Plasma Exchange versus Intravenous Immunoglobulin in Worsening Myasthenia Gravis: A Systematic Review and Meta-Analysis with Special Attention to Faster Relapse Control
Abstract
:1. Introduction
2. Methods
2.1. Clinical Question
2.2. Hypothesis
2.3. Eligibility Criteria
2.4. Information Resources
2.5. Search Strategy
2.6. Selection Process
2.7. Data Collection Process
2.8. Data Items
2.9. Study Risk of Bias Assessment and Grading
2.10. Synthesis Methods
3. Results
3.1. Search and Selection
3.2. Basic Characteristics of Included Studies
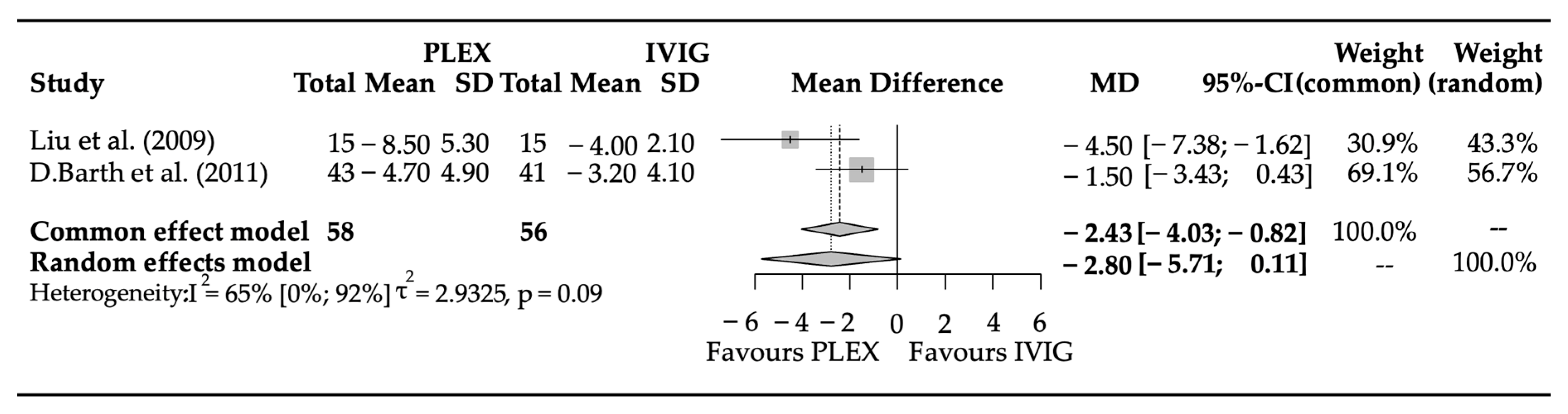
3.3. Efficacy Parameters
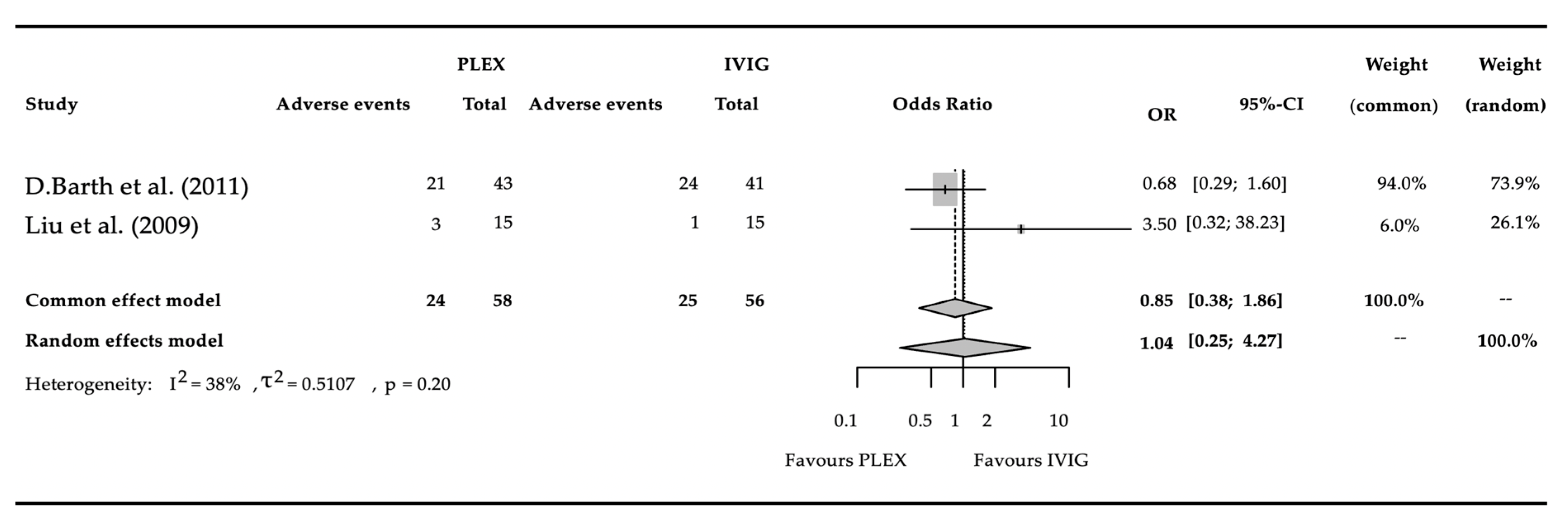
3.4. Adverse Events
3.5. Risk of Bias Assessment
3.6. Publication Bias and Heterogeneity
4. Discussion
5. Conclusions
5.1. Strengths and Limitations
5.2. Implications
5.2.1. Implications for Research
5.2.2. Implications for Practice
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChR+ | acetylcholine receptor-positive |
| AE | adverse event |
| FcRn | neonatal fragment crystallizable (Fc) receptor |
| ICU | intensive care unit |
| IVIG | intravenous immunoglobulin |
| MD | medical doctor |
| MG | myasthenia gravis |
| MG-ADL | Myasthenia Gravis Activities of Daily Living |
| MGFA | Myasthenia Gravis Foundation of America |
| MuSK+ | anti muscle-specific kinase-positive |
| OR | odds ratio |
| PLEX | plasma exchange |
| PRO | patient-reported outcome |
| QMG | quantitative myasthenia gravis scores |
| RCT | randomized controlled trial |
| RoB | risk of bias assessment |
References
- Keesey, J.C. Clinical evaluation and management of myasthenia gravis. Muscle Nerve 2004, 29, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; McConville, J.; Farrugia, M.E.; Bowen, J.; Plested, P.; Tang, T.; Evoli, A.; Matthews, I.; Sims, G.; Dalton, P.; et al. Antibodies in myasthenia gravis and related disorders. Ann. N. Y. Acad. Sci. 2003, 998, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.S.; Cardwell, C.R.; McCarron, P.O.; McConville, J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, D.C.; Zhang, C.; Li, Z.; Zhai, Y.; Xiu, Y.; Gu, H.; Li, H.; Wang, Y.; Shi, F.D. Incidence, mortality, and economic burden of myasthenia gravis in China: A nationwide population-based study. Lancet Reg. Health West. Pac. 2020, 5, 100063. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.B.; Jensen, T.S.; Tsiropoulos, I.; Sørensen, T.; Kjaer, M.; Højer-Pedersen, E.; Rasmussen, M.J.; Lehfeldt, E. Mortality and survival in myasthenia gravis: A Danish population based study. J. Neurol. Neurosurg. Psychiatry 1998, 64, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bedlack, R.S.; Sanders, D.B. On the concept of myasthenic crisis. J. Clin. Neuromuscul. Dis. 2002, 4, 40–42. [Google Scholar] [CrossRef]
- Rózsa, C.; Mikor, A.; Kasa, K.; Illes, Z.; Komoly, S. Long-term effects of combined immunosuppressive treatment on myasthenic crisis. Eur. J. Neurol. 2009, 16, 796–800. [Google Scholar] [CrossRef]
- Wendell, L.C.; Levine, J.M. Myasthenic crisis. Neurohospitalist 2011, 1, 16–22. [Google Scholar] [CrossRef]
- O'Riordan, J.I.; Miller, D.H.; Mottershead, J.P.; Hirsch, N.P.; Howard, R.S. The management and outcome of patients with myasthenia gravis treated acutely in a neurological intensive care unit. Eur. J. Neurol. 1998, 5, 137–142. [Google Scholar] [CrossRef]
- Stetefeld, H.; Schroeter, M. SOP myasthenic crisis. Neurol. Res. Pract. 2019, 1, 19. [Google Scholar] [CrossRef]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef]
- Pinching, A.J.; Peters, D.K. Remission of myasthenia gravis following plasma-exchange. Lancet 1976, 2, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, H.; Barth, D.; Bril, V. Safety of plasma exchange therapy in patients with myasthenia gravis. Muscle Nerve 2013, 47, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Skeie, G.O.; Apostolski, S.; Evoli, A.; Gilhus, N.E.; Illa, I.; Harms, L.; Hilton-Jones, D.; Melms, A.; Verschuuren, J.; Horge, H.W. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur. J. Neurol. 2010, 17, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ipe, T.S.; Davis, A.R.; Raval, J.S. Therapeutic Plasma Exchange in Myasthenia Gravis: A Systematic Literature Review and Meta-Analysis of Comparative Evidence. Front. Neurol. 2021, 12, 662856. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, P.; Chevret, S.; Toyka, K. Plasma exchange for myasthenia gravis. Cochrane Database Syst. Rev. 2002, 2002, Cd002275. [Google Scholar] [CrossRef] [PubMed]
- Arsura, E.L.; Bick, A.; Brunner, N.G.; Grob, D. Effects of repeated doses of intravenous immunoglobulin in myasthenia gravis. Am. J. Med. Sci. 1988, 295, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Barak, Y.; Miron, S.; Sarova-Pinhas, I. Immunoglobulin treatment in refractory Myasthenia gravis. Muscle Nerve 2000, 23, 551–555. [Google Scholar] [CrossRef]
- Cosi, V.; Lombardi, M.; Piccolo, G.; Erbetta, A. Treatment of myasthenia gravis with high-dose intravenous immunoglobulin. Acta Neurol. Scand. 1991, 84, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Jongen, J.L.; van Doorn, P.A.; van der Meché, F.G. High-dose intravenous immunoglobulin therapy for myasthenia gravis. J. Neurol. 1998, 245, 26–31. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Choudhry, M.A.; Akbar, M.S.; Mohammad, Y.; Chua, H.C.; Yahia, A.M.; Ulatowski, J.A.; Krendel, D.A.; Leshner, R.T. Plasma exchange versus intravenous immunoglobulin treatment in myasthenic crisis. Neurology 1999, 52, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, G.I.; Barohn, R.J.; Foster, B.M.; Jackson, C.E.; Kissel, J.T.; Day, J.W.; Thornton, C.A.; Nations, S.P.; Bryan, W.W.; Amato, A.A.; et al. Randomized, controlled trial of intravenous immunoglobulin in myasthenia gravis. Muscle Nerve 2002, 26, 549–552. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Wolfe, G.; Benatar, M.; Cea, G.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.; et al. International Consensus Guidance for Management of Myasthenia Gravis. 2020 Update. Neurology 2021, 96, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.; Herbelin, L.; Dimachkie, M.M.; Barohn, R.J. Measuring Clinical Treatment Response in Myasthenia Gravis. Neurol. Clin. 2018, 36, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Jaretzki, A., 3rd; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia gravis: Recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000, 55, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Meta-Analysis with R; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.; Ebert, D.D. Dmetar: Companion R Package For The Guide ‘Doing Meta-Analysis in R’; Chapman & Hall/CRC Press: London, UK, 2019. [Google Scholar]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical Aspects of the Analysis of Data from Retrospective Studies of Disease. JNCI J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar] [CrossRef]
- Thompson, S.G.; Turner, R.M.; Warn, D.E. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat. Methods Med. Res. 2001, 10, 375–392. [Google Scholar] [CrossRef]
- Paule, R.C.; Mandel, J. Consensus Values and Weighting Factors. J. Res. Natl. Bur. Stand. 1982, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman and Hall/CRC: London, UK, 2021. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016, 6, e010247. [Google Scholar] [CrossRef] [PubMed]
- Rønager, J.; Ravnborg, M.; Hermansen, I.; Vorstrup, S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artif. Organs 2001, 25, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, P.; Chevret, S.; Clair, B.; Tranchant, C.; Chastang, C. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann. Neurol. 1997, 41, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Nabavi Nouri, M.; Ng, E.; Nwe, P.; Bril, V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011, 76, 2017–2023. [Google Scholar] [CrossRef]
- Liu, J.F.; Wang, W.X.; Xue, J.; Zhao, C.B.; You, H.Z.; Lu, J.H.; Gu, Y. Comparing the autoantibody levels and clinical efficacy of double filtration plasmapheresis, immunoadsorption, and intravenous immunoglobulin for the treatment of late-onset myasthenia gravis. Ther. Apher. Dial. 2010, 14, 153–160. [Google Scholar] [CrossRef]
- Guptill, J.T.; Sanders, D.B.; Evoli, A. Anti-MuSK antibody myasthenia gravis: Clinical findings and response to treatment in two large cohorts. Muscle Nerve 2011, 44, 36–40. [Google Scholar] [CrossRef]
- Murthy, J.M.; Meena, A.K.; Chowdary, G.V.; Naryanan, J.T. Myasthenic crisis: Clinical features, complications and mortality. Neurol. India 2005, 53, 37–40, discussion 40. [Google Scholar] [CrossRef]
- Ortiz-Salas, P.; Velez-Van-Meerbeke, A.; Galvis-Gomez, C.A.; Rodriguez, Q.J. Human Immunoglobulin Versus Plasmapheresis in Guillain-Barre Syndrome and Myasthenia Gravis: A Meta-Analysis. J. Clin. Neuromuscul. Dis. 2016, 18, 1–11. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Karachaliou, E.; Chroni, E.; Zouvelou, V.; Tzanetakos, D.; Salakou, S.; Papadopoulou, M.; Tzartos, S.; Voumvourakis, K.; Kilidireas, C.; et al. Immunotherapies in MuSK-positive Myasthenia Gravis; an IgG4 antibody-mediated disease. Front. Immunol. 2023, 14, 1212757. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiong, W.C.; Mei, L. Neuromuscular Junction Formation, Aging, and Disorders. Annu. Rev. Physiol. 2018, 80, 159–188. [Google Scholar] [CrossRef] [PubMed]
- Aurangzeb, S.; Tariq, M.; Irshad, M.; Badshah, M.; Khan, R.S. Relationship between anti-acetylcholine receptor antibody titres and severity of myasthenia gravis. J. Pak. Med. Assoc. 2009, 59, 289–292. [Google Scholar] [PubMed]
- Berger, B.; Schröter, N. Changes in antibody titers and clinical course in myasthenia gravis retrospective study. Prog. Neurol. Psychiatry 2022, 26, 28–33. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P.; Ma, M.; Yang, H.; Qi, G. Efficacy and safety of double-filtration plasmapheresis treatment of myasthenia gravis: A systematic review and meta-analysis. Medicine 2021, 100, e25622. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.; Katzberg, H.; Nabavi, M.; Bril, V. The quantitative myasthenia gravis score: Comparison with clinical, electrophysiological, and laboratory markers. J. Clin. Neuromuscul. Dis. 2012, 13, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, F.; Mantegazza, R. Antibody Therapies in Autoimmune Neuromuscular Junction Disorders: Approach to Myasthenic Crisis and Chronic Management. Neurotherapeutics 2022, 19, 897–910. [Google Scholar] [CrossRef]
- Di Stefano, V.; Alonge, P.; Rini, N.; Militello, M.; Lupica, A.; Torrente, A.; Brighina, F. Efgartigimod beyond myasthenia gravis: The role of FcRn-targeting therapies in stiff-person syndrome. J. Neurol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Saccà, F.; Barnett, C.; Vu, T.; Peric, S.; Phillips, G.A.; Zhao, S.; Qi, C.Z.; Gelinas, D.; Chiroli, S.; Verschuuren, J. Efgartigimod improved health-related quality of life in generalized myasthenia gravis: Results from a randomized, double-blind, placebo-controlled, phase 3 study (ADAPT). J. Neurol. 2023, 270, 2096–2105. [Google Scholar] [CrossRef]
- Howard, J.F., Jr.; Utsugisawa, K.; Benatar, M.; Murai, H.; Barohn, R.J.; Illa, I.; Jacob, S.; Vissing, J.; Burns, T.M.; Kissel, J.T.; et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): A phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017, 16, 976–986. [Google Scholar] [CrossRef]


| Aspect | Plasma Exchange | Intravenous Immunoglobulin |
|---|---|---|
| Advantages | Suitable for immediate intervention Rapid removal of pathogenic antibodies | Suitable for immediate intervention Requires minimally invasive intervention Suitable for outpatient setting Does not remove the applied therapy |
| Disadvantages | Invasive procedure requiring vascular access Requires special instruments, and trained personnel Removed the applied therapy | Expensive treatment option Shortage of supply may occur |
| Country | Number of Centers | Randomized | Blinded | Mean Age of PLEX Group | Number of Patients/ Sexes in PLEX Group | Baseline QMG of PLEX Group | Number of PLEX Treatments | Mean Age of IVIG Group | Number of Patients/ Sexes in IVIG Group | Baseline QMG of IVIG Group | IVIG Dosage | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barth, D. et al. (2011) [38] | Canada | Single-centered | Yes | Evaluator masked | 58 ± 17 | 17M/24F | 14.44 ± 3.8 | 5/every second day | 57 ± 18 | 19M/24F | 14.26 ± 4.0 | 2/2 day (1g/kg/d) |
| Liu et al. (2009) [39] | China | Single-centered | Yes | Evaluator masked | 55.2 ± 1.4 | 9M/6F | 19.4 ± 2.2 | 3/24–48 h | 53.2 ± 1.7 | 8M/7F | 16.5 ± 1.7 | 5/5day (0.4 g/kg/d) |
| Efficacy related parameters | D1: Randomization process D2: Deviations from the intended interventions D3: Missing outcome data D4: Measurement of the outcome D5: Selection of the reported result | ||||||
| D1 | D2 | D3 | D4 | D5 | Overall | ||
| Barth [38] (2011) | (+) | (+) | (+) | (+) | (+) | (+) | |
| Liu (2009) [39] | (+) | (+) | (+) | (+) | (+) | (+) | |
| Adverse events related parameters | |||||||
| Barth [38] (2011) | (+) | (−) | (+) | (−) | (+) | (−) | |
| Liu (2009) [39] | (+) | (−) | (+) | (−) | (!) | (−) | |
| Low risk (+) | Some concerns (!) | High risk (−) | |||||
| Reported Side Effects in PLEX | Reported Side Effects in IVIG |
|---|---|
| hypertension (2) hematoma (1) citrate reaction (6) poor venous access delaying treatment (4) vasospasm (8) vasovagal reaction (2) myocardial infarction (1) | allergic reaction (2) nausea and vomiting (8) headache (8) chills (2) fever (3) hemolytic anemia (1) hypertension (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlekovics, M.; Engh, M.A.; Lugosi, K.; Szabo, L.; Hegyi, P.; Terebessy, T.; Csukly, G.; Molnar, Z.; Illes, Z.; Lovas, G. Plasma Exchange versus Intravenous Immunoglobulin in Worsening Myasthenia Gravis: A Systematic Review and Meta-Analysis with Special Attention to Faster Relapse Control. Biomedicines 2023, 11, 3180. https://doi.org/10.3390/biomedicines11123180
Pavlekovics M, Engh MA, Lugosi K, Szabo L, Hegyi P, Terebessy T, Csukly G, Molnar Z, Illes Z, Lovas G. Plasma Exchange versus Intravenous Immunoglobulin in Worsening Myasthenia Gravis: A Systematic Review and Meta-Analysis with Special Attention to Faster Relapse Control. Biomedicines. 2023; 11(12):3180. https://doi.org/10.3390/biomedicines11123180
Chicago/Turabian StylePavlekovics, Mark, Marie Anne Engh, Katalin Lugosi, Laszlo Szabo, Peter Hegyi, Tamas Terebessy, Gabor Csukly, Zsolt Molnar, Zsolt Illes, and Gabor Lovas. 2023. "Plasma Exchange versus Intravenous Immunoglobulin in Worsening Myasthenia Gravis: A Systematic Review and Meta-Analysis with Special Attention to Faster Relapse Control" Biomedicines 11, no. 12: 3180. https://doi.org/10.3390/biomedicines11123180
APA StylePavlekovics, M., Engh, M. A., Lugosi, K., Szabo, L., Hegyi, P., Terebessy, T., Csukly, G., Molnar, Z., Illes, Z., & Lovas, G. (2023). Plasma Exchange versus Intravenous Immunoglobulin in Worsening Myasthenia Gravis: A Systematic Review and Meta-Analysis with Special Attention to Faster Relapse Control. Biomedicines, 11(12), 3180. https://doi.org/10.3390/biomedicines11123180






