Iodixanol as a New Contrast Agent for Cyanoacrylate Embolization: A Preliminary In Vivo Swine Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Swine Renal-Artery Embolization
2.2. Micro-Computed Tomography
2.2.1. Acquisition and Reconstruction Parameters
2.2.2. Objectively Assessed µCT Outcome Measures
2.2.3. Subjectively Assessed µCT Outcome Measures
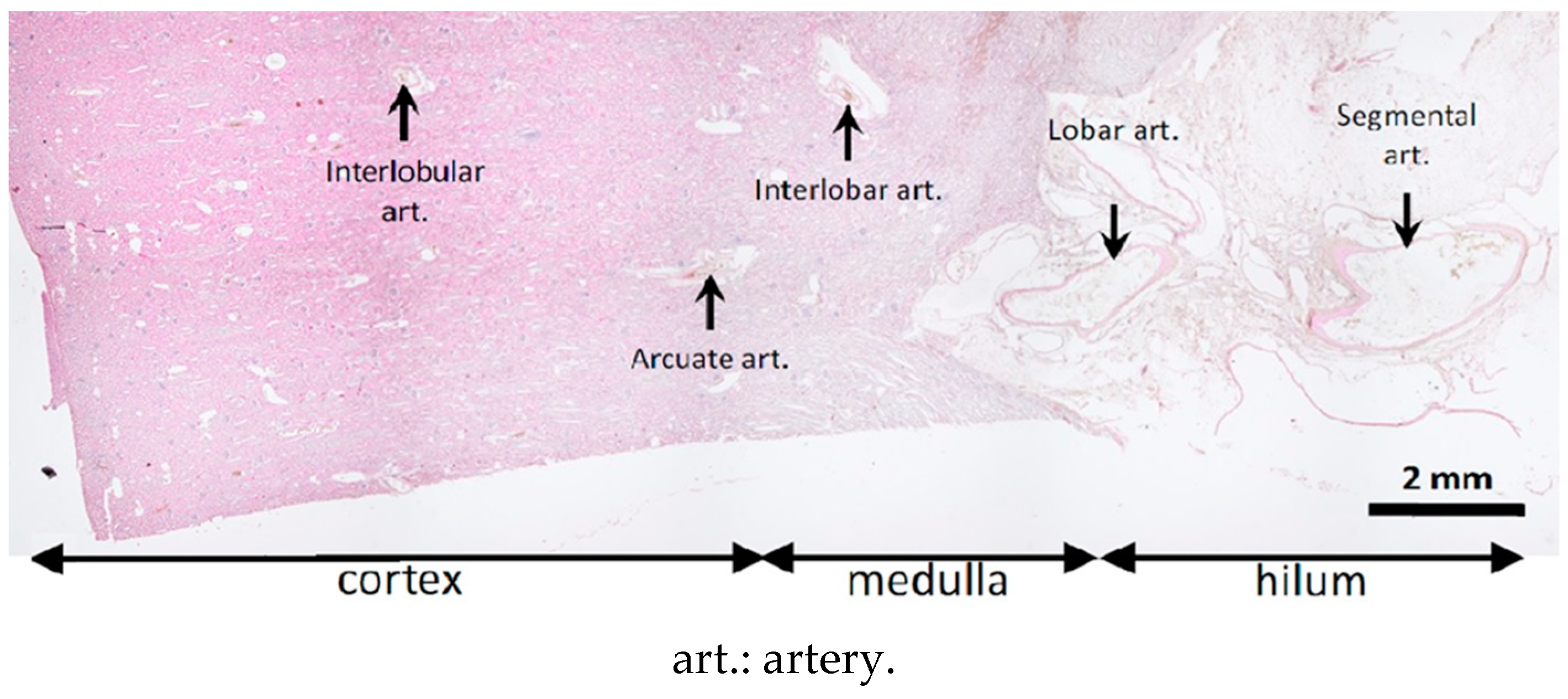
2.3. Histological Evaluation
2.4. Statistical Analysis
3. Results
3.1. Micro-Computed Tomography Parameters
3.1.1. Objectively Assessed µCT Parameters
3.1.2. Subjectively Assessed µCT Parameters
3.2. Histological Parameters
3.3. Dose-Response Effect and Binary Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, A.; Blanzy, J.; Gómez, K.J.R.; Preul, M.C.; Vernon, B.L. Liquid embolic agents for endovascular embolization: A review. Gels 2023, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, B.A.; Shi, H.-B.; Liu, S.; Alqurashi, T.A.; Sabri, Z.J. N-butyl cyanoacrylate glue versus nonspherical polyvinyl alcohol particles for prostatic arterial embolization to treat benign prostatic hyperplasia: Safety and efficacy. Urol. J. 2023, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.; Zetterlund, P.B.; Aldabbagh, F. Radical Polymerization of Alkyl 2-Cyanoacrylates. Molecules 2018, 23, 465. [Google Scholar] [CrossRef]
- Schmitt, N.; Wucherpfennig, L.; Hohenstatt, S.; Weyland, C.S.; Sommer, C.M.; Bendszus, M.; Möhlenbruch, M.A.; Vollherbst, D.F. Visibility of liquid embolic agents in fluoroscopy: A systematic in vitro study. J. NeuroInterv. Surg. 2023, 15, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, C.; Seiji, K.; Matsunaga, K.; Matsuhashi, T.; Ohta, M.; Shida, S.; Takase, K.; Takahashi, S. Properties of N-butyl cyanoacrylate-iodized oil mixtures for arterial embolization: In vitro and in vivo experiments. J. Vasc. Interv. Radiol. 2012, 23, 1215–1221.e1. [Google Scholar] [CrossRef] [PubMed]
- Guillen, K.; Comby, P.O.; Salsac, A.V.; Falvo, N.; Lenfant, M.; Oudot, A.; Sikner, H.; Dencausse, A.; Laveissiere, E.; Aho-Glele, S.L.; et al. X-ray Microtomography to Assess Determinants of In Vivo N-Butyl Cyanoacrylate Glubran®2 Polymerization: A Rabbit-Model Study. Biomedicines 2022, 10, 2625. [Google Scholar] [CrossRef]
- Li, Y.J.; Barthes-Biesel, D.; Salsac, A.V. Polymerization kinetics of n-butyl cyanoacrylate glues used for vascular embolization. J. Mech. Behav. Biomed. Mater. 2017, 69, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Sheng, J.; Tan, H.; Mao, J. Pancreas Lipiodol Embolism Induced Acute Necrotizing Pancreatitis Following Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Case Report and Literature Review. Medicine 2019, 98, e18095. [Google Scholar] [CrossRef]
- Naorungroj, T.; Naksanguan, T.; Chinthammitr, Y. Pulmonary Lipiodol Embolism after Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Case Report and Literature Review. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2013, 96 (Suppl. 2), S270–S275. [Google Scholar]
- Ishimaru, H.; Morikawa, M.; Sakugawa, T.; Sakamoto, I.; Motoyoshi, Y.; Ikebe, Y.; Uetani, M. Cerebral Lipiodol Embolism Related to a Vascular Lake during Chemoembolization in Hepatocellular Carcinoma: A Case Report and Review of the Literature. World J. Gastroenterol. 2018, 24, 4291–4296. [Google Scholar] [CrossRef]
- Batcheller, L.; Thaller, M.; Wright, B. Cerebral Lipiodol Embolisation. Pract. Neurol. 2022, 22, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Lamby, P.; Prantl, L.; Wiggermann, P.; Jung, E.; Krüger-Genge, A.; Franke, R. Post-mortem distribution of iodinated contrast media (ICM) (iodixanol versus iopromide) in the porcine kidney after multiple bolus injections in vivo into the supra-renal aorta. Clin. Hemorheol. Microcirc. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Renal Safety of Iodixanol. Expert Rev. Cardiovasc. Ther. 2006, 4, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Terrenato, I.; Sperati, F.; Musicco, F.; Pozzi, A.F.; di Turi, A.; Caterino, M.; de Lutio di Castelguidone, E.; Setola, S.V.; Bellomi, M.; Neumaier, C.E.; et al. Iodixanol versus Iopromide in Cancer Patients: Evidence from a Randomized Clinical Trial. J. Cell. Physiol. 2018, 233, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Guillen, K.; Salsac, A.V.; Comby, P.O.; Aho-Glele, L.S.; Chevallier, O.; Loffroy, R. In Vitro Characteristics of a Cyanoacrylate/Water-Soluble Contrast Emulsion: Preliminary Data from Light Microscopy Approach. CardioVasc. Interv. Radiol. 2023, 46, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Higashino, N.; Sonomura, T.; Okuhira, R.; Koike, M.; Ikoma, A.; Kawai, N.; Minamiguchi, H. Determination of the Optimal Ratio and the Relationship between Viscosity and Adhesion of n-Butyl Cyanoacrylate-Lipiodol-Iopamidol for Balloon-Assisted Embolization of Wide-Neck Aneurysms in Swine. CardioVasc. Interv. Radiol. 2022, 45, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Higashino, N.; Sonomura, T.; Fukuda, K.; Ikoma, A.; Okuhira, R.; Ueda, S.; Kawai, N. Feasibility and Safety of n-Butyl Cyanoacrylate-Lipiodol-Iopamidol as an Alternative Liquid Embolic Material. CardioVasc. Interv. Radiol. 2021, 44, 482–488. [Google Scholar] [CrossRef]
- Ikoma, A.; Sonomura, T.; Higashino, N.; Fukuda, K.; Ihira, H.; Furotani, H.; Koike, M.; Sato, H.; Murata, S.-I.; Minamiguchi, H. Feasibility of the glue-in-plug technique using a novel liquid embolic material in a swine model. J. Vasc. Interv. Radiol. 2023, 34, 2233–2239. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Wang, Z.; Chen, K.; Xue, C.; Yu, M.; Wang, Y.; Kong, F.; Liu, K.; Qin, K. Raman spectroscopic characterization of polymerization kinetics of cyanoacrylate embolic glues for vascular embolization. Polymers 2021, 13, 3362. [Google Scholar] [CrossRef]
- Guillen, K.; Comby, P.O.; Chevallier, O.; Salsac, A.V.; Loffroy, R. In Vivo Experimental Endovascular Uses of Cyanoacrylate in Non-Modified Arteries: A Systematic Review. Biomedicines 2021, 9, 1282. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Groningen, M.C.-V.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814. [Google Scholar] [CrossRef]
- Kawai, K.; Sato, Y.; Hokama, J.Y.; Kawakami, R.; Konishi, T.; Ghosh, S.K.B.; Virmani, R.; Finn, A.V. Histology, OCT, and micro-CT evaluation of coronary calcification treated with intravascular lithotripsy: Atherosclerotic cadaver study. JACC Cardiovasc. Interv. 2023, 16, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Ausk, B.J.; Tucker, A.N.; Huber, P.; Firoozabadi, R.; Gross, J.M.; Gross, T.S.; Bain, S.D. A microCT-based platform to quantify drug targeting. Eur. Radiol. Exp. 2023, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, T.; Kawai, N.; Sato, M.; Sonomura, T.; Takasaka, I.; Nakai, M.; Minamiguchi, H.; Sahara, S.; Iwasaki, Y.; Naka, T.; et al. Comparison of hemostatic durability between N-butyl cyanoacrylate and gelatin sponge particles in transcatheter arterial embolization for acute arterial hemorrhage in a coagulopathic condition in a swine model. Cardiovasc. Interv. Radiol. 2010, 33, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Boulton, M.; Lee, D.H.; Pelz, D.M.; Lownie, S.P.; Boulton, M.; Lee, D.H.; Pelz, D.M.; Lownie, S.P. A systematic characterization of the factors influencing polymerization and dynamic behavior of n-butyl cyanoacrylate. J. Neurointerv. Surg. 2018, 10, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Meier, P.; Tamhane, U.U.; Welch, K.B.; Moscucci, M.; Gurm, H.S. The Relative Renal Safety of Iodixanol Compared with Low-Osmolar Contrast Media: A Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2009, 2, 645–654. [Google Scholar] [CrossRef]
- Biondi-Zoccai, G.; Lotrionte, M.; Thomsen, H.S.; Romagnoli, E.; D’Ascenzo, F.; Giordano, A.; Frati, G. Nephropathy after Administration of Iso-Osmolar and Low-Osmolar Contrast Media: Evidence from a Network Meta-Analysis. Int. J. Cardiol. 2014, 172, 375–380. [Google Scholar] [CrossRef]
- Díaz, L.; Zambrano, E.; Flores, M.E.; Contreras, M.; Crispín, J.C.; Alemán, G.; Bravo, C.; Armenta, A.; Valdés, V.J.; Tovar, A.; et al. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev. Investig. Clin. 2020, 73, 199–209. [Google Scholar] [CrossRef]






| Parameters | Mean ± SD (Range) |
|---|---|
| Indexed cast ratio, % | 1.10 ± 1.31 (0.07–3.36) |
| Renal-artery ROI (HU) | 2955.33 ± 2456.21 (204.16–6918.28) |
| Cast-capsule distance, mm | 5.84 ± 460 (0.83–1467) |
| Post-embolization renal-artery diameter, mm | 461 ± 1.10 (2.80–680) |
| Volume of glue–ICA injected, mL | 3.30 ± 1.92 (1.80–7.00) |
| SE | p > |t| | 95% CI | ||
|---|---|---|---|---|
| Obliteration | 0.86 | 0.064 | −4.09 | 0.15 |
| Banff score | 0.55 | 0.456 | −0.95 | 1.85 |
| Necrosis | 0.89 | 0.347 | −1.33 | 3.16 |
| Indexed cast ratio | 0.02 | 0.095 | −0.08 | 0.01 |
| Renal-artery ROI | 4115.78 | 0.378 | −13,902.78 | 6090.25 |
| Cast heterogeneity | 2.42 | 0.880 | −5.51 | 6.27 |
| Cast-capsule distance | 2.67 | 0.001 | 10.78 | 24.33 |
| Post-embolization renal-artery diameter | 1.82 | 0.454 | −2.99 | 5.91 |
| Glue–ICA volume injected | 3.04 | 0.206 | −11.68 | 3.09 |
| SE | p > |t| | 95%CI | ||
|---|---|---|---|---|
| Obliteration | 0.48 | 0.217 | −1.72 | 0.45 |
| Banff score | 0.25 | 0.340 | −0.35 | 0.88 |
| Necrosis | 0.43 | 0.277 | −0.54 | 1.57 |
| Indexed cast ratio | 0.00 | 0.000 | −0.04 | −0.02 |
| Renal-artery ROI | 974.49 | 0.001 | −6956.25 | −2567.02 |
| Cast heterogeneity | 1.07 | 0.376 | −1.42 | 3.42 |
| Cast-capsule distance | 2.50 | 0.042 | 0.28 | 12.16 |
| Post-embolization renal-artery diameter | 0.83 | 0.365 | −2.69 | 1.10 |
| Glue–ICA volume injected | 1.55 | 0.638 | −2.75 | 4.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillen, K.; Comby, P.-O.; Oudot, A.; Salsac, A.-V.; Falvo, N.; Virely, T.; Poupardin, O.; Guillemin, M.; Chevallier, O.; Loffroy, R. Iodixanol as a New Contrast Agent for Cyanoacrylate Embolization: A Preliminary In Vivo Swine Study. Biomedicines 2023, 11, 3177. https://doi.org/10.3390/biomedicines11123177
Guillen K, Comby P-O, Oudot A, Salsac A-V, Falvo N, Virely T, Poupardin O, Guillemin M, Chevallier O, Loffroy R. Iodixanol as a New Contrast Agent for Cyanoacrylate Embolization: A Preliminary In Vivo Swine Study. Biomedicines. 2023; 11(12):3177. https://doi.org/10.3390/biomedicines11123177
Chicago/Turabian StyleGuillen, Kévin, Pierre-Olivier Comby, Alexandra Oudot, Anne-Virginie Salsac, Nicolas Falvo, Thierry Virely, Olivia Poupardin, Mélanie Guillemin, Olivier Chevallier, and Romaric Loffroy. 2023. "Iodixanol as a New Contrast Agent for Cyanoacrylate Embolization: A Preliminary In Vivo Swine Study" Biomedicines 11, no. 12: 3177. https://doi.org/10.3390/biomedicines11123177
APA StyleGuillen, K., Comby, P.-O., Oudot, A., Salsac, A.-V., Falvo, N., Virely, T., Poupardin, O., Guillemin, M., Chevallier, O., & Loffroy, R. (2023). Iodixanol as a New Contrast Agent for Cyanoacrylate Embolization: A Preliminary In Vivo Swine Study. Biomedicines, 11(12), 3177. https://doi.org/10.3390/biomedicines11123177






