Assessment of the Severity and the Remission Criteria in Eosinophilic Esophagitis
Abstract
:1. Introduction
2. Clinical Symptom Assessment in EoE
3. Evaluation of Endoscopic Features in EoE
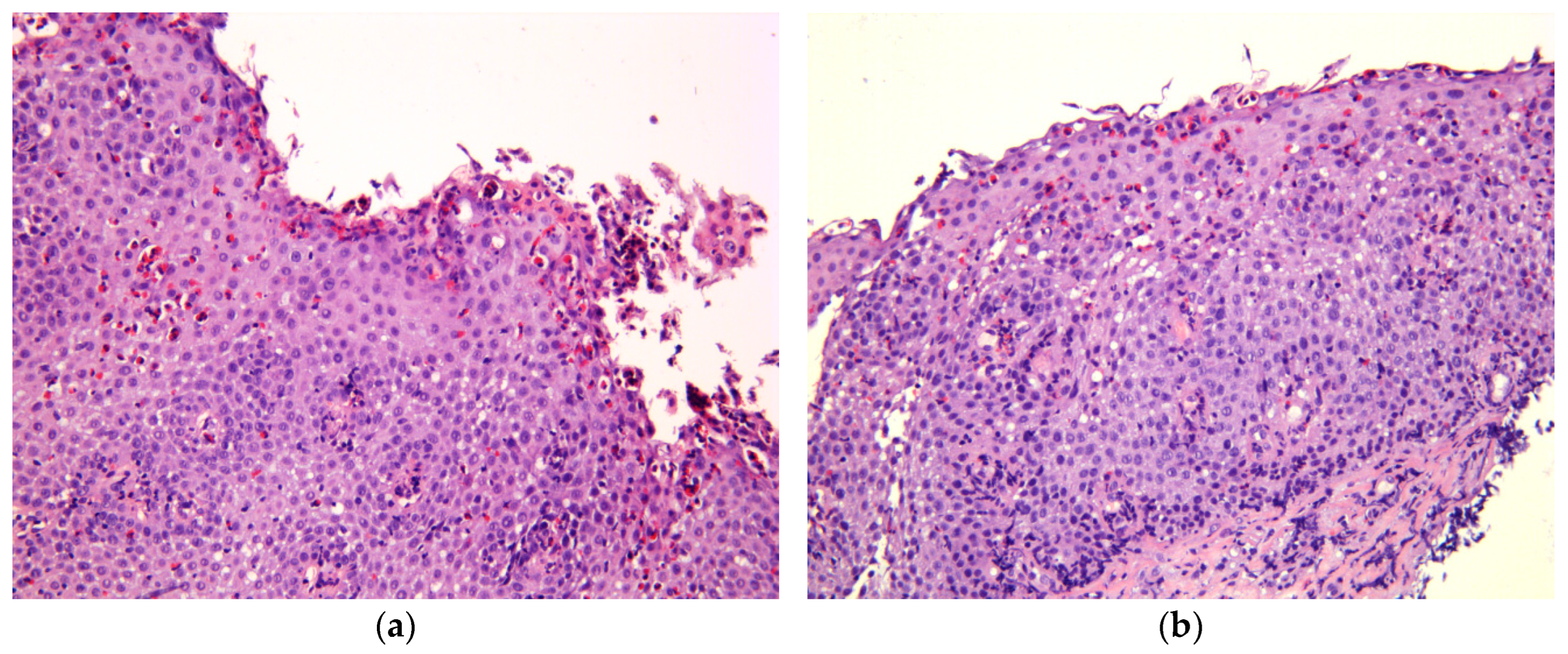
4. Histological Criteria of the Remission in EoE
5. Multidisciplinary Approach to Estimate Severity of EoE
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucendo, A.J.; Molina-Infante, J.; Arias, A.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; Gonzalez-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Liacouras, C.A.; Molina-Infante, J.; Furuta, G.T.; Spergel, J.M.; Zevit, N.; Spechler, S.J.; Attwood, S.E.; Straumann, A.; Aceves, S.S.; et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018, 155, 1022–1033.e10. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Gonsalves, N.; Hirano, I.; Furuta, G.T.; Liacouras, C.A.; Katzka, D.A. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am. J. Gastroenterol. 2013, 108, 679–692; quiz 693. [Google Scholar] [CrossRef] [PubMed]
- Kaibysheva, V.O.; Mikhaleva, L.M.; Nikonov, E.L.; Shapovalyants, S.G. Epidemiology, etiology and pathogenesis of eosinophilic esophagitis. The latest data. Russ. J. Evid.-Based Gastroenterol. = Dokazatel’naya Gastroenterol. 2019, 8, 50–72. [Google Scholar] [CrossRef]
- Maslenkina, K.S.; Mikhaleva, L.M.; Motylev, E.N.; Gushchin, M.U.; Kaibysheva, V.O.; Atyakshin, D.A.; Kudryavtseva, Y.Y.; Kudryavtsev, G.Y. Clinical and morphological diagnosis of eosinophilic esophagitis. Clin. Exp. Morphol. 2023, 12, 5–18. [Google Scholar] [CrossRef]
- Gonzalez-Cervera, J.; Arias, A.; Redondo-Gonzalez, O.; Cano-Mollinedo, M.M.; Terreehorst, I.; Lucendo, A.J. Association between atopic manifestations and eosinophilic esophagitis: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2017, 118, 582–590.e2. [Google Scholar] [CrossRef]
- Capucilli, P.; Hill, D.A. Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications. Clin. Rev. Allergy Immunol. 2019, 57, 111–127. [Google Scholar] [CrossRef]
- Hill, D.A.; Grundmeier, R.W.; Ramos, M.; Spergel, J.M. Eosinophilic Esophagitis Is a Late Manifestation of the Allergic March. J. Allergy Clin. Immunol. Pract. 2018, 6, 1528–1533. [Google Scholar] [CrossRef]
- Ram, G.; Lee, J.; Ott, M.; Brown-Whitehorn, T.F.; Cianferoni, A.; Shuker, M.; Wang, M.L.; Verma, R.; Liacouras, C.A.; Spergel, J.M. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann. Allergy Asthma Immunol. 2015, 115, 224–228.e1. [Google Scholar] [CrossRef]
- Reed, C.C.; Iglesia, E.G.A.; Commins, S.P.; Dellon, E.S. Seasonal exacerbation of eosinophilic esophagitis histologic activity in adults and children implicates role of aeroallergens. Ann. Allergy Asthma Immunol. 2019, 122, 296–301. [Google Scholar] [CrossRef]
- Bohm, M.; Jacobs, J.W., Jr.; Gupta, A.; Gupta, S.; Wo, J.M. Most children with eosinophilic esophagitis have a favorable outcome as young adults. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Hirano, I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology 2018, 154, 319–332.e3. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Safroneeva, E.; Bussmann, C.; Kuchen, T.; Portmann, S.; Simon, H.U.; Straumann, A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013, 145, 1230–1236.e2. [Google Scholar] [CrossRef] [PubMed]
- Warners, M.J.; Oude Nijhuis, R.A.B.; de Wijkerslooth, L.R.H.; Smout, A.; Bredenoord, A.J. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am. J. Gastroenterol. 2018, 113, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Kim, H.P.; Sperry, S.L.; Rybnicek, D.A.; Woosley, J.T.; Shaheen, N.J. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest. Endosc. 2014, 79, 577–585.e4. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.; Donohue, K.; Lee, J.J. Developing Pharmacologic Treatments for Eosinophilic Esophagitis: Draft Guidance from the United States Food and Drug Administration. Gastroenterology 2019, 157, 275–277. [Google Scholar] [CrossRef]
- Franciosi, J.P.; Gordon, M.; Sinopoulou, V.; Dellon, E.S.; Gupta, S.K.; Reed, C.C.; Gutierrez-Junquera, C.; Venkatesh, R.D.; Erwin, E.A.; Egiz, A.; et al. Medical treatment of eosinophilic esophagitis. Cochrane Database Syst. Rev. 2023, 7, CD004065. [Google Scholar] [CrossRef]
- Hirano, I.; Furuta, G.T. Approaches and Challenges to Management of Pediatric and Adult Patients with Eosinophilic Esophagitis. Gastroenterology 2020, 158, 840–851. [Google Scholar] [CrossRef]
- Greuter, T.; Hirano, I.; Dellon, E.S. Emerging therapies for eosinophilic esophagitis. J. Allergy Clin. Immunol. 2020, 145, 38–45. [Google Scholar] [CrossRef]
- Racca, F.; Pellegatta, G.; Cataldo, G.; Vespa, E.; Carlani, E.; Pelaia, C.; Paoletti, G.; Messina, M.R.; Nappi, E.; Canonica, G.W.; et al. Type 2 Inflammation in Eosinophilic Esophagitis: From Pathophysiology to Therapeutic Targets. Front. Physiol. 2021, 12, 815842. [Google Scholar] [CrossRef]
- Feo-Ortega, S.; Lucendo, A.J. Evidence-based treatments for eosinophilic esophagitis: Insights for the clinician. Ther. Adv. Gastroenterol. 2022, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.A.; Sharaf, R.N.; Furuta, G.T.; Aceves, S.S.; Greenhawt, M.; Spergel, J.M.; Falck-Ytter, Y.T.; Dellon, E.S.; Chachu, K.A.; Day, L.; et al. Technical Review on the Management of Eosinophilic Esophagitis: A Report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology 2020, 158, 1789–1810.e15. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Falk, G.W. Eosinophilic Esophagitis: A Review. JAMA 2021, 326, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, L.T.; Westmark, S.; Melgaard, D.; Krarup, A.L. Effectiveness of PPI treatment and guideline adherence in 236 patients with eosinophilic oesophagitis-Results from the population-based DanEoE cohort shows a low complication rate. United Eur. Gastroenterol. J. 2021, 9, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Dellon, E.S.; Moawad, F.J.; Furuta, G.T.; Aceves, S.S.; Rothenberg, M.E. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J. Allergy Clin. Immunol. 2015, 135, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Zhang, X.; Huo, X.; Yu, C.; Zhang, Q.; Wang, D.H.; Spechler, S.J.; Souza, R.F. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 2013, 62, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Zhang, X.; Wilson, K.S.; Wang, D.H.; Park, J.Y.; Huo, X.; Yu, C.; Zhang, Q.; Spechler, S.J.; Souza, R.F. JAK-STAT6 Pathway Inhibitors Block Eotaxin-3 Secretion by Epithelial Cells and Fibroblasts from Esophageal Eosinophilia Patients: Promising Agents to Improve Inflammation and Prevent Fibrosis in EoE. PLoS ONE 2016, 11, e0157376. [Google Scholar] [CrossRef]
- van Rhijn, B.D.; Weijenborg, P.W.; Verheij, J.; van den Bergh Weerman, M.A.; Verseijden, C.; van den Wijngaard, R.M.; de Jonge, W.J.; Smout, A.J.; Bredenoord, A.J. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1815–1823.e2. [Google Scholar] [CrossRef]
- Miehlke, S.; Schlag, C.; Lucendo, A.J.; Biedermann, L.; Vaquero, C.S.; Schmoecker, C.; Hayat, J.; Hruz, P.; Ciriza de Los Rios, C.; Bredenoord, A.J.; et al. Budesonide orodispersible tablets for induction of remission in patients with active eosinophilic oesophagitis: A 6-week open-label trial of the EOS-2 Programme. United Eur. Gastroenterol. J. 2022, 10, 330–343. [Google Scholar] [CrossRef]
- Massironi, S.; Mulinacci, G.; Gallo, C.; Elvevi, A.; Danese, S.; Invernizzi, P.; Vespa, E. Mechanistic Insights into Eosinophilic Esophagitis: Therapies Targeting Pathophysiological Mechanisms. Cells 2023, 12, 2473. [Google Scholar] [CrossRef]
- Dellon, E.S.; Woosley, J.T.; Arrington, A.; McGee, S.J.; Covington, J.; Moist, S.E.; Gebhart, J.H.; Tylicki, A.E.; Shoyoye, S.O.; Martin, C.F.; et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology 2019, 157, 65–73.e5. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Desai, N.K.; Lan, L.; Williams, J.; Dellon, E.S.; et al. Budesonide Oral Suspension Improves Outcomes in Patients with Eosinophilic Esophagitis: Results from a Phase 3 Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 525–534.e10. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.J.; Molina-Infante, J.; Lucendo, A.J.; Cantrell, S.E.; Tmanova, L.; Douglas, K.M. Systematic review with meta-analysis: Endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017, 46, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, M.; Runge, T.M.; Eluri, S.; Dellon, E.S. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: A systematic review and meta-analysis. Gastrointest. Endosc. 2017, 86, 581–591.e3. [Google Scholar] [CrossRef] [PubMed]
- Pentiuk, S.; Putnam, P.E.; Collins, M.H.; Rothenberg, M.E. Dissociation between symptoms and histological severity in pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.A.; Jung, K.W.; Arora, A.S.; Enders, F.; Katzka, D.A.; Kephardt, G.M.; Kita, H.; Kryzer, L.A.; Romero, Y.; Smyrk, T.C.; et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2012, 10, 742–749.e1. [Google Scholar] [CrossRef] [PubMed]
- Safroneeva, E.; Straumann, A.; Coslovsky, M.; Zwahlen, M.; Kuehni, C.E.; Panczak, R.; Haas, N.A.; Alexander, J.A.; Dellon, E.S.; Gonsalves, N.; et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults with Eosinophilic Esophagitis. Gastroenterology 2016, 150, 581–590.e4. [Google Scholar] [CrossRef]
- Dellon, E.S.; Gupta, S.K. A Conceptual Approach to Understanding Treatment Response in Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2149–2160. [Google Scholar] [CrossRef]
- Hirano, I.; Spechler, S.; Furuta, G.; Dellon, E.S. White Paper AGA: Drug Development for Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1173–1183. [Google Scholar] [CrossRef]
- Reed, C.C.; Dellon, E.S. Eosinophilic Esophagitis. Med. Clin. N. Am. 2019, 103, 29–42. [Google Scholar] [CrossRef]
- Kaibysheva, V.O.; Kashin, S.V.; Mikhaleva, L.M.; Vidyayeva, N.S.; Kuvaev, R.O.; Galkova, Z.V.; Ilchishina, T.A.; Pechnikova, V.V.; Nikonov, E.L.; Shapovalyants, S.G. Eosinophilic esophagitis: Current view on the problem and own clinical observations. Russ. J. Evid.-Based Gastroenterol. = Dokazatel’naya Gastroenterol. 2019, 8, 58–83. [Google Scholar] [CrossRef]
- Laserna-Mendieta, E.J.; Navarro, P.; Casabona-Frances, S.; Savarino, E.V.; Perez-Martinez, I.; Guagnozzi, D.; Barrio, J.; Perello, A.; Guardiola-Arevalo, A.; Betore-Glaria, M.E.; et al. Differences between childhood- and adulthood-onset eosinophilic esophagitis: An analysis from the EoE connect registry. Dig Liver Dis. 2023, 55, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.K.; Avis, K.T.; Dimmitt, R.A.; Goodin, B.R. Topical Review: Eosinophilic Esophagitis in Children: Implications for Health-Related Quality of Life and Potential Avenues for Future Research. J. Pediatr. Psychol. 2015, 40, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, T.; Toto, E.; Ho, N.; Taft, T.; Hirano, I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J. Gastroenterol. 2012, 18, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.K.; Stecevic, V.; Chang, C.H.; Goldstein, N.S.; Badizadegan, K.; Furuta, G.T. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest. Endosc. 2005, 61, 795–801. [Google Scholar] [CrossRef]
- Dellon, E.S.; Irani, A.M.; Hill, M.R.; Hirano, I. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment. Pharmacol. Ther. 2013, 38, 634–642. [Google Scholar] [CrossRef]
- Hudgens, S.; Evans, C.; Phillips, E.; Hill, M. Psychometric validation of the Dysphagia Symptom Questionnaire in patients with eosinophilic esophagitis treated with budesonide oral suspension. J. Patient. Rep. Outcomes 2017, 1, 3. [Google Scholar] [CrossRef]
- Dellon, E.S.; Katzka, D.A.; Collins, M.H.; Hamdani, M.; Gupta, S.K.; Hirano, I.; Investigators, M.P. Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared with Placebo in Patients with Eosinophilic Esophagitis. Gastroenterology 2017, 152, 776–786.e5. [Google Scholar] [CrossRef]
- Dellon, E.S.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Goodwin, B.; Eisner, J.D.; Lan, L.; Desai, N.K.; et al. Long-Term Treatment of Eosinophilic Esophagitis with Budesonide Oral Suspension. Clin. Gastroenterol. Hepatol. 2022, 20, 1488–1498.e11. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Straumann, A.; Panczak, R.; Coslovsky, M.; Kuehni, C.E.; Maurer, E.; Haas, N.A.; Romero, Y.; Hirano, I.; Alexander, J.A.; et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014, 147, 1255–1266.e21. [Google Scholar] [CrossRef]
- Albinsson, S.; Tuomi, L.; Wenneras, C.; Larsson, H. Patient-Reported Dysphagia in Adults with Eosinophilic Esophagitis: Translation and Validation of the Swedish Eosinophilic Esophagitis Activity Index. Dysphagia 2022, 37, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Hommel, K.A.; DeBrosse, C.W.; Greenberg, A.B.; Greenler, A.J.; Abonia, J.P.; Rothenberg, M.E.; Varni, J.W. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: Qualitative methods. BMC Gastroenterol. 2011, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients with Eosinophilic Esophagitis. Gastroenterology 2019, 156, 592–603.e10. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Dellon, E.S.; Hamilton, J.D.; Collins, M.H.; Peterson, K.; Chehade, M.; Schoepfer, A.M.; Safroneeva, E.; Rothenberg, M.E.; Falk, G.W.; et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults with Active Eosinophilic Esophagitis. Gastroenterology 2020, 158, 111–122.e10. [Google Scholar] [CrossRef] [PubMed]
- Safroneeva, E.; Pan, Z.; King, E.; Martin, L.J.; Collins, M.H.; Yang, G.Y.; Capocelli, K.E.; Arva, N.C.; Abonia, J.P.; Atkins, D.; et al. Long-Lasting Dissociation of Esophageal Eosinophilia and Symptoms After Dilation in Adults with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2022, 20, 766–775.e4. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.Y.; LeSuer, W.E.; Horsley-Silva, J.L.; Putikova, A.; Buras, M.R.; Gibson, J.B.; Pyon, G.C.; Simmons, T.D.; Doyle, A.D.; Wright, B.L. Food-Specific IgG4 Is Elevated Throughout the Upper Gastrointestinal Tract in Eosinophilic Esophagitis. Dig Dis. Sci. 2023, 68, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Collins, M.H.; Rothenberg, M.E.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.; Straumann, A.; Safroneeva, E.; Rodriguez, C.; et al. Long-term Efficacy and Tolerability of RPC4046 in an Open-Label Extension Trial of Patients with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 473–483.e17. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Mitlyng, B.L.; Iuga, A.; Bookhout, C.E.; Cortright, L.M.; Walker, K.B.; Gee, T.S.; McGee, S.J.; Cameron, B.A.; et al. Mepolizumab for treatment of adolescents and adults with eosinophilic oesophagitis: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Gut 2023, 72, 1828–1837. [Google Scholar] [CrossRef]
- Straumann, A.; Conus, S.; Degen, L.; Felder, S.; Kummer, M.; Engel, H.; Bussmann, C.; Beglinger, C.; Schoepfer, A.; Simon, H.U. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010, 139, 1526–1537.e1. [Google Scholar] [CrossRef]
- Miehlke, S.; Hruz, P.; Vieth, M.; Bussmann, C.; von Arnim, U.; Bajbouj, M.; Schlag, C.; Madisch, A.; Fibbe, C.; Wittenburg, H.; et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 2016, 65, 390–399. [Google Scholar] [CrossRef]
- Lorenz, N.J.; Link, A.; Czapiewski, P.; Arnim, U.V. Eosinophilic esophagitis: Comparison of clinical, endoscopic and histological scoring systems. Z. Gastroenterol. 2022, 60, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, N.; Yang, G.Y.; Doerfler, B.; Ritz, S.; Ditto, A.M.; Hirano, I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012, 142, 1451–1459.e1; quiz e1414–1455. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, J.; Barrio-Andres, J.; Nantes Castillejo, O.; Valdivieso-Cortazar, E.; Perez-Martinez, I.; Boumidi, A.; Olmos-Jerez, J.A.; Payeras-Llodra, G.; Alcaide-Suarez, N.; Ruiz-Rebollo, L.; et al. The Endoscopic Reference Score shows modest accuracy to predict either clinical or histological activity in adult patients with eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017, 45, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Grudell, A.B.; Alexander, J.A.; Enders, F.B.; Pacifico, R.; Fredericksen, M.; Wise, J.L.; Locke, G.R., 3rd; Arora, A.; Zais, T.; Talley, N.J.; et al. Validation of the Mayo Dysphagia Questionnaire. Dis. Esophagus 2007, 20, 202–205. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, J.; Lohse, M.R.; Arora, A.S.; Peloquin, J.M.; Geno, D.M.; Kuntz, M.M.; Enders, F.B.; Fredericksen, M.; Abdalla, A.A.; Khan, Y.; et al. The Mayo Dysphagia Questionnaire-30: Documentation of reliability and validity of a tool for interventional trials in adults with esophageal disease. Dysphagia 2010, 25, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Sheikh, A.; Speck, O.; Woodward, K.; Whitlow, A.B.; Hores, J.M.; Ivanovic, M.; Chau, A.; Woosley, J.T.; Madanick, R.D.; et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012, 143, 321–324.e1. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.C.; Wolf, W.A.; Cotton, C.C.; Dellon, E.S. A visual analogue scale and a Likert scale are simple and responsive tools for assessing dysphagia in eosinophilic oesophagitis. Aliment. Pharmacol. Ther. 2017, 45, 1443–1448. [Google Scholar] [CrossRef]
- Martin, L.J.; Franciosi, J.P.; Collins, M.H.; Abonia, J.P.; Lee, J.J.; Hommel, K.A.; Varni, J.W.; Grotjan, J.T.; Eby, M.; He, H.; et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J. Allergy Clin. Immunol. 2015, 135, 1519–1528.e8. [Google Scholar] [CrossRef]
- Aceves, S.S.; Newbury, R.O.; Dohil, M.A.; Bastian, J.F.; Dohil, R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann. Allergy Asthma Immunol. 2009, 103, 401–406. [Google Scholar] [CrossRef]
- Dohil, R.; Newbury, R.; Fox, L.; Bastian, J.; Aceves, S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 2010, 139, 418–429. [Google Scholar] [CrossRef]
- Gupta, S.K.; Vitanza, J.M.; Collins, M.H. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2015, 13, 66–76.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Vance, R.B.; Shaheen, N.J.; Dellon, E.S. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 988–996.e5. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Moy, N.; Heckman, M.G.; Thomas, C.S.; Gonsalves, N.; Achem, S.R. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: Validation of a novel classification and grading system. Gut 2013, 62, 489–495. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.D.; Warners, M.J.; Curvers, W.L.; van Lent, A.U.; Bekkali, N.L.; Takkenberg, R.B.; Kloek, J.J.; Bergman, J.J.; Fockens, P.; Bredenoord, A.J. Evaluating the endoscopic reference score for eosinophilic esophagitis: Moderate to substantial intra- and interobserver reliability. Endoscopy 2014, 46, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Cotton, C.C.; Gebhart, J.H.; Higgins, L.L.; Beitia, R.; Woosley, J.T.; Shaheen, N.J. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin. Gastroenterol. Hepatol. 2016, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kuchen, T.; Straumann, A.; Safroneeva, E.; Romero, Y.; Bussmann, C.; Vavricka, S.; Netzer, P.; Reinhard, A.; Portmann, S.; Schoepfer, A.M. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy 2014, 69, 1248–1254. [Google Scholar] [CrossRef]
- van Rhijn, B.D.; Verheij, J.; Smout, A.J.; Bredenoord, A.J. The Endoscopic Reference Score shows modest accuracy to predict histologic remission in adult patients with eosinophilic esophagitis. Neurogastroenterol. Motil. 2016, 28, 1714–1722. [Google Scholar] [CrossRef]
- Chen, J.W.; Pandolfino, J.E.; Lin, Z.; Ciolino, J.D.; Gonsalves, N.; Kahrilas, P.J.; Hirano, I. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 2016, 48, 794–801. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Bolton, S.M.; Amsden, K.; Wershil, B.K.; Hirano, I.; Kagalwalla, A.F. Eosinophilic Esophagitis Reference Score Accurately Identifies Disease Activity and Treatment Effects in Children. Clin. Gastroenterol. Hepatol. 2018, 16, 1056–1063. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Panczak, R.; Zwahlen, M.; Kuehni, C.E.; Coslovsky, M.; Maurer, E.; Haas, N.A.; Alexander, J.A.; Dellon, E.S.; Gonsalves, N.; et al. How do gastroenterologists assess overall activity of eosinophilic esophagitis in adult patients? Am. J. Gastroenterol. 2015, 110, 402–414. [Google Scholar] [CrossRef]
- Greuter, T.; Safroneeva, E.; Bussmann, C.; Biedermann, L.; Vavricka, S.R.; Katzka, D.A.; Schoepfer, A.M.; Straumann, A. Maintenance Treatment of Eosinophilic Esophagitis with Swallowed Topical Steroids Alters Disease Course over a 5-Year Follow-Up Period in Adult Patients. Clin. Gastroenterol. Hepatol. 2019, 17, 419–428.e6. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, M.A.; Hirano, I.; Kahrilas, P.J.; Rothe, J.; Luger, D.; Pandolfino, J.E. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology 2011, 140, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nicodeme, F.; Hirano, I.; Chen, J.; Robinson, K.; Lin, Z.; Xiao, Y.; Gonsalves, N.; Kwasny, M.J.; Kahrilas, P.J.; Pandolfino, J.E. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2013, 11, 1101–1107.e1. [Google Scholar] [CrossRef] [PubMed]
- Nasi, M.; De Gaetano, A.; Carnevale, G.; Bertoni, L.; Selleri, V.; Zanini, G.; Pisciotta, A.; Caramaschi, S.; Reggiani Bonetti, L.; Farinetti, A.; et al. Effects of Energy Drink Acute Assumption in Gastrointestinal Tract of Rats. Nutrients 2022, 14, 1928. [Google Scholar] [CrossRef] [PubMed]
- Wolf, W.A.; Cotton, C.C.; Green, D.J.; Hughes, J.T.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Evaluation of Histologic Cutpoints for Treatment Response in Eosinophilic Esophagitis. J. Gastroenterol. Hepatol. Res. 2015, 4, 1780–1787. [Google Scholar] [CrossRef]
- Reed, C.C.; Wolf, W.A.; Cotton, C.C.; Rusin, S.; Perjar, I.; Hollyfield, J.; Woosley, J.T.; Shaheen, N.J.; Dellon, E.S. Optimal Histologic Cutpoints for Treatment Response in Patients with Eosinophilic Esophagitis: Analysis of Data from a Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2018, 16, 226–233.e2. [Google Scholar] [CrossRef]
- Eke, R.; Li, T.; White, A.; Tariq, T.; Markowitz, J.; Lenov, A. Systematic review of histological remission criteria in eosinophilic esophagitis. JGH Open 2018, 2, 158–165. [Google Scholar] [CrossRef]
- Konikoff, M.R.; Noel, R.J.; Blanchard, C.; Kirby, C.; Jameson, S.C.; Buckmeier, B.K.; Akers, R.; Cohen, M.B.; Collins, M.H.; Assa’ad, A.H.; et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 2006, 131, 1381–1391. [Google Scholar] [CrossRef]
- Andreae, D.A.; Hanna, M.G.; Magid, M.S.; Malerba, S.; Andreae, M.H.; Bagiella, E.; Chehade, M. Swallowed Fluticasone Propionate Is an Effective Long-Term Maintenance Therapy for Children with Eosinophilic Esophagitis. Am. J. Gastroenterol. 2016, 111, 1187–1197. [Google Scholar] [CrossRef]
- Dellon, E.S.; Speck, O.; Woodward, K.; Covey, S.; Rusin, S.; Shaheen, N.J.; Woosley, J.T. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod. Pathol. 2015, 28, 383–390. [Google Scholar] [CrossRef]
- Hiremath, G.; Choksi, Y.A.; Acra, S.; Correa, H.; Dellon, E.S. Factors Associated with Adequate Lamina Propria Sampling and Presence of Lamina Propria Fibrosis in Children with Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1814–1823.e1811. [Google Scholar] [CrossRef]
- Rajan, J.; Newbury, R.O.; Anilkumar, A.; Dohil, R.; Broide, D.H.; Aceves, S.S. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J. Allergy Clin. Immunol. 2016, 137, 147–156.e148. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Martin, L.J.; Alexander, E.S.; Boyd, J.T.; Sheridan, R.; He, H.; Pentiuk, S.; Putnam, P.E.; Abonia, J.P.; Mukkada, V.A.; et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.A.; Godwin, B.C.; Wilkins, B.; Elci, O.U.; Benitez, A.; DeMarshall, M.; Sharma, M.; Gross, J.; Klein-Szanto, A.J.; Liacouras, C.A.; et al. Persistent Basal Cell Hyperplasia Is Associated with Clinical and Endoscopic Findings in Patients With Histologically Inactive Eosinophilic Esophagitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1475–1482.e1. [Google Scholar] [CrossRef] [PubMed]
- Bolton, S.M.; Kagalwalla, A.F.; Arva, N.C.; Wang, M.Y.; Amsden, K.; Melin-Aldana, H.; Dellon, E.S.; Bryce, P.J.; Wershil, B.K.; Wechsler, J.B. Mast Cell Infiltration Is Associated with Persistent Symptoms and Endoscopic Abnormalities Despite Resolution of Eosinophilia in Pediatric Eosinophilic Esophagitis. Am. J. Gastroenterol. 2020, 115, 224–233. [Google Scholar] [CrossRef]
- Hiremath, G.; Sun, L.; Correa, H.; Acra, S.; Collins, M.H.; Bonis, P.; Arva, N.C.; Capocelli, K.E.; Falk, G.W.; King, E.; et al. Development and Validation of Web-Based Tool to Predict Lamina Propria Fibrosis in Eosinophilic Esophagitis. Am. J. Gastroenterol. 2022, 117, 272–279. [Google Scholar] [CrossRef]
- Collins, M.H.; Martin, L.J.; Wen, T.; Abonia, J.P.; Putnam, P.E.; Mukkada, V.A.; Rothenberg, M.E. Eosinophilic Esophagitis Histology Remission Score: Significant Relations to Measures of Disease Activity and Symptoms. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 598–603. [Google Scholar] [CrossRef]
- Dellon, E.S.; Khoury, P.; Muir, A.B.; Liacouras, C.A.; Safroneeva, E.; Atkins, D.; Collins, M.H.; Gonsalves, N.; Falk, G.W.; Spergel, J.M.; et al. A Clinical Severity Index for Eosinophilic Esophagitis: Development, Consensus, and Future Directions. Gastroenterology 2022, 163, 59–76. [Google Scholar] [CrossRef]

| Features | DSQ | DSD | EEsAI | SDI | DSS | MDQ | PEESSv2 | SST | CSS |
|---|---|---|---|---|---|---|---|---|---|
| Age | |||||||||
| For adults | + | + | + | + | + | + | |||
| For children | + | + | + | ||||||
| Dysphagia | |||||||||
| Presence | + | + | + | + | + | + | + | + | + |
| Duration | + | + | + | ||||||
| Frequency | + | + | + | + | + | ||||
| Severity | + | + | + | + | + | + | + | + | |
| Presence of pain | + | + | + | ||||||
| Behavior adaptation | + | + | + | + | + | + | |||
| Heartburn | + | + | + | + | |||||
| Regurgitation | + | + | + | + | |||||
| Odynophagia | + | + | + | ||||||
| Presence of allergies or asthma | + | ||||||||
| Medication/treatment used | + | ||||||||
| Chest pain | + | ||||||||
| Abdominal pain | + | + | + | ||||||
| Vomiting | + | + | + | ||||||
| Nausea | + | + | + | ||||||
| Poor appetite | + | ||||||||
| Nocturnal awakening | + | + | |||||||
| Gastrointestinal hemorrhage | + | ||||||||
| Anorexia or early satiety | + | + |
| Endoscopic Feature | Description | Grade |
|---|---|---|
| Major features | ||
| Fixed rings | None | 0 |
| Mild (subtle circumferential ridges) | 1 | |
| Moderate (distinct rings that do not impair passage of a standard diagnostic adult endoscope (outer diameter 8–9.5 mm)) | 2 | |
| Severe (distinct rings that do not permit passage of a diagnostic endoscope) | 3 | |
| Exudates (white spots, plaques) | None | 0 |
| Mild (lesions involving <10% of the esophageal surface area) | 1 | |
| Severe (lesions involving >10% of the esophageal surface area) | 2 | |
| Furrows (vertical lines, longitudinal furrows) | Absent | 0 |
| Present | 1 | |
| Edema (vascular pattern, mucosal pallor) | Absent (distinct vascularity present) | 0 |
| Loss of clarity or absence of vascular markings | 1 | |
| Stricture | Absent | 0 |
| Present | 1 | |
| Minor features | ||
| Crepe paper esophagus | Absent | 0 |
| Present | 1 |
| Histological Criteria | Grade Score | Stage Score |
|---|---|---|
| Eosinophilic inflammation | 0—Intraepithelial eosinophils not present | 0—Intraepithelial eosinophils 0–14/hpf, |
| 1—PEC < 15/hpf | 1—PEC ≥ 15/hpf in <33% of hpfs | |
| 2—PEC 15–59/hpf | 2—PEC ≥ 15/hpf in 33–66% of hpfs | |
| 3—PEC > 60/hpf | 3—PEC ≥ 15/hpf in >66% of hpfs | |
| Epithelial basal zone | 0—BZH not present | 0—BZH not present |
| 1—basal zone occupies > 15% but <33% of total epithelial thickness | 1—BZH (any grade > 0) in <33% of epithelium | |
| 2—basal zone occupies 33–66% of total epithelial thickness | 2—BZH (any grade > 0) in 33–66% of epithelium | |
| 3—basal zone occupies > 66% of total epithelial thickness | 3—BZH (any grade > 0) in >66% of epithelium | |
| Eosinophil abscess (EA) | 0—groups or aggregates of eosinophils not present | 0—groups or aggregates of eosinophils not present |
| 1—group of 4–9 eosinophils | 1—EA (any grade > 0) in <33% of epithelium | |
| 2—group of 10–20 eosinophils | 2—EA (any grade > 0) in 33–66% of epithelium | |
| 3—group of >20 eosinophils | 3—EA (any grade > 0) in >66% of epithelium | |
| Eosinophil surface layering (SL) | 0—absent SL (fewer than 3 aligned eosinophils) | 0—absent SL |
| 1—SL of 3–4 eosinophils | 1—SL (any grade > 0) in <33% of epithelium | |
| 2—SL of 5–10 eosinophils | 2—SL (any grade > 0) in 33–66% of epithelium | |
| 3—SL of >10 eosinophils | 3—SL (any grade > 0) in >66% of epithelium. | |
| Dilated intercellular spaces (DIS) | 0—DIS not seen at any magnification | 0—DIS not seen at any magnification |
| 1—Intercellular bridges in DIS visible at 400× magnification only | 1—DIS (any grade > 0) in <33% of epithelium | |
| 2—Intercellular bridges in DIS visible at 200× magnification | 2—DIS (any grade > 0) in 33–66% of epithelium | |
| 3—Intercellular bridges in DIS visible at 100× magnification or lower | 3—DIS (any grade > 0) in >66% of epithelium | |
| Surface epithelial alteration (SEA) | 0—SEA not present | 0—SEA not present |
| 1—SEA without eosinophils | 1—SEA (any grade > 0) in <33% of epithelium | |
| 2—SEA with any eosinophils | 2—SEA (any grade > 0) in 33–66% of epithelium | |
| 3—shed altered surface epithelium admixed with numerous eosinophils consistent with exudate | 3—SEA (any grade > 0) in >66% of epithelium | |
| Dyskeratotic epithelial cells (DECs) | 0—DEC not present | 0—DEC not present |
| 1—1 DEC/HPF | 1—DEC (any grade > 0) in <33% of epithelium | |
| 2—2–5 DEC/HPF | 2—DEC (any grade > 0) in 33–66% of epithelium | |
| 3—>5 DEC/HPF | 3—DEC (any grade > 0) in >66% of epithelium | |
| Lamina propria fibrosis (LPF) | 0—LPF not present | 0—LPF not present |
| 1—fibers are cohesive and interfiber spaces cannot be demarcated | 1—LPF (any grade > 0) in <33% of lamina propria | |
| 2—fiber diameter equals the diameter of a basal cell nucleus | 2—LPF (any grade > 0) in 33–66% of lamina propria | |
| 3—fiber diameter exceeds the diameter of a basal cell nucleus | 3—LPF (any grade > 0) in >66% of lamina propria |
| Points per Feature | 1 Point | 2 Points | 4 Points | 15 Points |
|---|---|---|---|---|
| Symptoms and complications | ||||
| Symptoms | Weekly | Daily | Multiple times per day or disrupting social functioning | |
| Complications | - | Food impaction with ER visit or endoscopy (patient ≥18 years) |
|
|
| Inflammatory features | ||||
| Endoscopy (edema, furrows, and/or exudates) Histology | Localized 15–60 eos/hpf | Diffuse >60 eos/hpf | - - | - - |
| Fibrostenotic features | ||||
| Endoscopy (rings, strictures) | Present, but endoscope passes easily | Present, but requires dilation or a snug fit when passing a standard endoscope | - | Cannot pass standard upper endoscope; repeated dilations (in an adult ≤ 18 years); or any dilation (in a child < 18 years) |
| Histology | - | BZH or LPF (or DEC/SEA if no LP) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslenkina, K.; Mikhaleva, L.; Mikhalev, A.; Kaibysheva, V.; Atiakshin, D.; Motilev, E.; Buchwalow, I.; Tiemann, M. Assessment of the Severity and the Remission Criteria in Eosinophilic Esophagitis. Biomedicines 2023, 11, 3204. https://doi.org/10.3390/biomedicines11123204
Maslenkina K, Mikhaleva L, Mikhalev A, Kaibysheva V, Atiakshin D, Motilev E, Buchwalow I, Tiemann M. Assessment of the Severity and the Remission Criteria in Eosinophilic Esophagitis. Biomedicines. 2023; 11(12):3204. https://doi.org/10.3390/biomedicines11123204
Chicago/Turabian StyleMaslenkina, Ksenia, Liudmila Mikhaleva, Alexander Mikhalev, Valeria Kaibysheva, Dmitri Atiakshin, Eugeny Motilev, Igor Buchwalow, and Markus Tiemann. 2023. "Assessment of the Severity and the Remission Criteria in Eosinophilic Esophagitis" Biomedicines 11, no. 12: 3204. https://doi.org/10.3390/biomedicines11123204
APA StyleMaslenkina, K., Mikhaleva, L., Mikhalev, A., Kaibysheva, V., Atiakshin, D., Motilev, E., Buchwalow, I., & Tiemann, M. (2023). Assessment of the Severity and the Remission Criteria in Eosinophilic Esophagitis. Biomedicines, 11(12), 3204. https://doi.org/10.3390/biomedicines11123204






