In Vitro Chondrogenesis Induction by Short Peptides of the Carboxy-Terminal Domain of Transforming Growth Factor β1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Eukaryotic Cell Line, Media, and Chemicals
2.2. Cloning
2.3. Overexpression and Purification of C-Terminal TGF-β1
2.4. Peptide Synthesis
2.5. Cell Culture and Differentiation
2.6. RNA Extraction
2.7. cDNA Synthesis
2.8. Real-Time Polymerase Chain Reaction
2.9. Alcian Blue Staining and Semi-Quantification
2.10. Western Blot Analysis
2.11. Rheological Measurements
2.12. Docking Procedures
2.13. Molecular Dynamics Simulations
2.14. Statistical Analysis
3. Results
3.1. Overproduction and Purification of L83-S112 C-Terminal Domain of TGF-β1
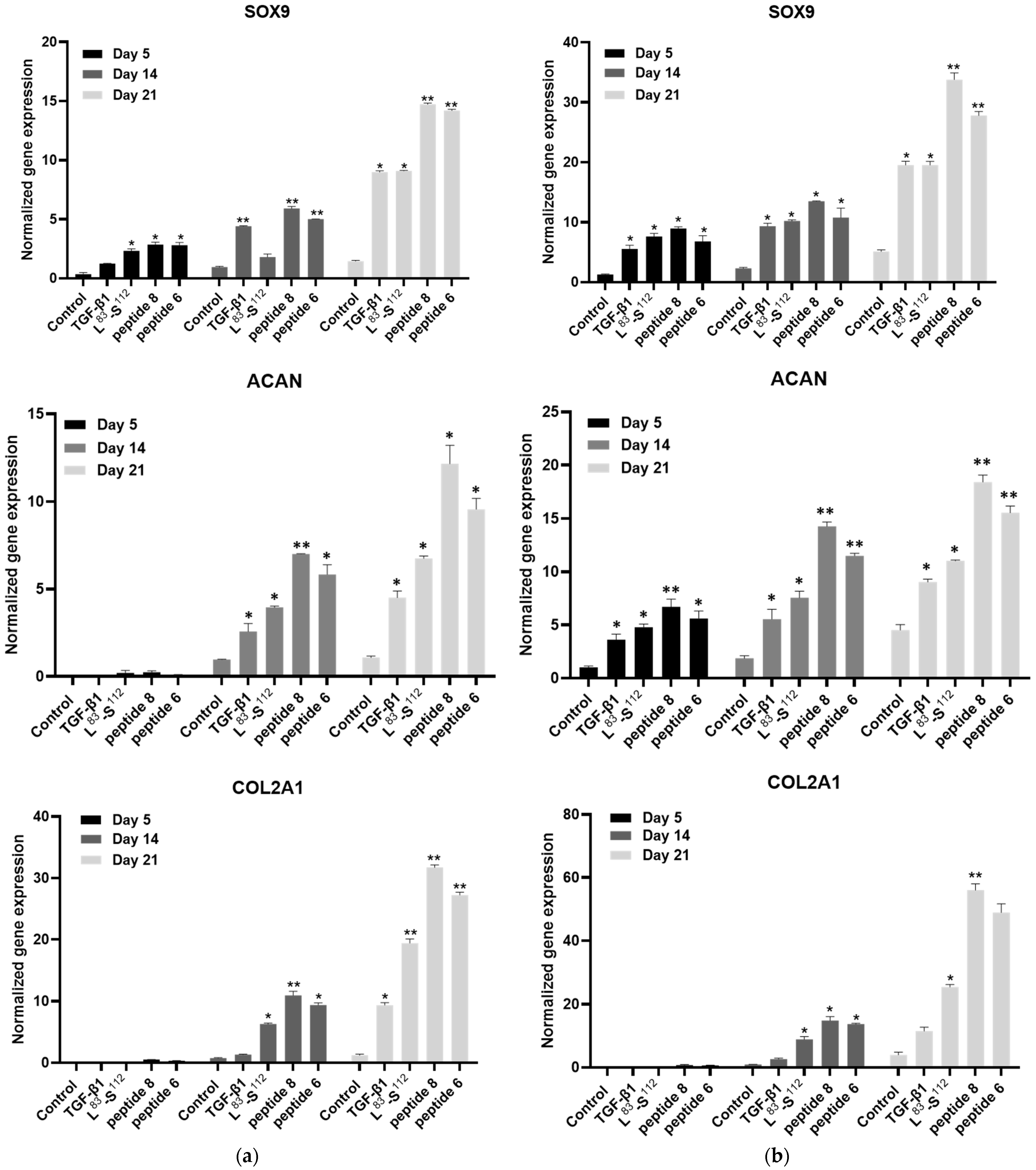
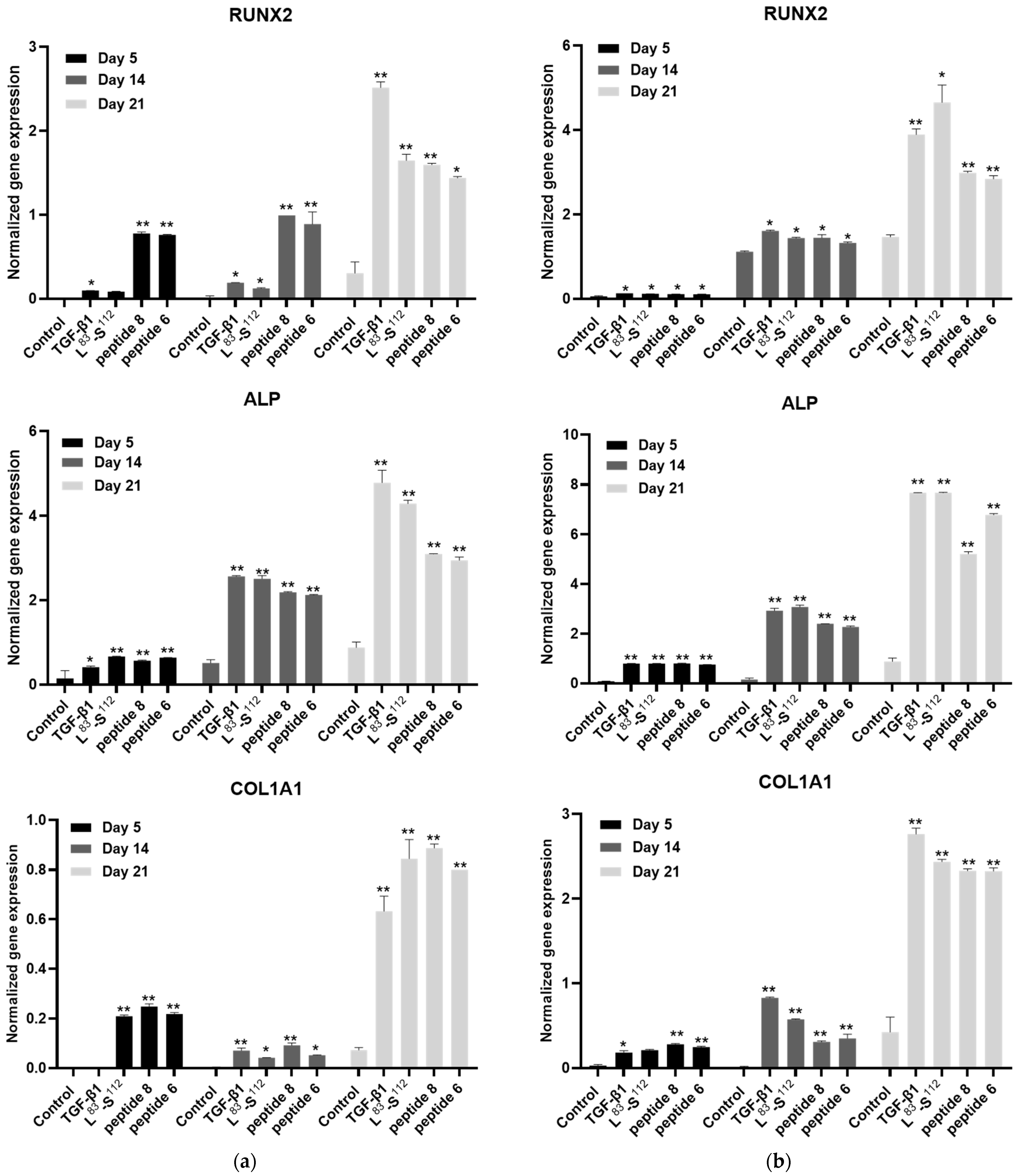
3.2. In Vitro Chondrogenic Differentiation of hDPSCs Is More Effective When Cells Are Treated with the Peptides Derived from the C-Terminal Domain in Monolayer Cultures
3.3. In Vitro Chondrogenic Differentiation of hDPSCs Is More Effective When Cells Are Treated with the Peptides Derived from the C-Terminal Domain in 3D Cultures
3.4. In Vitro Chondrogenic Differentiation of hDPSCs Affects the Rheological Properties of the Gelatin Type B–Alginate Gels
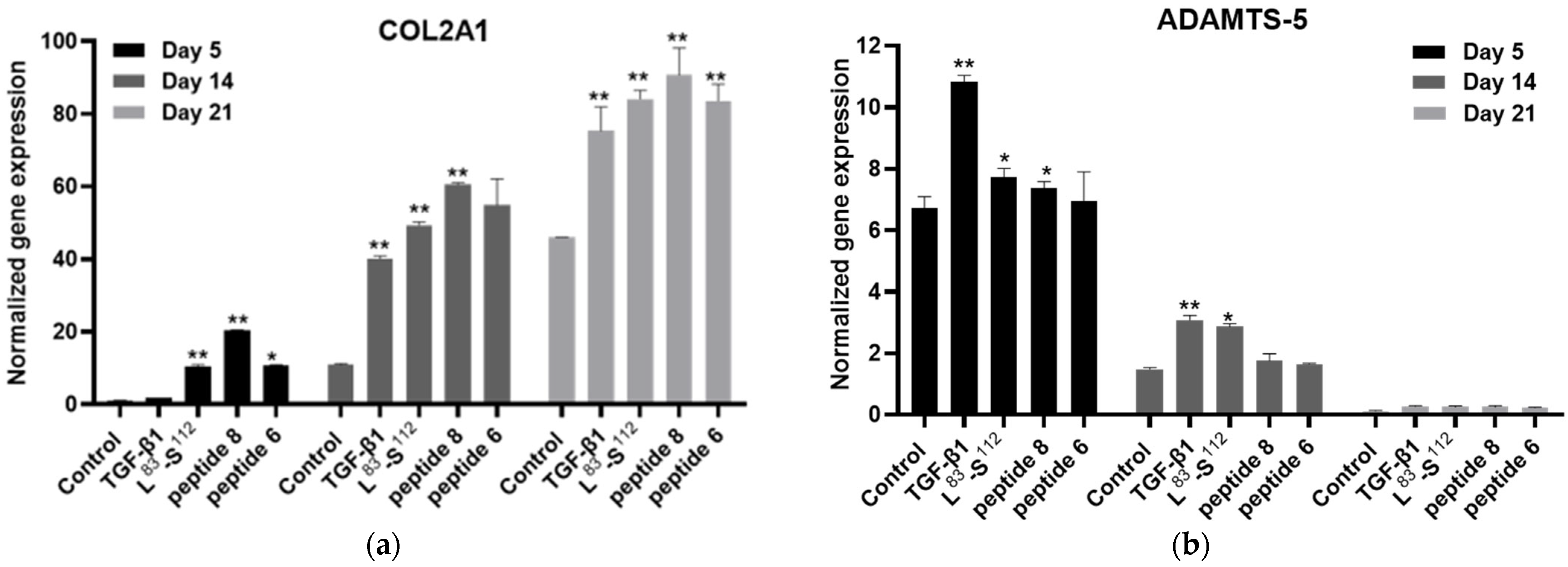
3.5. Implication of TGF-β1 Functional Peptides in Extracellular Matrix Homeostasis in Monolayer Cultures
3.6. Interaction of the Peptides with the Receptors
Hypothesis Testing
- (a)
- TGF is absent. In this case, the peptide can bind to the TβRII-TβRI complex, stabilizing it. This can be carried out if the peptide can bind to both subunits at the same time, e.g., in the space between the two subunits. Therefore, our docking results should find such associations more stable than TβRII-TβRI alone, which turned out to be the case. This mode of peptide action promotes the interaction of the intracellular domains of TβRII and TβRI and the subsequent activation of the type I receptor kinase. Since reliable structural models of the full-length receptors (extracellular + transmembrane + intracellular) are not yet available, it is not possible to argue about further interactions between the intracellular domains of the dimers. Nevertheless, since more TβRII-TβRI dimers will be present with a peptide bound than without, a higher outcome of the Smad pathway branch is expected. TβRII-TβRI dimers can also approach each other, and they may interact intracellularly in several modes. One of these modes could be similar to that dictated by TGF. Moreover, peptide binding has a kinetic advantage over TGF. Ignoring the lateral diffusion of the receptors, one can estimate the ratios of the translational (Dt,pept/Dt,TGF) and rotational (Dr,pept/Dr,TGF) diffusion constants of the peptides and TGF using the HullRad software version 9 [30]. This calculation shows that the peptides reach their target 4 times faster and may scan the binding sites rotationally 60 times faster than the TGF dimer does.
- (b)
- TGF is present. In this case, the peptide can bind to the (TβRII-TβRI)-TGF2-(TβRII-TβRI) hexamer at the same receptor intersubunit position as in case (a), enhancing its stability. Our docking results, combined with MDS, should find the hexamer + peptide more stable than the hexamer alone. In order to save simulation time, we actually tested the stability of the (TβRII-TβRI)-pept-TGF1 complex.
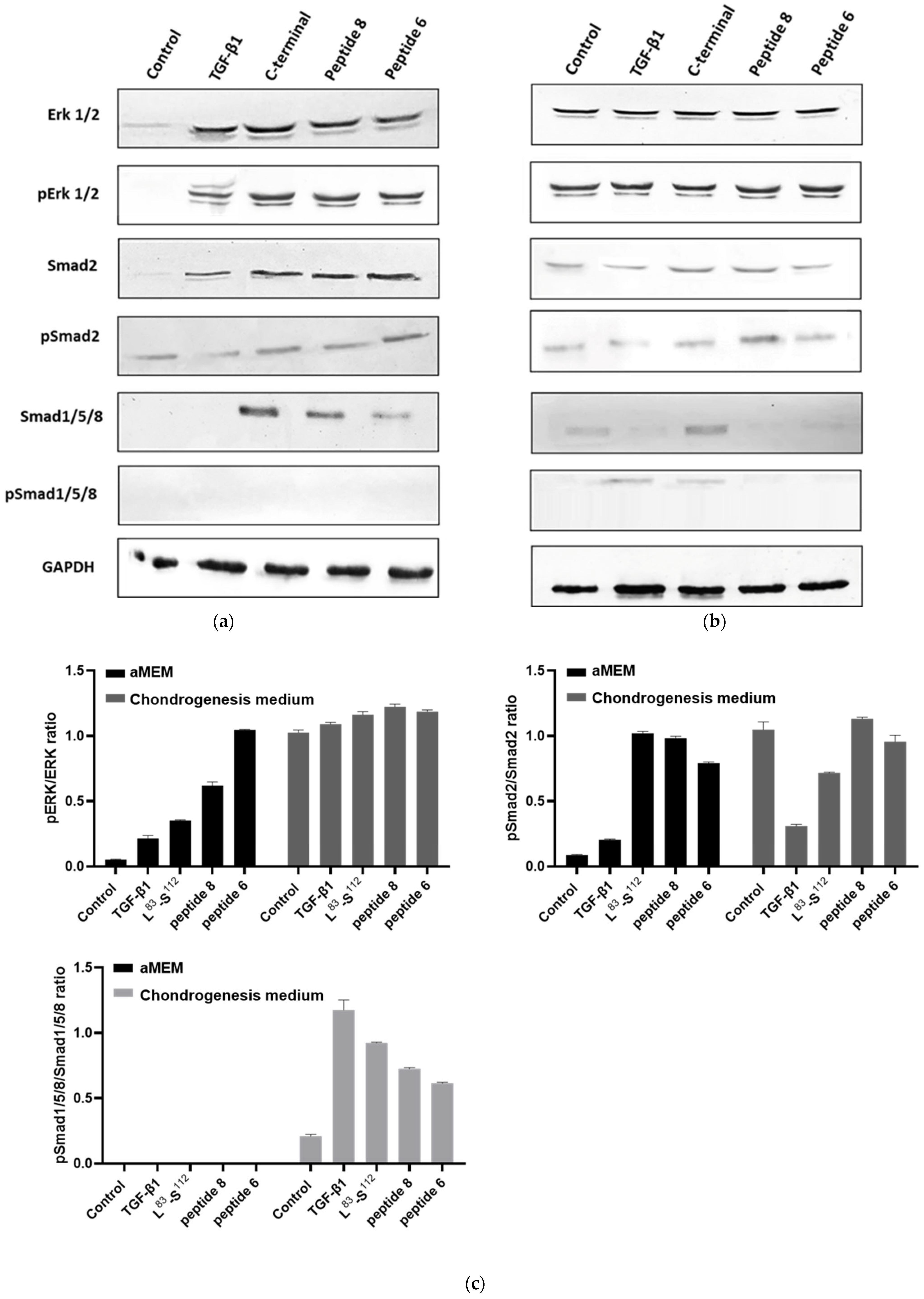
3.7. Peptides Promote the Activation of the MAPK and Smad2 Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.A.; Ahmed, G.; Dakin, S.G.; Kendrick, B.; Price, A. Osteoarthritis: A narrative review of molecular approaches to disease management. Arthritis Res. Ther. 2023, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis. 2002, 61, ii78–ii81. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Donell, S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, diagnosis, and treatment options. Med. Clin. 2020, 104, 293–311. [Google Scholar]
- Weber, A.E.; Bolia, I.K.; Trasolini, N.A. Biological strategies for osteoarthritis: From early diagnosis to treatment. Int. Orthop. 2021, 45, 335–344. [Google Scholar] [CrossRef]
- Tiku, M.L.; Sabaawy, H.E. Cartilage regeneration for treatment of osteoarthritis: A paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar] [CrossRef]
- Li, M.H.; Xiao, R.; Li, J.B.; Zhu, Q. Regenerative approaches for cartilage repair in the treatment of osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1577–1587. [Google Scholar] [CrossRef]
- Alraies, A.; Alaidaroos, N.Y.; Waddington, R.J.; Moseley, R.; Sloan, A.J. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol. 2017, 18, 12. [Google Scholar] [CrossRef]
- Van der Kraan, P.M. Differential Role of Transforming Growth Factor-beta in an Osteoarthritic or a Healthy Joint. J. Bone Metab. 2018, 25, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Q.; Liu, Y.; Xie, J. The role of TGF-β2 in cartilage development and diseases. Bone Jt. Res. 2021, 10, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Rim, Y.A.; Ju, J.H. The role of transforming growth factor beta in joint homeostasis and cartilage regeneration. Tissue Eng. Part C Methods 2022, 28, 570–587. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L.; Facchini, A. Signaling pathways in cartilage repair. Int. J. Mol. Sci. 2014, 15, 8667–8698. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Ying, J.; Xiao, D.; O’Keefe, R.J. FoxO1 is a crucial mediator of TGF-β/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc. Natl. Acad. Sci. USA 2020, 117, 30488–30497. [Google Scholar] [CrossRef]
- Hinck, A.P. Structural studies of the TGF-βs and their receptors—Insights into evolution of the TGF-β superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef]
- Cherifi, C.; Monteagudo, S.; Lories, R.J. Promising targets for therapy of osteoarthritis: A review on the Wnt and TGF-β signalling pathways. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211006959. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Papachristou, E.; Koidis, P.; Geurtsen, W. Wnt/β-catenin signaling regulates Dental Pulp Stem Cells’ responses to pulp injury by resinous monomers. Dent. Mater. 2015, 31, 542–555. [Google Scholar] [CrossRef]
- Xia, P.; Wang, X.; Qu, Y.; Lin, Q.; Cheng, K.; Gao, M.; Ren, S.; Zhang, T.; Li, X. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res. Ther. 2017, 8, 281. [Google Scholar] [CrossRef]
- Ragni, E.; Viganò, M.; Rebulla, P.; Giordano, R.; Lazzari, L. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: How to choose the most reliable housekeeping genes. J. Cell. Mol. Med. 2013, 17, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Honorato, R.V.; Koukos, P.I.; Jimenez-Garcia, B.; Tsaregorodtsev, A.; Verlato, M.; Giachetti, A.; Rosato, A.; Bonvin, A.M.J.J. Structural biology in the clouds: The WeNMR-EOSC Ecosystem. Front. Mol. Biosci. 2021, 8, 729513. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Rodrigues, J.; Kastritis, P.; Bonvin, A.M.J.J.; Vangone, A. PRODIGY: A web-server for predicting the binding affinity in protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Sheng-You, H. HPEPDOCK: A web server for blind peptide–protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, C.; Zou, X. MDockPeP: An ab-initio protein-peptide docking server. J. Comput. Chem. 2018, 39, 2409–2413. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Seeber, M.; Felline, A.; Raimondi, F.; Muff, S.; Friedman, R.; Rao, F.; Caflisch, A.; Fanelli, F. Wordom: A user-friendly program for the analysis of molecular structures, trajectories, and free energy surfaces. J. Comput. Chem. 2011, 32, 1183–1194. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, version 1.2r3pre; Schrödinger, LLC: New York, NY, USA, 2000; 3p.
- Fleming, P.J.; Fleming, K.G. HullRad: Fast Calculations of Folded and Disordered Protein and Nucleic Acid Hydrodynamic Properties. Biophys. J. 2018, 114, 856–869. [Google Scholar] [CrossRef]
- Thielen, N.; van der Kraan, P.; van Caam, A. TGFβ/BMP Signaling Pathway in Cartilage Homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; O’Keefe, R.J.; Chen, D. TGF-beta signaling in chondrocytes. Front. Biosci. J. Virtual Libr. 2005, 10, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.M.; Neefjes, M.; Vitters, E.L.; van Beuningen, H.M.; Blom, A.B.; Koenders, M.I.; van Lent, P.L.E.M.; van de Loo, F.A.J.; Blaney Davidson, E.N.; van Caam, A.P.M.; et al. Identification of Transcription Factors Responsible for a Transforming Growth Factor-β-Driven Hypertrophy-like Phenotype in Human Osteoarthritic Chondrocytes. Cells 2022, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.W.; Burmester, J.K.; Tsang, M.L.; Weatherbee, J.A.; Hinck, A.P.; Ohlsen, D.J.; Sporn, M.B.; Roberts, A.B. Binding affinity of transforming growth factor-beta for its type II receptor is determined by the C-terminal region of the molecule. J. Biol. Chem. 1996, 271, 30656–30662. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, S.; Arab, S.S.; Behmanesh, M.; Sajedi, R.H. Directed Blocking of TGF-β Receptor I Binding Site Using Tailored Peptide Segments to Inhibit its Signaling Pathway. Iran. J. Biotechnol. 2020, 18, e2561. [Google Scholar] [CrossRef]
- Boesen, C.C.; Radaev, S.; Motyka, S.A.; Patamawenu, A.; Sun, P.D. The 1.1 A crystal structure of human TGF-beta type II receptor ligand binding domain. Structure 2002, 10, 913–919. [Google Scholar] [CrossRef]
- Bai, Y.; Cheng, X.; Liu, X.; Guo, Q.; Wang, Z.; Fu, Y.; He, W.; Yu, Q. Transforming growth factor-β1 promotes early odontoblastic differentiation of dental pulp stem cells via activating AKT, Erk1/2 and p38 MAPK pathways. J. Dent. Sci. 2023, 18, 87–94. [Google Scholar] [CrossRef]
- Mantsou, A.; Papachristou, E.; Keramidas, P.; Lamprou, P.; Pitou, M.; Papi, R.M.; Dimitriou, K.; Aggeli, A.; Choli-Papadopoulou, T. Fabrication of a Smart Fibrous Biomaterial That Harbors an Active TGF-β1 Peptide: A Promising Approach for Cartilage Regeneration. Biomedicines 2023, 11, 1890. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Ansari, S.; Xu, X.; Chee, W.W.; Schricker, S.R.; Shi, S. Alginate hydrogel as a promising scaffold for dental-derived stem cells: An in vitro study. J. Mater. Sci. Mater. Med. 2012, 23, 3041–3051. [Google Scholar] [CrossRef]
- Ma, N.; Teng, X.; Zheng, Q.; Chen, P. The regulatory mechanism of p38/MAPK in the chondrogenic differentiation from bone marrow mesenchymal stem cells. J. Orthop. Surg. Res. 2019, 14, 434. [Google Scholar] [CrossRef]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2, 14002. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zou, C.; Chen, Y.; Zhu, W.; Liu, W.; Huang, J.; Liu, Q.; Wang, D.; Duan, L.; Xiong, J.; et al. TGFβ1 induces hypertrophic change and expression of angiogenic factors in human chondrocytes. Oncotarget 2017, 24, 91316–91327. [Google Scholar] [CrossRef] [PubMed]


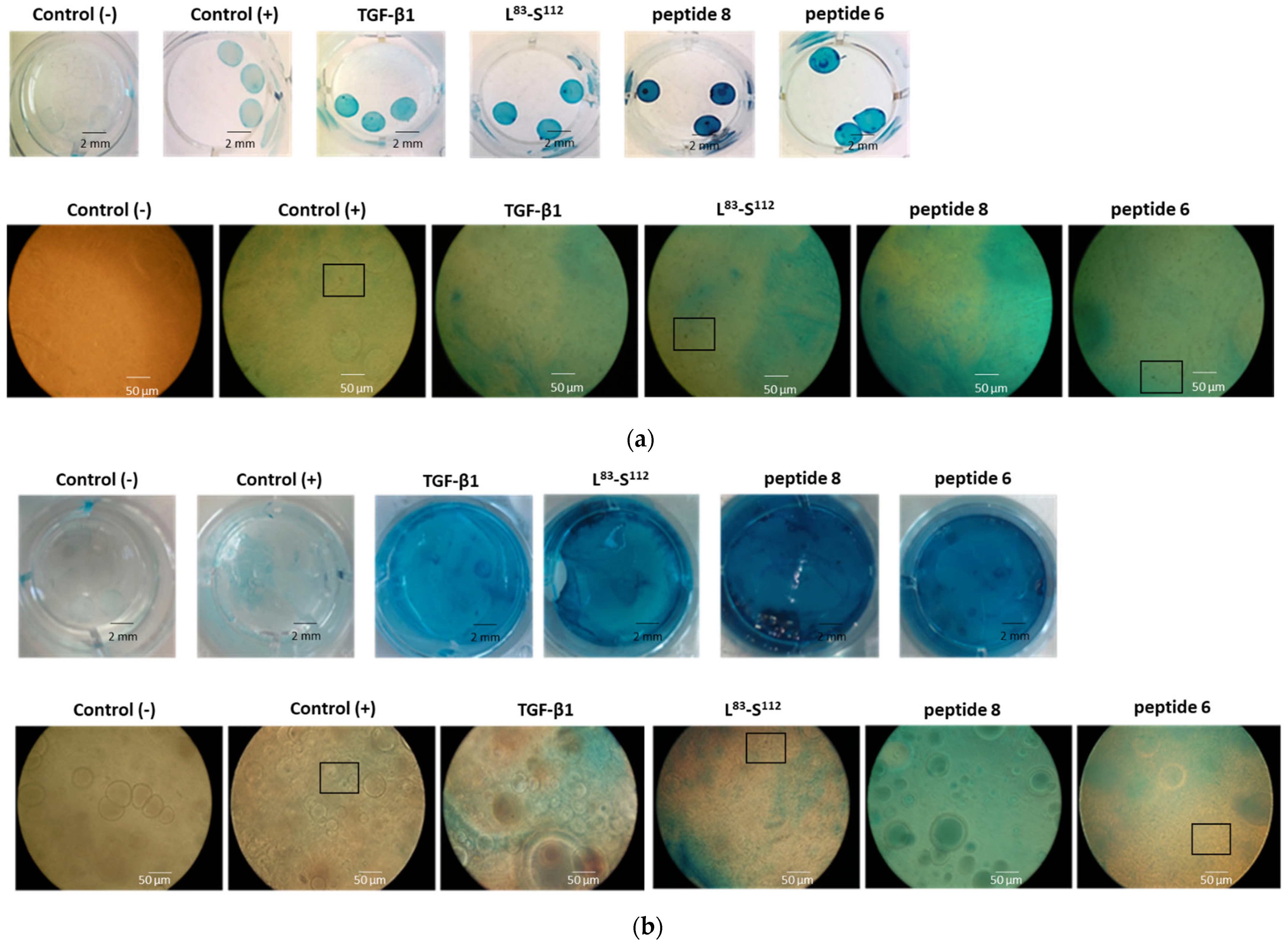
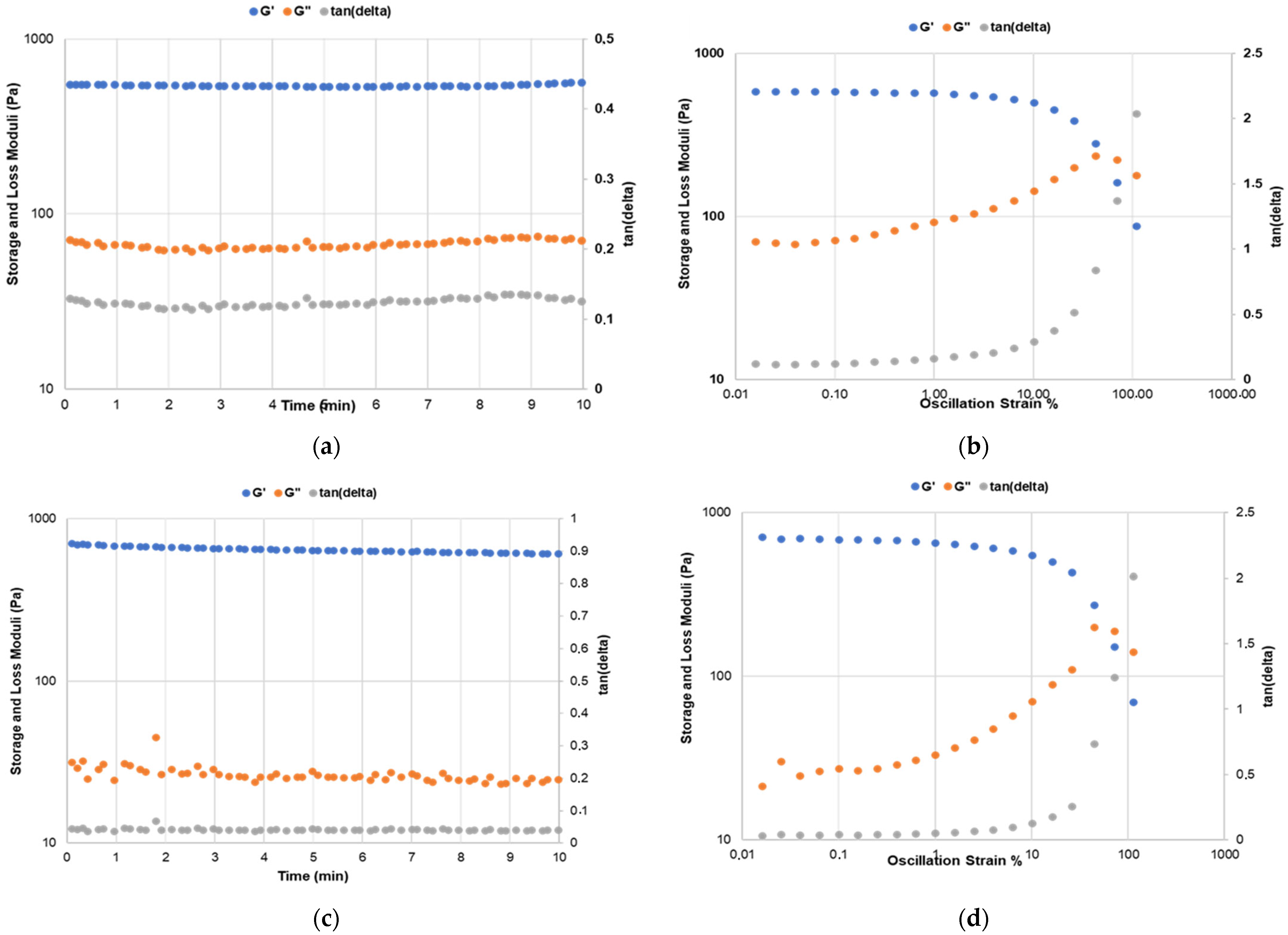




| Primer Sequence (5′ to 3′) | Restriction Enzyme | Plasmid Vector |
|---|---|---|
| 5′-CCGCCATATGTCCGGC CTGAACGACATCTTCG-3΄ Forward | NdeI | pET-29c(+) |
| 5΄-CCGCAAGCTTCTAGTG GATGTGGTGTCAGCTCACTTGCAGGAGCG -3΄Reverse | HindIII | pET-29c(+) |
| Amino Acid Sequence | |
|---|---|
| Full-length mature TGF-β1 | ALDTNYCFSSTEKNCCVRQLYIDFRKDLGWKWIHEPKGYHANFCLGPCPYIWSLDTQYSKVLALYNQHNPGASAAPCCVPQALEPLPIVYYVGRKPKVEQLSNMIVRSCKCS |
| C-terminal region L83-S112 | LEPLPIVYYVGRKPKVEQLSNMIVRSCKCS |
| Peptide 8 | YYVGRKPK |
| Peptide 6 | YVGRKP |
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| GUSB | CATTTTGTCGGCTGGGTGTG | GCCATGGCTTGACGTTGAA |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
| COL2A1 | CAACCAGGACCAAAGGGACA | ACCTTTGTCACCACGATCCC |
| ADAMTS-5 | TGGAAGTGAGCAAGAACGCT | ATGCCCACATAAATCCTCCCG |
| MMP13 | AAGGAGCATGGCGACTTCT | TGGCCCAGGAGGAAAAGC |
| COL1A1 | CAGTGTGGCCCAGAAGAACT | CCGCCATACTCGAACTGGAAT |
| COL10A1 | AGAATCCATCTGAGAATATGC | CCTCTTACTGCTATACCTTTAC |
| RUNX2 | TCATGGCGGGTAACGATGAA | GTGAAACTCTTGCCTCGTCC |
| SOX9 | GGCAAGCTCTGGAGACTTCTG | CCCGTTCTTCACCGACTTCC |
| ALP | CCGAGATACAAGCACTCCCA | TCCGTCACGTTGTTCCTGTT |
| ACAN | GGAAGGGAGGGGAACCATTG | TGATGGCTGTCCACTGACAC |
| Day 0 | ||
| Without cells | Untreated cells | |
| G′ (Pa) | 1597 ± 25 | 1556 ± 28 |
| G″ (Pa) | 262 ± 35 | 278 ± 38 |
| Day 14 | ||
| Without cells | Untreated cells | |
| G′ (Pa) | 1750 ± 35 | 1926 ± 39 |
| G″ (Pa) | 116 ± 25 | 227 ± 17 |
| u | Complex | Model | <ΔG> [kcal/mol] * |
|---|---|---|---|
| HADDOCK | TβRII-TβRI-peptide 6 | 163 | −14.1 |
| TβRII-TβRI-peptide 8 | 140 | −15.1 | |
| HPEPDOCK | TβRII-TβRI-peptide 6 | 15 | −15.5 |
| TβRII-TβRI-peptide 8 | 1 | −18.5 | |
| MDOCKPEP | TβRII-TβRI-peptide 6 | 9 | −16.7 |
| TβRII-TβRI-peptide 8 | 10 | −17.4 | |
| TβRII-TβRI | −7.3 | ||
| HADDOCK | TβRII-TβRI-TGF2 | −31.3 | |
| TβRII-peptide 6t-TβRI-TGF2 | −36.4 |
| Complex | Amino Acid Composition | |
|---|---|---|
| HADDOCK | TβRII-TβRI-peptide 6 | TβRII: A21, V22, F24, L27, S117, D118, E119 TβRI: C62, A63, P64, S65, S66, K67 |
| TβRII-TβRI-peptide 6-TGF2 | TβRII: K23, S117, D118, E119, C120 TβRI: E36, C62, A63, P64, K67, T68, G69, S70, V71, T72, T73, T74, S93, S94, P95, G96 | |
| TβRII-TβRI-peptide 8 | TβRII: K23, F24, P25, Q26, A94, S95, P96, S116, D118 TβRI: E36, R58, P64, S65, V71, T72, T73, T74, Y75, P95, G96 | |
| TβRII-TβRI-peptide 8-TGF2 | TβRII: V22, K23, F24, Q26, P57, Q58, E59, V60, A93, A94, P96, S116, S117, D118 TβRI: C62, A63, S66, T74 | |
| HPEPDOCK | TβRII-TβRI-peptide 6 | TβRII: K23, F24, L27, D118, E119 TβRI: E36, R58, C62, A63, S65, S66, K67, G69, S70, V71, T73, T74, Y75 |
| TβRII-TβRI-peptide 8 | TβRII: V22, K23, F24, P25, Q26, L27, P57, Q58, E59, P96, K97, S116, S117, D118, E119 TβRI: R58, A63, P64, S65, S66, K67, G69, S70, V71, T72, T74, Y75, C76 | |
| MDOCKPEP | TβRII-TβRI-peptide 6 | TβRII: A21, V22, K23, F24, Q26, P57, A94, S95, P96, S116, S117 TβRI: T73, T74, Y75, Q79, N83, I85, P88, T89, T90 |
| TβRII-TβRI-peptide 8 | TβRII: A21, K23, F24, P25, Q26, P57, P96, S116, S117, D118 TβRI: E36, R58, C62, A63, P64, S65, V71, T72, T73, T74, Y75, E86, T89, V91, K92, S93, S94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitou, M.; Papachristou, E.; Bratsios, D.; Kefala, G.-M.; Tsagkarakou, A.S.; Leonidas, D.D.; Aggeli, A.; Papadopoulos, G.E.; Papi, R.M.; Choli-Papadopoulou, T. In Vitro Chondrogenesis Induction by Short Peptides of the Carboxy-Terminal Domain of Transforming Growth Factor β1. Biomedicines 2023, 11, 3182. https://doi.org/10.3390/biomedicines11123182
Pitou M, Papachristou E, Bratsios D, Kefala G-M, Tsagkarakou AS, Leonidas DD, Aggeli A, Papadopoulos GE, Papi RM, Choli-Papadopoulou T. In Vitro Chondrogenesis Induction by Short Peptides of the Carboxy-Terminal Domain of Transforming Growth Factor β1. Biomedicines. 2023; 11(12):3182. https://doi.org/10.3390/biomedicines11123182
Chicago/Turabian StylePitou, Maria, Eleni Papachristou, Dimitrios Bratsios, Georgia-Maria Kefala, Anastasia S. Tsagkarakou, Demetrios D. Leonidas, Amalia Aggeli, Georgios E. Papadopoulos, Rigini M. Papi, and Theodora Choli-Papadopoulou. 2023. "In Vitro Chondrogenesis Induction by Short Peptides of the Carboxy-Terminal Domain of Transforming Growth Factor β1" Biomedicines 11, no. 12: 3182. https://doi.org/10.3390/biomedicines11123182





