The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis
Abstract
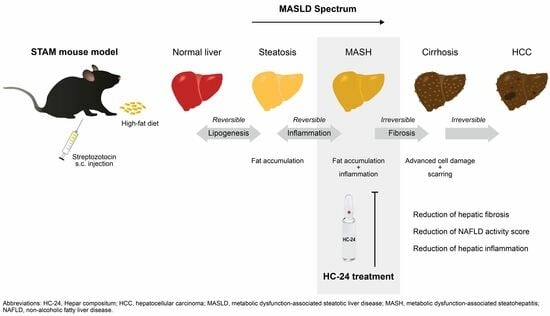
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Medication
2.3. Experimental Design
2.4. Whole Blood and Plasma Biochemistry
2.5. Liver Biochemistry
2.6. Liver Histopathology
2.7. Statistical Analysis
3. Results
3.1. HC-24 Does Not Affect Metabolic Risk Factors but Does Reduce NAFLD Activity Score (NAS) and Fibrosis in a Small-Scale Pilot Study
3.2. Effect of Treatments on Metabolic Risk Factors
3.3. HC-24 Reduces NAFLD Activity Score (NAS)
3.4. HC-24 Does Not Affect Hepatic Steatosis
3.5. HC-24 Reduces Hepatic Inflammation
3.6. HC-24 Reduces Hepatic Fibrosis
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The Prevalence and Incidence of NAFLD Worldwide: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.W.; Yan, H.Y.; Wang, Z.Y.; Zhao, S.H.; Wang, B. Obesity Is an Independent Risk Factor for Non-Alcoholic Fatty Liver Disease: Evidence from a Meta-Analysis of 21 Cohort Studies. Obes. Rev. 2016, 17, 510–519. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The Complex Link between NAFLD and Type 2 Diabetes Mellitus—Mechanisms and Treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-Term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wai-Sun Wong, V.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Bessone, F.; Razori, M.V.; Roma, M.G. Molecular Pathways of Nonalcoholic Fatty Liver Disease Development and Progression. Cell. Mol. Life Sci. 2019, 76, 99–128. [Google Scholar] [CrossRef]
- Huby, T.; Gautier, E.L. Immune Cell-Mediated Features of Non-Alcoholic Steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Borner, M.; Weiser, M. Biological Therapy of Liver Disorders—Results of Drug Monitoring with 801 Patients. Biol. Ther. 1995, XIII, 64–67. [Google Scholar]
- Vovk, A.D.; Solyanik, I. V Experience of Using of Antihomotoxic Preparations Hepar Compositum and Engystol in Complex Treatment Patients Suffering with Viral Hepatitis. Biol. Ther. 2003, 16–19. [Google Scholar]
- Tajmohammadi, A.; Razavi, B.M.; Hosseinzadeh, H. Silybum Marianum (Milk Thistle) and Its Main Constituent, Silymarin, as a Potential Therapeutic Plant in Metabolic Syndrome: A Review. Phyther. Res. 2018, 32, 1933–1949. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum Marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological Effects of Dandelion (Taraxacum Officinale) in Type 2 Diabetes. Rev. Diabet. Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef]
- Hfaiedh, M.; Brahmi, D.; Zourgui, L. Hepatoprotective Effect of T Araxacum Officinale Leaf Extract on Sodium Dichromate-Induced Liver Injury in Rats. Environ. Toxicol. 2016, 31, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jung, C.W.; Anh, N.H.; Kim, S.W.; Park, S.; Kwon, S.W.; Lee, S.J. Effects of Oats (Avena sativa L.) on Inflammation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2021, 8, 722866. [Google Scholar] [CrossRef]
- Debnath, T.; Kim, E.-K.; Das, G.; Nath, N.C.D.; Lee, K.-G. Protective Effect of Oat (Avena Sativa) Bran Extracts on Acute Hepatic Liver Damage in Mice. Food Agric. Immunol. 2019, 30, 34–46. [Google Scholar] [CrossRef]
- Mueller, A.M.; Kleemann, R.; Gart, E.; van Duyvenvoorde, W.; Verschuren, L.; Caspers, M.; Menke, A.; Krömmelbein, N.; Salic, K.; Burmeister, Y.; et al. Cholesterol Accumulation as a Driver of Hepatic Inflammation Under Translational Dietary Conditions Can Be Attenuated by a Multicomponent Medicine. Front. Endocrinol. 2021, 12, 601160. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Snabel, J.M.; de Ruiter, C.; Attema, J.; Caspers, M.P.M.; Lek, S.; van Heuven, B.J.; Speksnijder, A.G.C.L.; Giera, M.; et al. Translational Characterization of the Temporal Dynamics of Metabolic Dysfunctions in Liver, Adipose Tissue and the Gut during Diet-Induced NASH Development in Ldlr−/−.Leiden Mice. Heliyon 2023, 9, e13985. [Google Scholar] [CrossRef]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A Murine Model for Non-Alcoholic Steatohepatitis Showing Evidence of Association between Diabetes and Hepatocellular Carcinoma. Med. Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Fujii, M.; Sandel, J.; Shibazaki, Y.; Wakamatsu, K.; Mark, M.; Yoneyama, H. Linagliptin Alleviates Hepatic Steatosis and Inflammation in a Mouse Model of Non-Alcoholic Steatohepatitis. Med. Mol. Morphol. 2014, 47, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, E.F. Angiotensin Receptor Blockers in the Treatment of NASH/NAFLD: Could They Be a First-Class Option? Adv. Ther. 2008, 25, 1141–1174. [Google Scholar] [CrossRef] [PubMed]
- Gayban, A.J.B.; Souza, L.A.C.; Cooper, S.G.; Regalado, E.; Kleemann, R.; Feng Earley, Y. (Pro)Renin Receptor Antagonism Attenuates High-Fat-Diet–Induced Hepatic Steatosis. Biomolecules 2023, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Rensen, S.S.; Slaats, Y.; Nijhuis, J.; Jans, A.; Bieghs, V.; Driessen, A.; Malle, E.; Greve, J.W.; Buurman, W.A. Increased Hepatic Myeloperoxidase Activity in Obese Subjects with Nonalcoholic Steatohepatitis. Am. J. Pathol. 2009, 175, 1473–1482. [Google Scholar] [CrossRef]
- Wu, L.; Gao, X.; Guo, Q.; Li, J.; Yao, J.; Yan, K.; Xu, Y.; Jiang, X.; Ye, D.; Guo, J. The Role of Neutrophils in Innate Immunity-Driven Nonalcoholic Steatohepatitis: Lessons Learned and Future Promise. Hepatol. Int. 2020, 14, 652–666. [Google Scholar] [CrossRef]
- Pulli, B.; Ali, M.; Iwamoto, Y.; Zeller, M.W.G.; Schob, S.; Linnoila, J.J.; Chen, J.W. Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxidants Redox Signal. 2015, 23, 1255–1269. [Google Scholar] [CrossRef]
- Casini, A.; Ceni, E.; Salzano, R.; Biondi, P.; Parola, M.; Galli, A.; Foschi, M.; Caligiuri, A.; Pinzani, M.; Surrenti, C. Neutrophil-Derived Superoxide Anion Induces Lipid Peroxidation and Stimulates Collagen Synthesis in Human Hepatic Stellate Cells: Role of Nitric Oxide. Hepatology 1997, 25, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xu, M.J.; Cai, Y.; Wang, W.; Jiang, J.X.; Varga, Z.V.; Feng, D.; Pacher, P.; Kunos, G.; Torok, N.J.; et al. Neutrophil–Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cmgh 2018, 5, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Koop, A.C.; Thiele, N.D.; Steins, D.; Michaëlsson, E.; Wehmeyer, M.; Scheja, L.; Steglich, B.; Huber, S.; Schulze zur Wiesch, J.; Lohse, A.W.; et al. Therapeutic Targeting of Myeloperoxidase Attenuates NASH in Mice. Hepatol. Commun. 2020, 4, 1441–1458. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive Immunity: An Emerging Player in the Progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Yao, Y.; Zhang, X.; Guan, Y.; Zheng, F. CD4+ T Cell Activation and Inflammation in NASH-Related Fibrosis. Front. Immunol. 2022, 13, 967410. [Google Scholar] [CrossRef] [PubMed]
- Her, Z.; Tan, J.H.L.; Lim, Y.S.; Tan, S.Y.; Chan, X.Y.; Tan, W.W.S.; Liu, M.; Yong, K.S.M.; Lai, F.; Ceccarello, E.; et al. CD4+ T Cells Mediate the Development of Liver Fibrosis in High Fat Diet-Induced NAFLD in Humanized Mice. Front. Immunol. 2020, 11, 580968. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the Mechanism of Cell Death Resulting from Streptozotocin Challenge in Experimental Animals, Its Practical Use and Potential Risk to Humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis Stage but Not NASH Predicts Mortality and Time to Development of Severe Liver Disease in Biopsy-Proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased Risk of Mortality by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Daniels, S.J.; Holm Nielsen, S.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen Biology and Non-Invasive Biomarkers of Liver Fibrosis. Liver Int. 2020, 40, 736–750. [Google Scholar] [CrossRef]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and Resolution of Inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Dufour, J.F.; Caussy, C.; Loomba, R. Combination Therapy for Non-Alcoholic Steatohepatitis: Rationale, Opportunities and Challenges. Gut 2020, 69, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlton, M. Rational Combination Therapy for NASH: Insights from Clinical Trials and Error. J. Hepatol. 2023, 78, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Gallage, S.; Avila, J.E.B.; Ramadori, P.; Focaccia, E.; Rahbari, M.; Ali, A.; Malek, N.P.; Anstee, Q.M.; Heikenwalder, M. A Researcher’s Guide to Preclinical Mouse NASH Models. Nat. Metab. 2022, 4, 1632–1649. [Google Scholar] [CrossRef]






| Normal | Disease-Control | Telmisartan | Vehicle | HC-24 | |
|---|---|---|---|---|---|
| Body weight (g) | 25.0 ± 1.6 | 21.4 ± 1.6 ** | 18.2 ± 1.8 ## | 21.2 ± 2.4 | 20.8 ± 1.4 |
| Whole blood glucose (mg/dL) | 162.8 ± 20.8 | 572.8 ± 118.5 ** | 659.2 ± 236.7 | 585.8 ± 72.6 | 560.3 ± 108.2 |
| Plasma ALT (U/L) | 36.2 ± 17.0 | 62.3 ± 22.4 | 42.5 ± 5.9 # | 62.2 ± 24.2 | 39.5 ± 13.8 |
| Plasma triglycerides (mg/dL) | 94.8 ± 34.4 | 587.0 ± 439.6 ** | 937.5 ± 664.6 | 838.3 ± 607.7 | 447.0 ± 365.9 |
| Normal | Disease-Control | Telmisartan | Vehicle | HC-24 | |
|---|---|---|---|---|---|
| Body weight (g) | 22.4 ± 1.2 | 18.9 ± 1.8 *** | 16.3 ± 0.7 ## | 18.6 ± 1.3 | 18.3 ± 0.9 |
| Whole blood glucose (mg/dL) | 168.4 ± 28.3 | 560.2 ± 121.8 *** | 659.8 ± 177.7 | 556.9 ± 109.8 | 556.5 ± 112.3 |
| Plasma ALT (U/L) | 20.9 ± 7.8 | 71.3 ± 59.1 * | 46.9 ± 14.7 | 158.9 ± 167.2 | 87.1 ± 49.6 |
| Plasma triglycerides (mg/dL) | 75.9 ± 24.7 | 225.8 ± 186.2 * | 360.6 ± 394.8 | 550.0 ± 365.7 | 266.0 ± 122.0 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmeister, Y.; Weyer, K.; Dörre, A.; Seilheimer, B. The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis. Biomedicines 2023, 11, 3216. https://doi.org/10.3390/biomedicines11123216
Burmeister Y, Weyer K, Dörre A, Seilheimer B. The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis. Biomedicines. 2023; 11(12):3216. https://doi.org/10.3390/biomedicines11123216
Chicago/Turabian StyleBurmeister, Yvonne, Kathrin Weyer, Achim Dörre, and Bernd Seilheimer. 2023. "The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis" Biomedicines 11, no. 12: 3216. https://doi.org/10.3390/biomedicines11123216
APA StyleBurmeister, Y., Weyer, K., Dörre, A., & Seilheimer, B. (2023). The Multicomponent Medicinal Product Hepar Compositum Reduces Hepatic Inflammation and Fibrosis in a Streptozotocin- and High-Fat Diet-Induced Model of Metabolic Dysfunction-Associated Steatotic Liver Disease/Metabolic Dysfunction-Associated Steatohepatitis. Biomedicines, 11(12), 3216. https://doi.org/10.3390/biomedicines11123216