|---|---|---|---|
| 3 + 3 | 6 | 1 | Low | Individual, discrete, well-formed glands |  | Low-grade Cancer |
| 3 + 4 | 7 | 2 | Low to intermediate | Well-formed glands with a few poorly-formed/fused/cribriform glands |  | Intermediate-grade cancer |
| 4 + 3 | 7 | 3 | Intermediate | Poorly formed/fused/cribriform glands with lesser (5%) component of well-formed glands |  | |
| 4 + 4 | 8 | 4 | High | Poorly formed/fused/cribriform glands |  | High-grade cancer |
| 3 + 5 | Well-formed glands with a few areas lacking glands (or with necrosis) |  | ||||
| 5 + 3 | Lack glands (or with necrosis) and a few well-formed glands |  | ||||
| 4 + 5 5 + 4 5 + 5 | 9 or 10 | 5 | Very high | Lack gland formation (or with necrosis) with or without poorly-formed/fused/cribriform glands |  |
| Leukocyte | Inflammatory Mediator Produced | Effect | Refs. |
|---|---|---|---|
| Neutrophils | MMP-9 | MMP-9 produced by TANs and neutrophils degrade ECM leading to cancer progression in human xenografts and Mmp9-knockout mice | [104] |
| GM-CSF and CXCL8 | KRAS stimulated the expression of GM-CSF and CXCL8 in neutrophils which modulates the tumour microenvironment towards cancer progression in mouse models of ovarian cancer | [105] | |
| IL-8/CXCR2 | Overexpression of CXCR2 in neutrophils promotes their attachment in lung cancer regions in a K-RAS mutant mouse model of lung cancer | [106] | |
| NDE, ROS, RNE | NDE, ROS, and RNE release from neutrophils lead to hMSH2-dependent G2/M checkpoint arrest and for the presence of replication errors in a co-culture model that mimics intestinal inflammation in ulcerative colitis | [107] | |
| ARG-1 | Release of ARG-1 from neutrophils inhibit CD3-mediated T cell activation and proliferation leading to cancer progression in classic Hodgkin Lymphoma patients | [108] | |
| NET | Increased neutrophil and NET formation intended attenuate the rate of metastatic PCa in bones in vitro and an in vivo mouse model | [71] | |
| IL-1 | IL-1RA from neutrophils leads to inhibition of senescence promoting cancer progression | [109] | |
| TNF-α | TNFR1 KO mice with depletion of TNF receptor 1 developed smaller tumours with attenuated proliferation and absence of metastasis | [110] | |
| Cathepsin G | Cathepsin G release from neutrophils increases cancer cell adhesion, and aggregation, and metastasis in breast cancer cells | [111] | |
| Basophils | IL-4 | Basophils from pancreatic ductal adenocarcinomas secrete IL-4 which induce GATA-3 expression in Th2 cells in patient samples and Mcpt8Cre mice | [112] |
| CCL3 | Basophils express CCL3 to negatively regulate the normal hematopoietic process in MCPT8-DTR mice and bone marrow samples from patients with CML | [113] | |
| CCL3/CCL4 | Basophil plays a role in tumour rejection by increasing CD8+ T cell infiltration promoted by CCL3 and CCL4 in HCmel12-, B16-, and 616-OVA-induced transgenic FOXP3.LuciDTR-4 mice melanoma | [114] | |
| VEGFA | Immunologic activation by VEGF-2 of basophils induced the release of VEGF-A which induce basophil chemotaxis | [115] | |
| HGF | HGF is expressed in CML basophils in KU812-induced CML cell line | [116] | |
| ANGPT | Basophils express ANGPT1 and ANGPT2 mRNAs | [117] | |
| Eosinophils | IL-2 | IL-2 activate Tregs and Th17 cells involved in the promotion of cancer in a mouse model of PCa, and fibrosarcoma and head and neck human cancer tissues | [118] |
| IL-4 | IL-4 production promotes tumour growth and interaction with TAMs in a pancreatic-induced cancer mouse model | [78] | |
| IL-6 | Increase of IL-6 correlated in patients with metastatic PCa compared with localized PCa | [119] | |
| IL-5 and CCL17 | Eosinophils increase after Sipuleucel-T treatment of patients with metastatic castration-resistant PCa correlated with increase of IL-5 and CCL17, survival and maximal T-cell proliferation responses | [120] | |
| IFN-γ | IFN-γ induced CD4+ T cells to eliminate MHC II-negative cancer cells | [121] | |
| TNF-α | TNF-α correlated with increased extension of PCa in samples from PCa patients | [119] | |
| TGF-α | Overexpression of TGF-α decreased latency, increased growth, and tumour size of bladder cancer rat model | [122] | |
| VEGF | VEGF associated with poor prognosis of human small-cell lung carcinoma | [123] | |
| GM-CSF | Expression of GM-CSF correlated with NF-κB activation in bone-metastatic tumour tissues from individuals with metastatic breast cancer | [124] | |
| Mast cells | Chymase | Chymase released from human mast cell release latent TGF-β-binding protein from the matrix | [125] |
| Histamine | Histamine inhibition from mast cells inactivate EMT and cholangiocarcinoma growth via inhibition of c-Kit signalling in Mz-ChA-1-induced cholangiocarcinoma mouse model and human Mz-ChA-1 cells | [126] | |
| TNF-α | TNF-α released from mast cells amplifies and activates the functionality of CD8+ dendritic cells in Mcpt5-CreTNFfl/fl mice | [127] | |
| IL-1β | Overexpression of IL-1β promoted tumour invasiveness and metastasis by inducing the expression of angiogenic genes and growth factors | [128] | |
| IL1, IL-4, IL-6 | Decreased cell growth and participates in tumour rejection in breast cancer cells | [129] | |
| IL-8, IL-10 | Mast cell-derived IL-8 and IL-10 act as tumour suppressors contributing to tumour cell growth | [130,131] | |
| PGD2 | PGD2 secretion from mast cells attenuates angiogenesis in a Lewis lung carcinoma mouse model | [132] | |
| Macrophages | IL-1β | IL-1β induced Snail stabilization in Snail/MCF7 cells and this effect was dependent on cell types and IL-1β concentration | [133] |
| IL-8 | IL-8 produced by macrophages induce EMT in hepatocellular carcinoma samples via JAK2/STAT3/Snail pathway | [134] | |
| TNF-α | TNF-α induces the stabilization of Snail in a non-phosphorylated, functional form and thus enhances cell migration and invasion dependent on NF-κB activation | [133] | |
| TGF-β | TGF-β induced EMT phenotypes in A549 cells, including changes in cell morphology and induction of mesenchymal marker expression in part by NF-κB signalling | [135] | |
| MMP-2, MMP-9 | Macrophage-derived MMP-9 and MMP-2 related with fibrous capsule leading led to the migration and invasion of hepatocellular carcinoma cells in human samples | [136] | |
| CHI3L1 | M2 macrophage-secreted CHI3L1 promoted metastasis of gastric and breast cancer cells in vitro and in vivo; CHI3L1 interaction with IL-13Rα2 upregulates MMPs | [137] | |
| IL-23/IL-17 | Upregulation of IL-23 leads to tumour growth and progression and development of a tumoral IL-17 response which promote tumorigenesis in a mouse model of colorectal cancer | [138] | |
| IL-6 | TAM-derived IL-6 highly expressed in Hepatocellular carcinoma patients, which is correlated with disease grades and tumour progression | [139] | |
| PDGF | PDGF release from macrophages mediates the recruitment of pericytes in human melanoma cell lines and OCM-1-induced melanoma mouse model | [140] | |
| T cells | IL17-A | Inhibition of IL17-A release by Th17 cells prevent development of microinvasive PCa in mouse models | [141] |
| IL-17 | IL-17-producing T cells can promote PCa progression by enhancing inflammation and angiogenesis | [69] | |
| PD-1 | A high percentage of CD8+ T cells express PD-1 in PCa samples, which impair an effective immune response by these cells | [44] | |
| IL-3 | IL-3 expressed by T cells increase the recruitment of basophils and immune cells into the tumour microenvironment, which is linked with a poor survival | [112] | |
| IFN-γ | IFN-γ can enhance antigen presentation and contribute to immune surveillance in PCa | [142] | |
| TNF-α | TNF-α produced by activated T cells regulated apoptosis, angiogenesis, and inflammation in PCa | [143] | |
| TGF-β | TGF-β produced by T cells can suppress and promote tumour growth in PCa depending on the signal it receives | [144] | |
| B cells | Lymphotoxin | Lymphotoxin lead to CXCL13/IKKa/STAT3/E2F1/BMI1 (RNF51) activation, ubiquitination of histones within PCa cell nuclei and proliferation of androgen-deprived PCa cells in castration-resistant PCa in mice | [145] |
| TGF-β | Secretion of TGF-β by B-cells leads to anergy of CD8+ T cells | [146] | |
| IL-2 | IL-2 and IL-4 produced by B cells regulate the Th2 memory responses to Heligmosomoides polygyrus (Hp) in chimeric mice lacking AID infected with Hp | [147] | |
| IL-6 | Chimera’s mice with B cell lack IL-6 have impaired Th1 and Th17 responses to Salmonella | [148] | |
| GABA | B cell-derived GABA promotes monocyte differentiation into anti-inflammatory macrophages that secrete IL-10 and inhibit CD8+ T cell killer function in mice | [149] |
| Polyphenol | Model | Study Conditions | Effect | Ref. |
|---|---|---|---|---|
| Curcumin | PC-3 and DU-145 cells | 0–50 μM curcumin, 0–48 h, 37 °C | 5 μM curcumin reduced cell viability and proliferation in DU-145 cells; 25 μM curcumin reduced the survival and migration of DU-145 and PC-3 cells in 24 h | [250] |
| PC-3 and DU-145 cells | 10, 20, 30, 40 or 50 µmol/L curcumin at 37 °C for 12 h, 24 h or 48 h | 30 µmol/L curcumin for 24 h decreased cell proliferation, migration, and invasion in PC-3 and DU-145 cells by regulating the miR-30a-5p/PCLAF axis | [251] | |
| LNCaP xenografts mice | OA of 30 mg/kg curcumin 3×/week in athymic nude mice injected s.c with LNCaP cells | Increased TUNEL staining, decreased the expression of PCNA andKi67, and inhibition of the activation of NF-kB in LNCaP xenografts | [252] | |
| Immunodeficient mice | S.c injection of PC-3 cells and daily i.p injected after 4–6 weeks with curcumin analogues (10 μg/g body weight) for 31 days | Curcumin analogues inhibited growth and progression of PC-3 tumours | [253] | |
| BALB/c-nu/nu | PC-3 cells injected s.c in mice and 0.25 μmol Ca 37, 0.5 μmol Ca 37, or 6 μmol curcumin administered i.p daily for 16 days | Ca 37 analogue suppressed PCa tumour and promoted curcumin-induced growth inhibition of PCa cells | [254] | |
| Anacardic acid | C57BL/6 mice and nude mice | PC-3 cells s.c injected into mice and anacardic acid (2 mg/kg per day) s.c injected for 30 days | Inhibition of VEGF-induced cell proliferation, migration, and adhesion | [255] |
| LNCaP cells | 1–125 µmol/L anacardic acid at 30 °C for 24 h | 125 µmol/L anacardic acid inhibited LNCaP cell proliferation, induced G1/S cell cycle arrest and apoptosis of LNCaP cells | [256] | |
| Caffeic acid | PC-3, DU-145 and LNCaP cells | 10–106 nM CAPE for 72 h | CAPE attenuates proliferation and promotes and cytotoxic effect by reducing AKT, ERK and ER-a(Ser-167) phosphorylation in PC-3 cells | [257] |
| PC-3 cells | 20 µM CAPE for 24 h or 72 h | CAPE decreased protein expression of cyclin D1, cyclin E, SKP2, c-Myc, AKT, mTOR, and Bcl-2 | [258] | |
| Ellagic acid | LNCaP cells | 25 and 50 μM ellagic acid for 48 h | Increased ROS, TGF-β, IL-6, and tumour suppressor protein p21 levels and activated caspase-3 | [259] |
| PC-3 and PLS-10 cells | 0, 25, and 50 μM ellagic acid for 24 h, 37 °C | Decreased secretion of MMP-2 and proteolytic activity of collagenase/gelatinase secreted from PLS-10, inhibiting invasiveness of PCa cells | [260] | |
| Gallic acid | DU-145 cells | 24 h, 48 h, or 72 h | 100 mg/mL gallic acid promoted maximal growth inhibition at 72 h and 25 and 50 mg/mL maximal apoptotic death at 24 and 48 h in human DU-145 cells | [261] |
| DU-145 and 22Rv1 xenograft mice | Mice supplemented with 0.3% or 1% (w/v) gallic acid | Gallic acid feeding inhibited the growth of DU-145 and 22Rv1 PCa xenografts in nude mice | [262] | |
| Resveratrol | Immunodeficient (SCID) mice | C-3M-MM2 cells s.c injected, and 20 mg/kg resveratrol administered oral gavage every 2 days | Inhibited PCa growth and metastatic lung lesions associated with reduced miR-21 and pAKT, and elevated PDCD4 levels | [263] |
| BALB/cAnNCr-nu/nu mice | Supplemented with 50 and 100 mg/kg resveratrol. 2 weeks after LNCaP cells s.c injected | Delayed LNCaP tumour growth and inhibited expression of a marker for steroid hormone responses | [264] | |
| TRAMP-C1, TRAMP-C2, and TRAMP-C3 cells | 50 or 100 μM resveratrol for 0 h, 2 h, 4 h, 8 h, 12 h, and 16 h | TRAMP cells exposed to resveratrol showed mitochondria-mediated decreased cell viability, and altered cell morphology leading to aberrant expression of Bax and Bcl-2 proteins | [265] | |
| PC-3 and 22RV1 cells | 2.5–10 μM resveratrol | Resveratrol arrested cell cycle, promoted apoptosis, and sensitized PCa cells to ionization therapy, activated the ATM-AMPK-p53-p21cip1/p27kip1 and inhibit the AKT signaling pathways | [266] | |
| Piceatannol | DU-145, MLL, PC-3 and TRAMP-C2 cells | Treatment with EGF for 0 h, 6 h, 12 h or 24 h with 0 or 10 μmol/L piceatannol | Piceatannol reduced basal and EGF-induced migration and invasion of DU-145 cells-induced IL-6 secretion by IL-6/STAT3 inhibition | [267] |
| DU-145 cells | 0 or 40 μmol/L piceatannol for 24 h | Piceatannol increased the percentage of cells in G1 phase, cyclin A, cyclin D1, and reduced CDK4 and CDK2 activity | [268] | |
| DU-145 cells | 0–40 μM piceatannol for 24 h | Piceatannol reduced TNF-α-induced invasion and MMP-9 gene expression via suppression of NF-κB activity | [269] | |
| Pterostilbene | PC-3 and LNCaP cells | 0.1, 1, 10, 100, and 1000 mM pterostilbene for 24 h at 37 °C | A conjugate molecule caused 50% growth inhibition, reduced accumulation of cells in G2/M phase and induction of apoptosis by downregulation of PI3K/AKT and MAPK/ERK pathways | [270] |
| PC-3 and LNCaP cells | 0, 20, 40, 60, 80, and 100 mM pterostilbene for 48 h | 80 μM pterostilbene decreased lipid synthesis by decreasing FASN expression and inhibiting ACC activity, blocked cell cycle at G1 phase by inducing p53 and further up-regulating p21 expression | [271] | |
| Epigallocatechin-3-gallate (EGCG) | PC-3 cells | 1 and 25 μM EGCG for 48 h | 1 µM EGCG reduced PC-3 cell survival, promoted apoptosis by increasing the pro-apoptotic splice isoform of caspase-9 and enhanced apoptotic capacity of cisplatin | [272] |
| DU-145, PC-3 and LNCaP cells | 20, 40, 80, and 100 μg/mL EGCG for 24 h | EGCG inhibited cytokine and chemokine gene induction, activity of MMP-9 and -2, and NF-κB activity | [273] | |
| Fisetin | LNCaP cells | Treatment with fisetin 10–60 μM, 48 h | Fisetin induced apoptosis, PARP cleavage, modulation of Bcl-2 family protein expression, inhibition of PI3K, phosphorylation of AKT at Ser473 and Thr308, mitochondrial release of cytochrome c into cytosol, and activation of caspases-3, -8 and -9 | [274] |
| LNCaP cells | Treatment with fisetin 10–60 μmol/L 48 h | Fisetin acted as an AR ligand leading to decrease in AR stability and decreased transactivation of target genes including PSA | [275] | |
| PC-3, DU-145 and LNCaP cells | Cells treated with fisetin 10–120 μM for 24 h, 48 h, 72 h, and 96 h | Fisetin activated the mTOR repressor TSC2 through inhibition of AKT and activation of AMPK leading to inhibition of Cap-dependent translation and induction of autophagic cell death in PC-3 cells | [276] | |
| Quercetin | Cancer-induced (MNU and Testosterone treated) rats | Rats treated orally with 200 mg/kg quercetin 3×/week | Quercetin decreased expression of IGFIR, AKT, AR, cell proliferative and anti-apoptotic proteins | [277] |
| LNCaP, DU-145, and PC-3 cells | 5, 10, 20, 40, 80, and 160 μM quercetin for 24 h, 48 h, and 72 h | Apoptotic and necrotic cell death and AKT and NF-κB activation in PC-3 and LNCaP cells; reduction of AKT pathway and activation of Raf/MEK in DU-145 cells | [278] | |
| LNCaP cells | 0, 1, 10, 25, 50, 100, and 150 μM quercetin for 24 h to 5 days depending on the type of analysis | Inhibited expression and function of the AR in LNCaP cells, decreased mRNA levels of PSA, NKX3.1, and ODC and repressed AR expression | [279] | |
| LNCaP cells | 50–200 μM quercetin for 24 h and 48 h | Quercetin at 150 μM caused G0/G1 phase arrest via decreasing the levels of CDK2, cyclins E, and D proteins, stimulated the protein expression of ATF, GRP78, and GADD153, apoptotic cell death and DNA damage at 48 h, decreased Bcl-2, increased Bax, and activation of caspase-3, -8, and -9 | [280] | |
| BALB/cA nude mice | PC-3 cells injected s.c, 20 mg/kg/d quercetin injected i.p for 16 days | Reduced tumour growth, inhibited tumorigenesis by targeting angiogenesis, reduced cell viability and induced apoptosis correlated with downregulation of AKT, mTOR and P70S6K | [281] | |
| Apigenin | PC-3 xenograft mice | Orally administration of 20 and 50 μg/mouse/day apigenin for 8 weeks | Both doses of apigenin decreased tumour growth, HDAC activity, HDAC1 and HDAC3 protein expression, and bcl-2 expression shifting the Bax/Bcl-2 ratio in favour of apoptosis | [282] |
| LNCaP and PC-3 cells | 0, 10, 20, 40, and 80 μM apigenin for 72 h at 37 °C | Up-regulation of p21 expression, and p21 inhibits transcription of PLK-1 | [283] | |
| PC-3 and DU-145 cells | 5–40 μM apigenin for 24 h | Dose-dependent suppression of XIAP, c-IAP1, c-IAP2 and survivin protein levels, decrease in cell viability and apoptosis, decrease in Bcl-xL and Bcl-2, and inhibition of class I histone deacetylases and HDAC1 protein expression | [284] | |
| C57BL/TGN TRAMP mice | 20 and 50 µg/day of apigenin for 20 weeks | Inhibition of VEGF, uPA, MMP-2, and MMP-9, tumour growth, and metastasis, reduction of IGF-I, and increase of IGFBP-3 through inhibition of p-AKT and p-ERK1/2 | [285] | |
| TRAMP mice | 20 and 50 μg/mouse/day, 6 days/week for 20 weeks | Apigenin-treated mice showed reduced proliferation, reduced phosphorylation of AKT (Ser473) and FoxO3a (Ser253), and upregulation of FoxO-responsive proteins BIM and p27/Kip1 | [286] | |
| Proanthocyanidins | DU-145 cells | 0.1, 0.5 and 1.0 mg/mL PAC for 24 h | Down-regulation of MMP activity and up-regulation of TIMP activity | [287] |
| DU-145 and LNCaP cells | 20 μg/mL of blueberry fraction, 2.38 mM quercetin for 48 h (DU-145) or 72 h (LNCaP) | Inhibited growth of DU-145 and LNCaP cells | [288] | |
| DU-145, PC-3, and LNCaP cells | 1–100 mg/mL PAC complex for 48 h | Inhibited proliferation of PC-3 and DU-145 with higher effect in LNCaP cells by decreasing AR expressing, G1 cell cycle arrest, decreased cyclin-dependent kinases, and cyclins, stimulated p21 and p27, and increased phosphorylation of p44 and p42 | [289] |
| Nanoparticle (Size) | Model | Treatment | Effect | Ref. |
|---|---|---|---|---|
| Curcumin-CA-NP (12.53–60.23 nm) | DU-145 cells | 0.0832–260 μM 72 h | Uptake by PCa cells | [299] |
| Curcumin emulsome NP (184.21 nm) | LNCaP cells | 10–40 μM for 24 h, 48 h or 72 h | Decreased proliferation, cell cycle arrest at G2/M phase | [300] |
| Curcumin NPs (34.0–359.4 nm) | PC-3 cells | 50–600 μM overnight | Decreased cell viability and increased haemolytic effect | [301] |
| FA-RES + DTX-PBM NP (36.6 nm) | PC-3, C4-2B and LNCaP cells | 3 μM RES + 0.01 μM DTX 24 h, 48 h, or 72 h | Reduced expression of NF-kB p65, COX-2, pro- and anti-apoptotic genes | [302] |
| RSV-SLN (126.85 nm) | PC-3 cells and Charles Foster rats | 2 mL for 0–48 h and 2 mg/kg i.v. for 0–24 h | Internalization of NPs in PC-3 cells | [303] |
| RES-MSNs (60 nm) | PC-3 cells | 10–20 μg for 72 h | Increased anti-proliferative activity and sensitization of Docatexal | [304] |
| RL-loaded PLGA (202.8 nm) | LNCaP cells | 0–50 μM for 48 h | Decreased cell viability, G1-S phase arrest, increased apoptosis | [305] |
| EGCG-PA-PEG-NP (N.D.) | Tumour xenograft mice | 1 mg in food consumption | Proapoptotic and angiogenesis inhibitory effects, enhanced bioavailability | [306] |
| 198AuNP-EGCg (535 nm) | PC-3 xenograft SCID mice | 136 μCi I.T. for 42 days | 72% retention in tumours after 24 h and 80% reduction of tumour volumes after 28 days | [307] |
| EGCG-GA-MD-NPs (120 nm) | DU-145 cells | 0.9–60 mg/mL for 64 h | Reduced cell viability and apoptosis induction | [308] |
| Chitosan-based EGCG NP (150–200 nm) | Athymic nude xenograft mice | 3–6 mg/kg by O.A 5x week | Decreased tumour growth and PSA levels | [309] |
| EGCG-gold NPs (90.3 nm) | PC-3 cells | 0–200 μg/mL for 1–24 h | Increased NF-κB activity and apoptosis | [310] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.; Costa, C.; Fernandes, R.; Barros, A.N. Inflammation in Prostate Cancer: Exploring the Promising Role of Phenolic Compounds as an Innovative Therapeutic Approach. Biomedicines 2023, 11, 3140. https://doi.org/10.3390/biomedicines11123140
Fernandes R, Costa C, Fernandes R, Barros AN. Inflammation in Prostate Cancer: Exploring the Promising Role of Phenolic Compounds as an Innovative Therapeutic Approach. Biomedicines. 2023; 11(12):3140. https://doi.org/10.3390/biomedicines11123140
Chicago/Turabian StyleFernandes, Raquel, Cátia Costa, Rúben Fernandes, and Ana Novo Barros. 2023. "Inflammation in Prostate Cancer: Exploring the Promising Role of Phenolic Compounds as an Innovative Therapeutic Approach" Biomedicines 11, no. 12: 3140. https://doi.org/10.3390/biomedicines11123140
APA StyleFernandes, R., Costa, C., Fernandes, R., & Barros, A. N. (2023). Inflammation in Prostate Cancer: Exploring the Promising Role of Phenolic Compounds as an Innovative Therapeutic Approach. Biomedicines, 11(12), 3140. https://doi.org/10.3390/biomedicines11123140








