Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review
Abstract
:1. Introduction
1.1. Definition of Age-Related Macular Degeneration
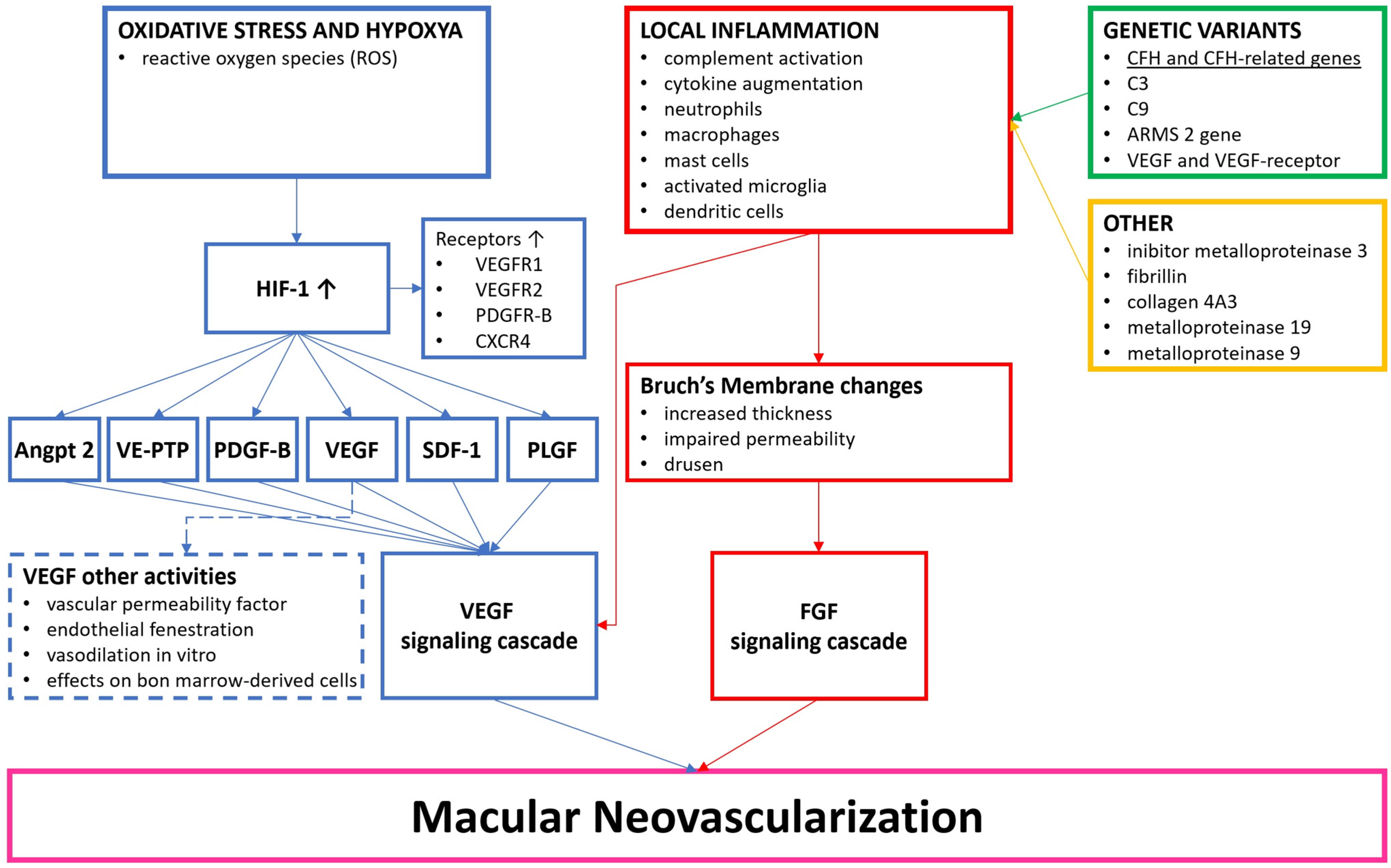
1.2. Pathophysiology of nAMD
1.3. Angiogenesis and VEGF Pathway
2. Current Treatment Landscape in nAMD
2.1. Anti-VEGF Therapy: The Gold Standard
2.2. Limitations of Anti-VEGF Therapy
2.3. Emerging Therapies and Need for Alternative Treatment Options
3. Gene Therapy Strategies for nAMD
3.1. Overview of Genes Targeted
3.2. Gene Silencing and Inhibition of VEGF Expression
3.3. Gene Delivery Approaches: Viral Vector-Based and Non-Viral Delivery
3.3.1. Viral Vector-Based Delivery
3.3.2. Non-Viral Delivery
3.4. Gene Editing Technologies and CRISPR/Cas9
4. Clinical Trials and Promising Gene Therapy Approaches
4.1. PEDF
4.2. Anti-VEGF
4.3. Endostatins and Angiostatins
4.4. Complement Cascade Inhibition
4.5. RNA Interference
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Cruickshanks, K.J. The Prevalence of Age-Related Maculopathy by Geographic Region and Ethnicity1This Paper Has Been Edited by Neville, N. Osborne, PhD, DSc, Nuffield Laboratory of Ophthalmology, University of Oxford, Walton Street, Oxford, UK; and Gerald, J. Chader, The Foundation Fighting Blindness, Hunt Valley, MS.1. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Wang, J.J. Prevalence of Age-Related Maculopathy in Australia. Ophthalmology 1995, 102, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.C.; Assink, J.J.; van Leeuwen, R.; Wolfs, R.C.; Vingerling, J.R.; Stijnen, T.; Hofman, A.; de Jong, P.T. Incidence and Progression Rates of Age-Related Maculopathy: The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2237–2241. [Google Scholar]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The Prevalence of Age-Related Macular Degeneration in Asians. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef]
- Wong, T.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The Natural History and Prognosis of Neovascular Age-Related Macular Degeneration. Ophthalmology 2008, 115, 116–126.e1. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Nusinowitz, S.; Wang, Y.; Kim, P.; Habib, S.; Baron, R.; Conley, Y.; Gorin, M. Retinal Structure in Pre-Clinical Age-Related Macular Degeneration. Curr. Eye Res. 2018, 43, 376–382. [Google Scholar] [CrossRef]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Fulceri, F.; Busceti, C.L.; Biagioni, F.; Fornai, F. Measurement of Drusen and Their Correlation with Visual Symptoms in Patients Affected by Age-Related Macular Degeneration. Arch. Ital. Biol. 2021, 3, 82–104. [Google Scholar] [CrossRef]
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-Related Macular Degeneration and Blindness Due to Neovascular Maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef]
- Chavakis, E.; Dimmeler, S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arter. Thromb. Vasc. Biol. 2002, 22, 887–893. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular Pathogenesis of Retinal and Choroidal Vascular Diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular Endothelial Growth Factor Acts as a Survival Factor for Newly Formed Retinal Vessels and Has Implications for Retinopathy of Prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Okubo, A.; Rosa, R.H.; Bunce, C.V.; Alexander, R.A.; Fan, J.T.; Bird, A.C.; Luthert, P.J. The Relationships of Age Changes in Retinal Pigment Epithelium and Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 1999, 40, 443–449. [Google Scholar]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration—Emerging pathogenetic and therapeutic concepts. Ann. Med. 2009, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Algvere, P.V.; Kvanta, A.; Seregard, S. Drusen maculopathy: A risk factor for visual deterioration. Acta Ophthalmol. 2016, 94, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef]
- Kelly, B.D.; Hackett, S.F.; Hirota, K.; Oshima, Y.; Cai, Z.; Berg-Dixon, S.; Rowan, A.; Yan, Z.; Campochiaro, P.A.; Semenza, G.L. Cell Type–Specific Regulation of Angiogenic Growth Factor Gene Expression and Induction of Angiogenesis in Nonischemic Tissue by a Constitutively Active Form of Hypoxia-Inducible Factor 1. Circ. Res. 2003, 93, 1074–1081. [Google Scholar] [CrossRef]
- Metzger, C.S.; Koutsimpelas, D.; Brieger, J. Transcriptional Regulation of the VEGF Gene in Dependence of Individual Genomic Variations. Cytokine 2015, 76, 519–526. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Peyman, G.A.; Fredj-Reygrobellet, D.; Gordon, W.C.; Lapalus, P.; Gastaud, P.; Bazan, N.G. Immunohistological Study of Subretinal Membranes in Age-Related Macular Degeneration. Jpn. J. Ophthalmol. 1992, 36, 443–451. [Google Scholar] [PubMed]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The Pivotal Role of the Complement System in Aging and Age-Related Macular Degeneration: Hypothesis Re-Visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Toomey, C.B.; Johnson, L.V.; Bowes Rickman, C. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog. Retin. Eye Res. 2018, 62, 38–57. [Google Scholar] [CrossRef]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A Common Haplotype in the Complement Regulatory Gene Factor H ( HF1/CFH ) Predisposes Individuals to Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R.; et al. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Zareparsi, S.; Branham, K.E.H.; Li, M.; Shah, S.; Klein, R.J.; Ott, J.; Hoh, J.; Abecasis, G.R.; Swaroop, A. Strong Association of the Y402H Variant in Complement Factor H at 1q32 with Susceptibility to Age-Related Macular Degeneration. Am. J. Hum. Genet. 2005, 77, 149–153. [Google Scholar] [CrossRef]
- The AMD Genetics Clinical Study Group; Gold, B.; Merriam, J.E.; Zernant, J.; Hancox, L.S.; Taiber, A.J.; Gehrs, K.; Cramer, K.; Neel, J.; Bergeron, J.; et al. Variation in Factor B (BF) and Complement Component 2 (C2) Genes Is Associated with Age-Related Macular Degeneration. Nat. Genet. 2006, 38, 458–462. [Google Scholar] [CrossRef]
- Lorés-Motta, L.; Paun, C.C.; Corominas, J.; Pauper, M.; Geerlings, M.J.; Altay, L.; Schick, T.; Daha, M.R.; Fauser, S.; Hoyng, C.B.; et al. Genome-Wide Association Study Reveals Variants in CFH and CFHR4 Associated with Systemic Complement Activation. Ophthalmology 2018, 125, 1064–1074. [Google Scholar] [CrossRef]
- Seddon, J.M.; Yu, Y.; Miller, E.C.; Reynolds, R.; Tan, P.L.; Gowrisankar, S.; Goldstein, J.I.; Triebwasser, M.; Anderson, H.E.; Zerbib, J.; et al. Rare Variants in CFI, C3 and C9 Are Associated with High Risk of Advanced Age-Related Macular Degeneration. Nat. Genet. 2013, 45, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, J.P.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A Functional Variant in the CFI Gene Confers a High Risk of Age-Related Macular Degeneration. Nat. Genet. 2013, 45, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R.; Sepp, T.; Matharu, B.K.; Khan, J.C.; Thurlby, D.A.; Shahid, H.; Clayton, D.G.; Hayward, C.; Morgan, J.; Wright, A.F.; et al. Complement C3 Variant and the Risk of Age-Related Macular Degeneration. N. Engl. J. Med. 2007, 357, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen Complement Components C3a and C5a Promote Choroidal Neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Bradt, B.M.; Kolb, W.P.; Cooper, N.R. Complement-Dependent Proinflammatory Properties of the Alzheimer’s Disease β-Peptide. J. Exp. Med. 1998, 188, 431–438. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s Aβ-Peptide Is Deposited at Sites of Complement Activation in Pathologic Deposits Associated with Aging and Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-Mediated Injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef]
- Wu, Z.; Lauer, T.W.; Sick, A.; Hackett, S.F.; Campochiaro, P.A. Oxidative Stress Modulates Complement Factor H Expression in Retinal Pigmented Epithelial Cells by Acetylation of FOXO3. J. Biol. Chem. 2007, 282, 22414–22425. [Google Scholar] [CrossRef]
- Hageman, G.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An Integrated Hypothesis That Considers Drusen as Biomarkers of Immune-Mediated Processes at the RPE-Bruch’s Membrane Interface in Aging and Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement Activation and Inflammatory Processes in Drusen Formation and Age Related Macular Degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef]
- Leung, K.W.; Barnstable, C.J.; Tombran-Tink, J. Bacterial Endotoxin Activates Retinal Pigment Epithelial Cells and Induces Their Degeneration through IL-6 and IL-8 Autocrine Signaling. Mol. Immunol. 2009, 46, 1374–1386. [Google Scholar] [CrossRef]
- Parmeggiani, F.; Romano, M.R.; Costagliola, C.; Semeraro, F.; Incorvaia, C.; D’Angelo, S.; Perri, P.; De Palma, P.; De Nadai, K.; Sebastiani, A. Mechanism of Inflammation in Age-Related Macular Degeneration. Mediat. Inflamm. 2012, 2012, 546786. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef]
- Skerka, C.; Chen, Q.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement Factor H Related Proteins (CFHRs). Mol. Immunol. 2013, 56, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Mattapallil, M.J.; Caspi, R.R. Compliments of Factor H: What’s in It for AMD? Immunity 2017, 46, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, K.M.; Yasuma, T.R.; Tomida, D.; Nakamura, M.; Ishikawa, K.; Kikuchi, M.; Ohmi, Y.; Niwa, T.; Hamajima, N.; Furukawa, K.; et al. C9-R95X Polymorphism in Patients with Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 508. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Retinal Macrophages Synthesize C3 and Activate Complement in AMD and in Models of Focal Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2977. [Google Scholar] [CrossRef]
- Grassmann, F.; Heid, I.M.; Weber, B.H.F.; International AMD Genomics Consortium (IAMDGC). Recombinant Haplotypes Narrow the ARMS2/HTRA1 Association Signal for Age-Related Macular Degeneration. Genetics 2017, 205, 919–924. [Google Scholar] [CrossRef]
- Lazzeri, S.; Orlandi, P.; Figus, M.; Fioravanti, A.; Cascio, E.; Di Desidero, T.; Agosta, E.; Canu, B.; Sartini, M.S.; Danesi, R.; et al. The Rs2071559 AA VEGFR-2 Genotype Frequency Is Significantly Lower in Neovascular Age-Related Macular Degeneration Patients. Sci. World J. 2012, 2012, 420190. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A. Association between Vascular Endothelial Growth Factor Polymorphisms and Age-Related Macular Degeneration: An Updated Meta-Analysis. Dis. Markers 2016, 2016, 8486406. [Google Scholar] [CrossRef]
- Mammadzada, P.; Corredoira, P.M.; André, H. The Role of Hypoxia-Inducible Factors in Neovascular Age-Related Macular Degeneration: A Gene Therapy Perspective. Cell Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.-J.; Goeddel, D.V.; Ferrara, N. Vascular Endothelial Growth Factor Is a Secreted Angiogenic Mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Plouët, J.; Schilling, J.; Gospodarowicz, D. Isolation and Characterization of a Newly Identified Endothelial Cell Mitogen Produced by AtT-20 Cells. EMBO J. 1989, 8, 3801–3806. [Google Scholar] [CrossRef] [PubMed]
- Michels, S.; Schmidt-Erfurth, U.; Rosenfeld, P.J. Promising New Treatments for Neovascular Age-Related Macular Degeneration. Expert Opin. Investig. Drugs 2006, 15, 779–793. [Google Scholar] [CrossRef]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta Growth Factor. Potentiation of Vascular Endothelial Growth Factor Bioactivity, in Vitro and in Vivo, and High Affinity Binding to Flt-1 but Not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar] [CrossRef]
- Barleon, B.; Sozzani, S.; Zhou, D.; Weich, H.A.; Mantovani, A.; Marmé, D. Migration of Human Monocytes in Response to Vascular Endothelial Growth Factor (VEGF) Is Mediated via the VEGF Receptor Flt-1. Blood 1996, 87, 3336–3343. [Google Scholar] [CrossRef]
- Kaiser, S.M.; Arepalli, S.; Ehlers, J.P. Current and future anti-VEGF agents for neovascular age-related macular degeneration. J. Exper. Pharmacol. 2021, 13, 905–912. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Do, D.V.; Schmidt-Erfurth, U.; Boyer, D.S.; Holz, F.G.; Heier, J.S.; Midena, E.; Kaiser, P.K.; Terasaki, H.; Marcus, D.M.; et al. Intravitreal aflibercept for DME. Ophthalmology 2014, 121, 2247–2254. [Google Scholar] [CrossRef]
- Folk, J.C.; Stone, E.M. Ranibizumab therapy for neovascular age-related macular degeneration. N. Engl. J. Med. 2010, 363, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Nasir, M.A.; Giust, M.J. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 363–372.e5. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Blum, C.A.; Copt, R.P.; Eandi, C.M.; Hatz, K.; Prünte, C.F.; Seelig, E.; Somfai, G.M. Brolucizumab in Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: Ophthalmology and Diabetology Treatment Aspects. Ophthalmol. Ther. 2023, 12, 639–655. [Google Scholar] [CrossRef] [PubMed]
- Monés, J.; Srivastava, S.K.; Jaffe, G.J.; Tadayoni, R.; Albini, T.A.; Kaiser, P.K.; Holz, F.G.; Korobelnik, J.F.; Kim, I.K.; Pruente, C.; et al. Risk of Inflammation, Retinal Vasculitis, and Retinal Occlusion-Related Events with Brolucizumab: Post Hoc Review of HAWK and HARRIER. Ophthalmology 2021, 128, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.A.; Finn, A.P.; Sternberg, P., Jr. Spotlight on Faricimab in the Treatment of Wet Age-Related Macular Degeneration: Design, Development and Place in Therapy. Drug Des. Dev. Ther. 2022, 16, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Ou, W.C.; Brown, D.M.; Croft, D.E.; Wang, R.; Payne, J.F.; Clark, W.L.; Abdelfattah, N.S.; Sadda, S.R.; TREX-AMD Study Group. TREX-AMD Study Group. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol. Retina 2017, 1, 314–321. [Google Scholar] [CrossRef]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of retinal gene therapy: Delivery of viral vector into the subretinal space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C.; Georgiadis, A.; Mowat, F.M.; Beattie, S.G.; Gardner, P.J.; et al. Long-Term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015, 372, 1887–1897. [Google Scholar] [CrossRef]
- Weleber, R.G.; Pennesi, M.E.; Wilson, D.J.; Kaushal, S.; Erker, L.R.; Jensen, L.; McBride, M.T.; Flotte, T.R.; Humphries, M.; Calcedo, R.; et al. Results at 2 years after gene therapy for Rpe65-deficient Leber congenital amaurosis and severe Early-Childhood-Onset retinal dystrophy. Ophthalmology 2016, 123, 1606–1620. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.E.; Fenner, B.J.; Barathi, V.A.; Tun, S.B.B.; Wey, Y.S.; Tsai, A.S.H.; Su, X.; Lee, S.Y.; Cheung, C.M.G.; Wong, T.Y.; et al. Gene-based therapeutics for acquired retinal disease: Opportunities and progress. Front. Genet. 2021, 12, 795010. [Google Scholar] [CrossRef] [PubMed]
- Planul, A.; Dalkara, D. Vectors and gene delivery to the retina. Annu. Rev. Vis. Sci. 2017, 3, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Spooner, K.; Hong, T.; Wijeyakumar, W.; Chang, A.A. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: A systematic review with meta-analysis. Clin. Ophthalmol. 2017, 11, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Hong, T.; Chang, A.A. Treating the untreatable patient: Current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014, 92, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Garweg, J.G.; Traine, P.G.; Garweg, R.A.; Wons, J.; Gerhardt, C.; Pfister, I.B. Continued anti-VEGF treatment does not prevent recurrences in eyes with stable neovascular age-related macular degeneration using a treat-and-extend regimen: A retrospective case series. Eye 2022, 36, 862–868. [Google Scholar] [CrossRef]
- Khurana, R.N. Long-term management of neovascular age-related macular degeneration: To suspend or not to suspend? Ophthalmol. Retina 2019, 3, 621–622. [Google Scholar] [CrossRef]
- Torres-Costa, S.; Ramos, D.; Brandão, E.; Carneiro, Â.; Rosas, V.; Rocha-Sousa, A.; Falcão-Reis, F.; Falcão, M. Incidence of endophthalmitis after intravitreal injection with and without topical antibiotic prophylaxis. Eur. J. Ophthalmol. 2021, 31, 600–606. [Google Scholar] [CrossRef]
- Patil, N.S.; Dhoot, A.S.; Popovic, M.M.; Kertes, P.J.; Muni, R.H. Risk Of Intraocular Inflammation After Injection Of Antivascular Endothelial Growth Factor Agents: A Meta-Analysis. Retina 2022, 42, 2134–2142. [Google Scholar] [CrossRef]
- Levin, A.M.; Chaya, C.J.; Kahook, M.Y.; Wirostko, B.M. Intraocular Pressure Elevation Following Intravitreal Anti-VEGF Injections: Short- and Long-term Considerations. J. Glaucoma 2021, 30, 1019–1026. [Google Scholar] [CrossRef]
- Daien, V.; Nguyen, V.; Essex, R.W.; Guymer, R.; Arnold, J.J.; Munk, M.; Ceklic, L.; Gillies, M.C.; Barthelmes, D.; Fight Retinal Blindness! investigators. Prevalence and characteristics of macular atrophy in eyes with neovascular age-related macular degeneration. A study from a long-term observational dataset: The Fight Retinal Blindness project. Br. J. Ophthalmol. 2020, 104, 1064–1069. [Google Scholar] [CrossRef]
- Sadda, S.R.; Tuomi, L.L.; Ding, B.; Fung, A.E.; Hopkins, J.J. Macular Atrophy in the HARBOR Study for Neovascular Age-Related Macular Degeneration. Ophthalmology 2018, 125, 878–886. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K. Group S-US: Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, T.; Wu, Z.; Wu, Q.; Ke, X.; Luo, D.; Wang, H. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS ONE 2013, 8, e70544. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 study of OPT-302 inhibition of vascular endothelial growth factors C and D for neovascular age-related macular degeneration. Ophthalmol. Retina 2020, 4, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Aziz, A.A.; Jhingan, M.; Singh, S.R.; Khanani, A.M.; Chhablani, J. Emerging therapies in neovascular age-related macular degeneration in 2020. Asia Pac. J. Ophthalmol. 2020, 9, 250–259. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. KSI-301: Antibody biopolymer conjugate in retinal disorders. Ther. Adv. Ophthalmol. 2021, 13, 25158414211027708. [Google Scholar] [CrossRef]
- Chen, E.R.; Kaiser, P.K. Therapeutic potential of the ranibizumab port delivery system in the treatment of AMD: Evidence to date. Clin. Ophthalmol. 2020, 14, 1349–1355. [Google Scholar] [CrossRef]
- Xin, H.; Biswas, N.; Li, P.; Zhong, C.; Chan, T.C.; Nudleman, E.; Ferrara, N. Heparin-binding VEGFR1 variants as long-acting VEGF inhibitors for treatment of intraocular neovascular disorders. Proc. Natl. Acad. Sci. USA 2021, 118, e1921252118. [Google Scholar] [CrossRef]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Costanzo, E.; Scondotto, G.; Trifirò, G.; Virgili, G. Anti-VEGF and Other Novel Therapies for Neovascular Age-Related Macular Degeneration: An Update. BioDrugs 2021, 35, 673–692. [Google Scholar] [CrossRef]
- Khachigian, L.M.; Liew, G.; Teo, K.Y.C.; Wong, T.Y.; Mitchell, P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J. Transl. Med. 2023, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.O.; Ritter, R.; Iii Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement factor H polymorphism and age-related macular degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef] [PubMed]
- van Asten, F.; Simmons, M.; Singhal, A.; Keenan, T.D.; Ratnapriya, R.; Agrón, E.; Clemons, T.E.; Swaroop, A.; Lu, Z.; Chew, E.Y. Age-Related Eye Disease Study 2 Research Group. A deep phenotype association study reveals specific phenotype associations with genetic variants in age-related macular degeneration: Age-Related Eye Disease Study 2 (AREDS2) report no. 14. Ophthalmology 2018, 125, 559–568. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Cheng, C.Y.; Wen, F.; Tam, P.O.; Zhao, P.; Chen, H.; Li, Z.; Chen, L.; Tai, Z.; et al. A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat. Genet. 2016, 48, 640–647. [Google Scholar] [CrossRef]
- Botto, C.; Rucli, M.; Tekinsoy, M.D.; Pulman, J.; Sahel, J.-A.; Dalkara, D. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog. Retin Eye Res. 2021, 86, 100975. [Google Scholar] [CrossRef]
- De Guimaraes, T.A.C.; Georgiou, M.; Bainbridge, J.W.; Michaelides, M. Gene therapy for neovascular age-related macular degeneration: Rationale, clinical trials and future directions. Br. J. Ophthalmol. 2021, 105, 151–157. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, X.; Tang, Y.; Li, S.; Chen, J. Progress on ocular siRNA gene-silencing therapy and drug delivery systems. Fundam. Clin. Pharmacol. 2021, 35, 4–24. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Hyttinen, J.M.; Toropainen, E.; Kaarniranta, K. Endoplasmic reticulum stress in age-related macular degeneration: Trigger for neovascularization. Mol. Med. 2010, 16, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Patisiran: First global approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Small Interfering RNA (siRNA) Based Therapy; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Garba, A.O.; Mousa, S.A. Bevasiranib for the treatment of wet, age-related macular degeneration. Ophthalmol. Eye Dis. 2010, 2, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Symons, R.C.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. RNAi-Based Treatment for Neovascular Age-Related Macular Degeneration by Sirna-027. Am. J. Ophthalmol. 2010, 150, 33–39.e2. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Pogue, A.I.; Lukiw, W.J. Up–regulated pro–inflammatory MicroRNAs (miRNAs) in alzheimer’s disease (AD) and age–related macular degeneration (AMD). Cell Mol. Neurobiol. 2018, 38, 1021–1031. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, C.; Hanus, J.; Zhao, F.; Ma, J.; Yoshimura, A.; Wang, S. Strand and cell type–specific function of microRNA–126 in angiogenesis. Mol. Ther. 2016, 24, 1823–1835. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P. MicroRNAs as diagnostic and prognostic biomarkers of age–related macular degeneration: Advances and limitations. Neural Regen. Res. 2021, 16, 440–447. [Google Scholar] [CrossRef]
- Szemraj, M.; Bielecka-Kowalska, A.; Oszajca, K.; Krajewska, M.; Goś, R.; Jurowski, P.; Kowalski, M.; Szemraj, J. Serum micrornas as potential biomarkers of AMD. Med. Sci. Monitor. 2015, 21, 2734–2742. [Google Scholar] [CrossRef]
- Cruz-Aguilar, M.; Groman-Lupa, S.; Jimenez-Martınez, M.C. MicroRNAs as potential biomarkers and therapeutic targets in age-related macular degeneration. Front. Ophthalmol. 2023, 3, 1023782. [Google Scholar] [CrossRef]
- Hanlon, K.S.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Volak, A.; Spirig, S.E.; Muller, A.; Sousa, A.A.; Tsai, S.Q.; Bengtsson, N.E.; et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019, 10, 4439. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Jozic, A.; Su, G.L.; Herrera-Barrera, M.; Curtis, A.; Arrizabalaga, S.; Tschetter, W.; Ryals, R.C.; Sahay, G. Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat. Commun. 2023, 14, 6468. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.E.; Rolling, F.; Li, C.; Conrath, H.; Xiao, W.; Xiao, X.; Samulski, R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002, 76, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Day, T.P.; Byrne, L.C.; Schaffer, D.V.; Flannery, J.G. Advances in AAV vector development for gene therapy in the retina. Adv. Exp. Med. Biol. 2014, 801, 687–693. [Google Scholar] [PubMed]
- Grimm, D.; Büning, H. Small but increasingly mighty: Latest advances in AAV vector research, design, and evolution. Hum. Gene Ther. 2017, 28, 1075–1086. [Google Scholar] [CrossRef]
- Dismuke, D.J.; Tenenbaum, L.; Samulski, R.J. Biosafety of recombinant adeno-associated virus vectors. Curr. Gene Ther. 2013, 13, 434–452. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Han, I.C.; Burnight, E.R.; Ulferts, M.J.; Worthington, K.S.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; Stone, E.M.; Tucker, B.A.; Wiley, L.A. Helper-dependent adenovirus transduces the human and rat retina but elicits an inflammatory reaction when delivered subretinally in rats. Hum. Gene Ther. 2019, 30, 1371–1384. [Google Scholar] [CrossRef]
- Arbabi, A.; Liu, A.; Ameri, H. Gene therapy for inherited retinal degeneration. J. Ocul. Pharmacol. Ther. 2019, 35, 79–97. [Google Scholar] [CrossRef]
- Allocca, M.; Mussolino, C.; Garcia-Hoyos, M.; Sanges, D.; Iodice, C.; Petrillo, M.; Vandenberghe, L.H.; Wilson, J.M.; Marigo, V.; Surace, E.M.; et al. Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J. Virol. 2007, 81, 11372–11380. [Google Scholar] [CrossRef] [PubMed]
- Petrs-Silva, H.; Dinculescu, A.; Li, Q.; Deng, W.T.; Pang, J.J.; Min, S.H.; Chiodo, V.; Neeley, A.W.; Govindasamy, L.; Bennett, A.; et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 2011, 19, 293–301. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S. Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Gao, J.; Hussain, R.M.; Weng, C.Y. Voretigene Neparvovec in Retinal Diseases: A Review of the Current Clinical Evidence. Clin. Ophthalmol. 2020, 14, 3855–3869. [Google Scholar] [CrossRef] [PubMed]
- Gange, W.S.; Sisk, R.A.; Besirli, C.G.; Lee, T.C.; Havunjian, M.; Schwartz, H.; Borchert, M.; Sengillo, J.D.; Mendoza, C.; Berrocal, A.M.; et al. Perifoveal Chorioretinal Atrophy after Subretinal Voretigene Neparvovec-rzyl for RPE65-Mediated Leber Congenital Amaurosis. Ophthalmol. Retina 2022, 6, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.K.; Campochiaro, P.A.; Sohn, E.H.; Kelleher, M.; Harrop, R.; Loader, J.; Ellis, S.; Mitrophanous, K. Phase I Safety and Tolerability results for RetinoStat®, a Lentiviral Vector Expressing Endostatin and Angiostatin, in Patients with Advanced Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57. [Google Scholar]
- Mingozzi, F.; High, K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2011, 11, 321–330. [Google Scholar] [CrossRef]
- Oliveira, A.V.; Rosa da Costa, A.M.; Silva, G.A. Non-viral strategies for ocular gene delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1275–1289. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef]
- Cai, X.; Nash, Z.; Conley, S.M.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE 2009, 4, e5290. [Google Scholar] [CrossRef]
- Farjo, R.; Skaggs, J.; Quiambao, A.B.; Cooper, M.J.; Naash, M.I. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 2006, 1, e38. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Banworth, M.J.; Makkia, R.; Conley, S.M.; Al-Ubaidi, M.R.; Cooper, M.J.; Naash, M.I. Genomic DNA nanoparticles rescue rhodopsin-associated retinitis pigmentosa phenotype. FASEB J. 2015, 29, 2535–2544. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Conley, S.M.; Makkia, R.S.; Cooper, M.J.; Naash, M.I. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J. Clin. Investig. 2012, 122, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.K.; Jo, D.H.; Lee, S.N.; Cho, C.S.; Jeong, Y.K.; Jung, Y.; Yu, J.; Kim, J.H.; Woo, J.S.; Bae, S. High-purity production and precise editing of DNA base editing ribonucleoproteins. Sci. Adv. 2021, 7, eabg2661. [Google Scholar] [CrossRef] [PubMed]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.-K.; Liu, D.R. Cationic lipidmediated delivery of proteins enables efficient protein based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; López-Méndez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E.; et al. Niosomebased approach for in situ gene delivery to retina and brain cortex as immune-privileged tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef]
- Durak, S.; Esmaeili Rad, M.; Alp Yetisgin, A.; Eda Sutova, H.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease-recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Villate-Beitia, I.; Gallego, I.; Martínez-Navarrete, G.; Zárate, J.; López-Méndez, T.; Soto-Sánchez, C.; Santos-Vizcaíno, E.; Puras, G.; Fernández, E.; Pedraz, J.L. Polysorbate 20 non-ionic surfactant enhances retinal gene delivery efficiency of cationic niosomes after intravitreal and subretinal administration. Int. J. Pharm. 2018, 550, 388–397. [Google Scholar] [CrossRef]
- Yiu, G. Genome editing in retinal diseases using CRISPR technology. Ophthalmol. Retina 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child Educ. Pract. Ed. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Da Costa, B.L.; Levi, S.R.; Eulau, E.; Tsai, Y.T.; Quinn, P.M.J. Prime editing for inherited retinal diseases. Front. Genome Ed. 2021, 3, 775330. [Google Scholar] [CrossRef] [PubMed]
- Guha, T.K.; Wai, A.; Hausner, G. Programmable genome editing tools and their regulation for efficient genome engineering. Comput. Struct. Biotechnol. J. 2017, 15, 146–160. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Nishikawa, A.; Kume, S.; Chayama, K.; Yamamoto, T. Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci. Rep. 2014, 4, 5400. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.Z.; Wong, T.Y.; Ong, F.S. Genetic risk, ethnic variations and pharmacogenetic biomarkers in AMD and polypoidal choroidal vasculopathy. Expert Rev. Ophthalmol. 2013, 8, 127–140. [Google Scholar] [CrossRef]
- Imamura, Y.; Engelbert, M.; Iida, T.; Freund, K.B.; Yannuzzi, L.A. Polypoidal choroidal vasculopathy: A review. Surv. Ophthalmol. 2010, 55, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.W.; Kim, J.H.; Lee, S.H.; Kim, D.; Koo, T.; Kim, K.-e.; Kim, J.H.; Kim, J.-S. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 2017, 27, 419–426. [Google Scholar] [CrossRef]
- Ahmad, I. CRISPR/Cas9—A Promising Therapeutic Tool to Cure Blindness: Current Scenario and Future Prospects. Int. J. Mol. Sci. 2022, 23, 11482. [Google Scholar] [CrossRef]
- Lin, F.L.; Wang, P.Y.; Chuang, Y.F.; Wang, J.H.; Wong, V.H.Y.; Bui, B.V.; Liu, G.S. Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update. Mol. Ther. 2020, 28, 2120–2138. [Google Scholar] [CrossRef]
- Bennett, J.; Wilson, J.; Sun, D.; Forbes, B.; Maguire, A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Vis. Sci. 1994, 35, 2535–2542. [Google Scholar]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.; Benedict, W.; Bouck, N.P. Pigment epithelium-derived factor: A potent inhibitor of angio-genesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Bouck, N.; Volpert, O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am. J. Ophthalmol. 2002, 134, 220–227. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef] [PubMed]
- RRakoczy, E.P.; Lai, C.M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet 2015, 386, 2395–2403. [Google Scholar] [CrossRef]
- Rakoczy, E.P.; Magno, A.L.; Lai, C.M.; Pierce, C.M.; Degli-Esposti, M.A.; Blumenkranz, M.S.; Constable, I.J. Three-year follow-up of phase 1 and 2a rAAV.sFLT-1 subretinal gene therapy trials for exudative age-related macular degeneration. Am. J. Ophthalmol. 2019, 204, 113–123. [Google Scholar] [CrossRef]
- Constable, I.J.; Lai, C.M.; Magno, A.L.; French, M.A.; Barone, S.B.; Schwartz, S.D.; Blumenkranz, M.S.; Degli-Esposti, M.A.; Rakoczy, E.P. Gene therapy in neovascular age-related macular degeneration: Three-year follow-up of a phase 1 randomized dose-escalation trial. Am. J. Ophthalmol. 2017, 177, 150–158. [Google Scholar] [CrossRef]
- Adverum Biotechnologies. Transforming Gene Therapy. 2020. Available online: http://investors.adverum.com/static-files/c8256955-641c-45a3-bdda-5b99c2336a14 (accessed on 20 August 2023).
- Available online: https://investors.adverum.com/news/news-details/2021/Adverum-Provides-Update-on-ADVM-022-and-the-INFINITY-Trial-in-Patients-with-Diabetic-Macular-Edema/default.aspx (accessed on 20 August 2023).
- Available online: https://www.regenxbio.com/getmedia/d311cd48-532b-49ac-bf41-59d3d8907c8d/RGX-314_BobAvery-AAO-2021_11Nov21_FINAL.pdf?ext=.pdf (accessed on 20 August 2023).
- REGENXBIO Inc. Key Takeaways from the RGX-314 phase I/IIa Clinical Trial for Wet AMD (Cohorts 1–5). 2019. Available online: https://regenxbio.com/wp-content/uploads/2019/10/Key-Takeaways-From-The-RGX-314-Phase-I-IIa-Clinical-Trial-For-Wet-AMD-Cohorts-1-5.pdf (accessed on 20 August 2023).
- Kachi, S.; Binley, K.; Yokoi, K.; Umeda, N.; Akiyama, H.; Muramatu, D.; Iqball, S.; Kan, O.; Naylor, S.; Campochiaro, P.A. Equine infectious anemia viral vector-mediated codelivery of endostatin and angiostatin driven by retinal pigmented epithelium-specific VMD2 promoter inhibits choroidal neovascularization. Hum. Gene Ther. 2009, 20, 31–39. [Google Scholar] [CrossRef]
- Balaggan, K.S.; Binley, K.; Esapa, M.; MacLaren, R.E.; Iqball, S.; Duran, Y.; Pearson, R.A.; Kan, O.; Barker, S.E.; Smith, A.J.; et al. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Ther. 2006, 13, 1153–1165. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Lauer, A.K.; Sohn, E.H.; Mir, T.A.; Naylor, S.; Anderton, M.C.; Kelleher, M.; Harrop, R.; Ellis, S.; Mitrophanous, K.A. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum. Gene Ther. 2017, 28, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, R. The role of complement membrane attack complex in dry and wet AMD—From hypothesis to clinical trials. Exp. Eye Res. 2019, 184, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Schachar, R.A.; Nduaka, C.I.; Sperling, M.; Basile, A.S.; Klamerus, K.J.; Chi-Burris, K.; Yan, E.; Paggiarino, D.A.; Rosenblatt, I.; et al. PF-04523655 Study Group Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye 2012, 26, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Schachar, R.A.; Nduaka, C.I.; Sperling, M.; Klamerus, K.J.; Chi-Burris, K.; Yan, E.; Paggiarino, D.A.; Rosenblatt, I.; Aitchison, R.; et al. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study). Ophthalmology 2012, 119, 1867–1873. [Google Scholar] [CrossRef]
- Askou, A.L.; Alsing, S.; Benckendorff, J.N.E.; Holmgaard, A.; Mikkelsen, J.G.; Aagaard, L.; Bek, T.; Corydon, T.J. Suppression of choroidal neovascularization by AAV-based dual-acting antiangiogenic gene therapy. Mol. Ther. Nucleic Acids 2019, 16, 38–50. [Google Scholar] [CrossRef]
- Khanani, A.M.; Thomas, M.J.; Aziz, A.A.; Weng, C.Y.; Danzig, C.J.; Yiu, G.; Kiss, S.; Waheed, N.K.; Kaiser, P.K. Review of gene therapies for age-related macular degeneration. Eye 2022, 36, 303–311. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene therapy for retinal degenerative diseases: Progress, challenges, and future directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Buck, T.M.; Wijnholds, J. Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays. Int. J. Mol. Sci. 2020, 21, 4197. [Google Scholar] [CrossRef] [PubMed]
| Trial ID | Development | Tested Drug | Route of Administration | Mechanism | Results | |
|---|---|---|---|---|---|---|
| NCT00109499 | Phase I | AdGVPEDF.11D | Intravitreal injection | Induction of PEDF expression | Safe, MNV size stable or reduced with dose 1E8 or 1E9 PU | |
| NCT01024998 | Phase I | AAV2-sFLT01 | Intravitreal injection | Induction of gene AAV2-sFLT01 encoding for an anti-angiogenic fusion protein formed by FLT-1 and IgG1 Fc domain that neutralizes VEGF-A before it binds its receptor | Safe, good protein expression levels, but without significant anatomo-functional results | |
| NCT01494805 | Phase I/IIa | AAV (rAAV).sFLT-1 | Subretinal injection | Induction of gene encoding the natural anti-angiogenic protein FLT-1 that neutralizes VEGF-A before it binds its receptor | Safe, no significant anatomo-functional results | |
| NCT03748784 | Phase I | AAV.7m8-aflibercept | Intravitreal injection | Induction of endogenous aflibercept expression in confirmed exogenous aflibercept-responding patients | BCVA and retinal thickness maintenance in 12 patients out of 18 (10 of them not requiring rescue treatment for about 11 months) | |
| NCT03066258 | Phase I/IIa | RGX-314 (5 cohorts with different doses) | Subretinal injection | Induction of endogenous anti-VEGF protein (similar to ranibizumab) expression in confirmed ranibizumab-responding patients Induction of endogenous anti-VEGF protein (similar to ranibizumab) expression in confirmed ranibizumab-responding patients | Safe, good efficacy with functional and anatomical stabilization or improvement and less rescue treatments in patients treated with higher doses | |
| NCT04832724 | Phase II | RGX-314 (clinical vs. eventual commercial formulation) | Recruiting, data not available | |||
| NCT04514653 | Phase II | RGX-314 vs. ranibizumab | ||||
| NCT03999801 | Phase II | RGX-314 vs. ranibizumab and RGX-314 + local vs. RGX-314 + topical steroids | ||||
| NCT01301443 | Phase I | RetinoStat | Subretinal injection | Induction of supplemental endogenous endostatin and angiostatin expression | Safe. Non-significant effectiveness | |
| NCT03585556 | Phase I | AAVCAGsCD59 | Intravitreal injection | Induction of soluble CD59 expression to prevent MAC formation and cellular damage and apoptosis | Data not available | |
| NCT00722384 | Phase I | Bevasiranib | Intravitreal injection | Post-transcription silencing of VEGF mRNA | Safe | |
| NCT00259753 | Phase II | Bevasiranib | Vision loss and MNV extension | |||
| NCT00499590 | Phase III | Bevasiranib combined with intravitreal ranibizumab | Terminated due to missed primary endpoints | |||
| NCT00363714 | Phase I | AGN 211745 | Intravitreal injection | Post-transcription silencing of FLT-1 (VEGFR-2) mRNA | Safe | |
| NCT00725685 | Phase I | PF-04523655 | Intravitreal injection | Post-transcription silencing of hypoxia-induced gene RTP801 | Safe | |
| NCT00713518 | Phase II | PF-04523655 versus Ranibizumab | Intravitreal injection | A 19-nucleotide methylated double stranded siRNA targeting the RTP801 gene | Not significantly more effective than ranbizumab, but synergetic with it in improving BCVA | |
| NCT05657301 | Phase I | KH631 | Subretinal injection | Adeno-associated virus 8 vector that encodes a human VEGF receptor fusion protein | Recruiting, no results posted | |
| NCT05672121 | Phase I/II | KH631 | Subretinal injection | Adeno-associated virus 8 vector that encodes a human VEGF receptor fusion protein | Recruiting, no results posted | |
| NCT05536973 | Phase II | ADVM-022 (AAV.7m8-aflibercept) | Intravitreal injection | Induction of endogenous aflibercept expression in confirmed exogenous aflibercept-responding patients | Recruiting, no results posted | |
| NCT05197270 | Phase I/II | 4D-150 | Intravitreal injection | Dual transgene payload, expressing aflibercept and an anti-VEGF-C RNAi | Recruiting, no results posted | |
| NCT06031727 | Phase I | HG202 | Not specified | Knockdown of Vascular Endothelial Growth Factor A | Recruiting, no results posted | |
| NCT05903794 | Phase I | EXG102-031 | Not specified | Expressing a fusion protein that is able to bind all subtypes of VEGF as well as the angiopoietin 2 | Recruiting, no results posted | |
| NCT05099094 | Phase I | IDLV | Intravitreal/intracameral/subretinal | IDLV vector is engineered to carry the VEGFA antibody gene | Recruiting, no results posted | |
| NCT05407636 | Phase III | RGX-314 | Subretinal/suprachoroidal | Induction of endogenous anti-VEGF protein | Recruiting, no results posted | |
| NCT04704921 | Phase IIb/III | RGX-314 | Subretinal/suprachoroidal | Induction of endogenous anti-VEGF protein | Recruiting, no results posted | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finocchio, L.; Zeppieri, M.; Gabai, A.; Toneatto, G.; Spadea, L.; Salati, C. Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review. Biomedicines 2023, 11, 3221. https://doi.org/10.3390/biomedicines11123221
Finocchio L, Zeppieri M, Gabai A, Toneatto G, Spadea L, Salati C. Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review. Biomedicines. 2023; 11(12):3221. https://doi.org/10.3390/biomedicines11123221
Chicago/Turabian StyleFinocchio, Lucia, Marco Zeppieri, Andrea Gabai, Giacomo Toneatto, Leopoldo Spadea, and Carlo Salati. 2023. "Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review" Biomedicines 11, no. 12: 3221. https://doi.org/10.3390/biomedicines11123221
APA StyleFinocchio, L., Zeppieri, M., Gabai, A., Toneatto, G., Spadea, L., & Salati, C. (2023). Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review. Biomedicines, 11(12), 3221. https://doi.org/10.3390/biomedicines11123221









