Comparative Analysis of Diagnostic Performance of Automatic Breast Ultrasound, Full-Field Digital Mammography and Contrast-Enhanced Mammography in Relation to Breast Composition
Abstract
:1. Background
2. Materials and Methods
2.1. Histopathological Examinations
2.2. Statistical Methods
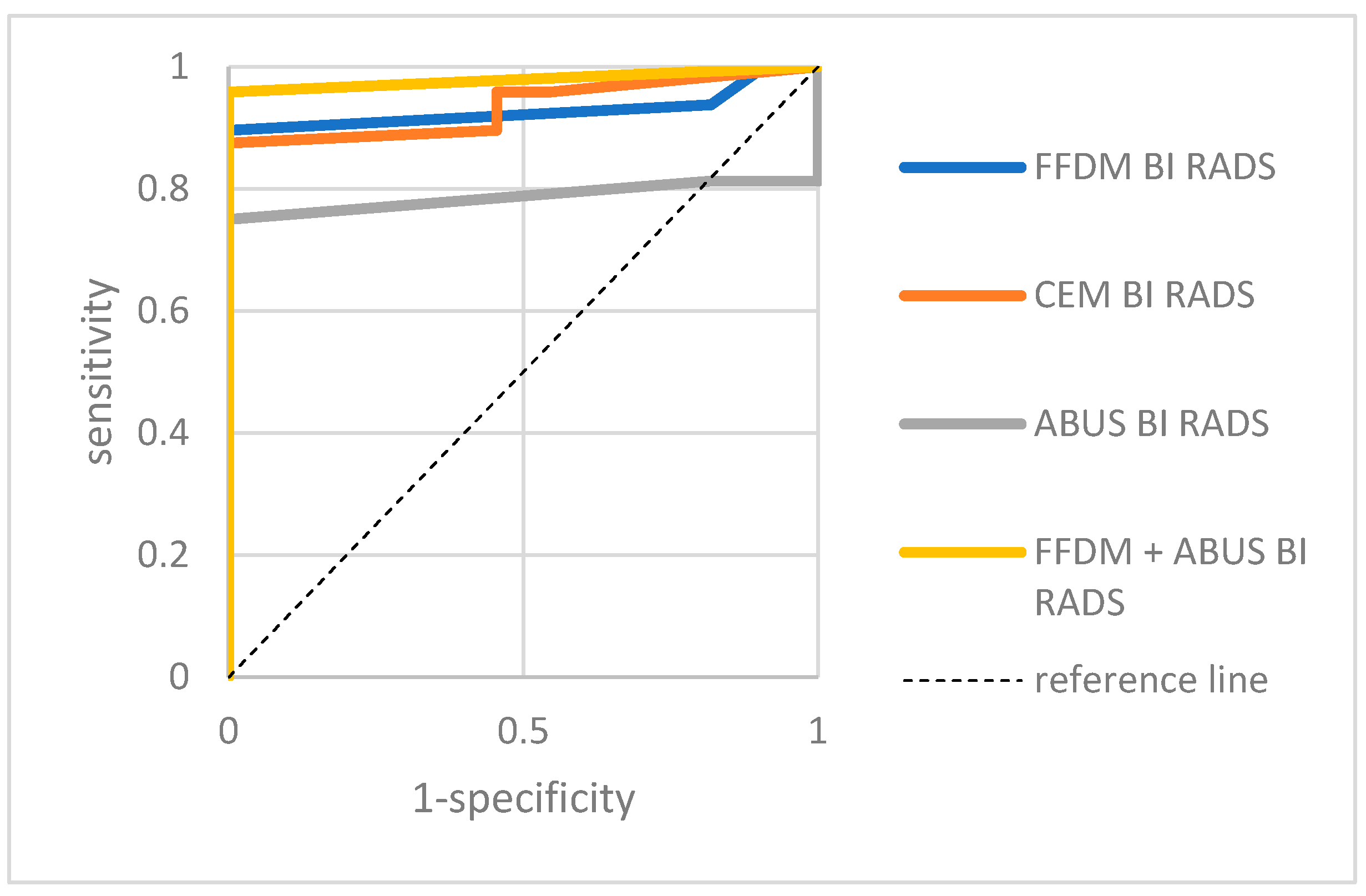
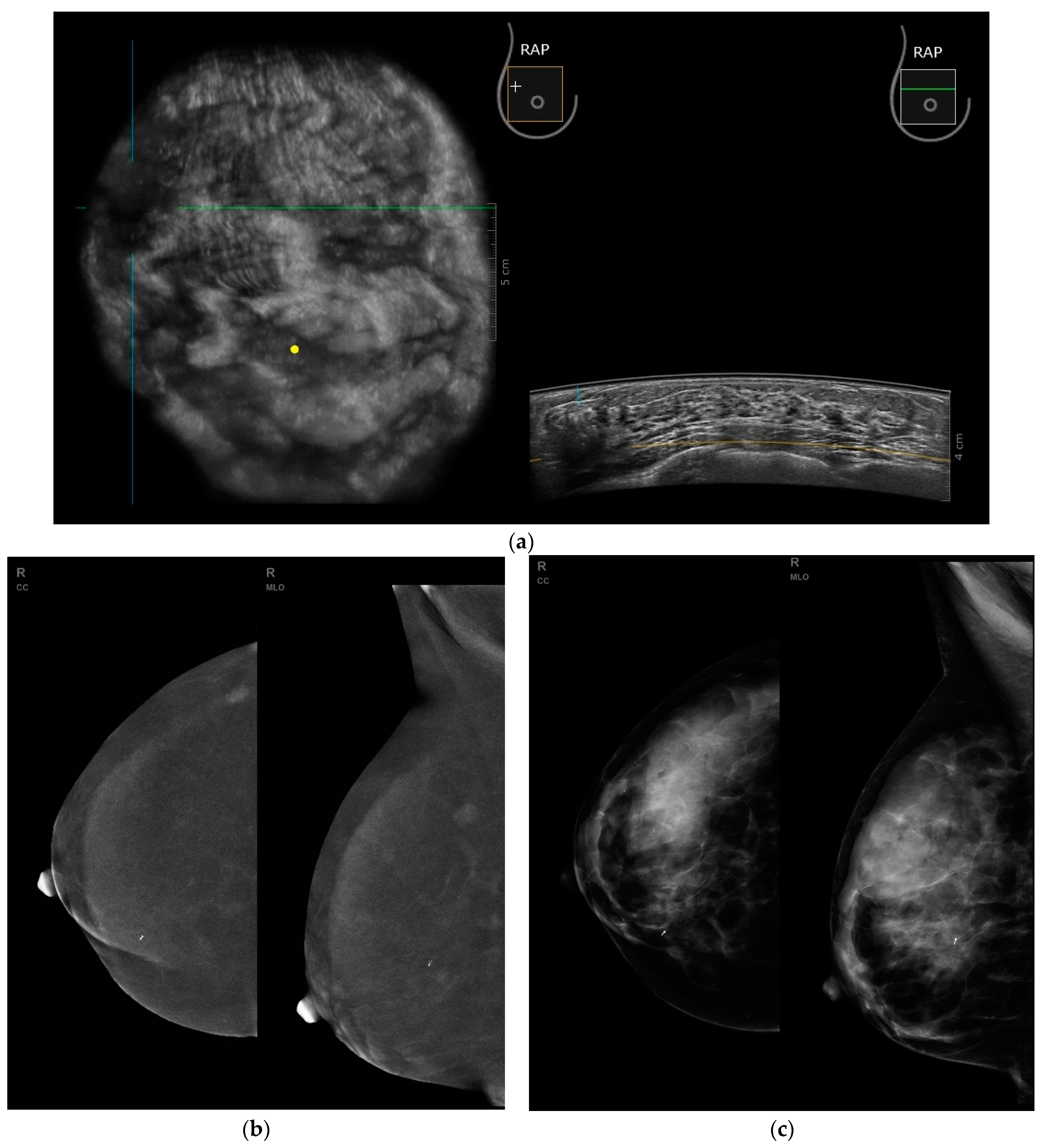
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Weiss, A.; Chavez-MacGregor, M.; Lichtensztajn, D.Y.; Yi, M.; Tadros, A.; Hortobagyi, G.N.; Giordano, S.H.; Hunt, K.K.; Mittendorf, E.A. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared with the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, R.E.; Smith, R.A.; Rutledge, J.H.; Smart, C.R. Benefit of screening mammography in women aged 40–49: A new meta-analysis of randomized controlled trials. J. Natl. Cancer Inst. Monogr. 1997, 22, 87–92. [Google Scholar] [CrossRef]
- Tabár, L.; Vitak, B.; Chen, H.H.; Yen, M.F.; Duffy, S.W.; Smith, R.A. Beyond randomized controlled trials: Organized mammographic screening substantially reduces breast carcinoma mortality. Cancer 2001, 91, 1724–1731. [Google Scholar] [CrossRef]
- Duffy, S.W.; Tabár, L.; Chen, H.H.; Holmqvist, M.; Yen, M.F.; Abdsalah, S.; Epstein, B.; Frodis, E.; Ljungberg, E.; Hedborg-Melander, C. The impact of organized mammography service screening on breast carcinoma mortality in seven Swedish counties. Cancer 2002, 95, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A.; D’Orsi, C.J.; Bassett, L.W.; Appleton, C.M.; Berg, W.A.; Burnside, E.S. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Sprague, B.L.; Gangnon, R.E.; Burt, V.; Trentham-Dietz, A.; Hampton, J.M.; Wellman, R.D.; Kerlikowske, K.; Miglioretti, D.L. Prevalence of mammographically dense breasts in the United States. J. Natl. Cancer Inst. 2014, 106, dju255. [Google Scholar] [CrossRef]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Duffy, S.; Yen, A.M.; Tabár, L.; Smith, R.A.; Chen, H.H. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1219–1228. [Google Scholar] [CrossRef]
- Berg, W.A. Tailored supplemental screening for breast cancer: What now and what next. AJR Am. J. Roentgenol. 2009, 192, 390–399. [Google Scholar] [CrossRef]
- Brekelmans, C.T.; Seynaeve, C.; Bartels, C.C.; Tilanus-Linthorst, M.M.; Meijers-Heijboer, E.J.; Crepin, C.M.; van Geel, A.N.; Menke, M.; Verhoog, L.C.; van Den Ouweland, A.; et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J. Clin. Oncol. 2001, 19, 924–930. [Google Scholar] [CrossRef]
- Chart, P.L.; Franssen, E. Management of women at increased risk for breast cancer: Preliminary results from a new program. CMAJ 1997, 157, 1235–1242. [Google Scholar]
- Macmillan, R.D. Screening women with a family history of breast cancer—Results from the British Familial Breast Cancer Group. Eur. J. Surg. Oncol. 2000, 26, 149–152. [Google Scholar] [CrossRef]
- Scheuer, L.; Kauff, N.; Robson, M.; Kelly, B.; Barakat, R.; Satagopan, J.; Ellis, N.; Hensley, M.; Boyd, J.; Borgen, P.; et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J. Clin. Oncol. 2002, 20, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Plewes, D.B.; Shumak, R.S.; Catzavelos, G.C.; Di Prospero, L.S.; Yaffe, M.J.; Goel, V.; Ramsay, E.; Chart, P.L.; Cole, D.E.; et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J. Clin. Oncol. 2001, 19, 3524–3531. [Google Scholar] [CrossRef] [PubMed]
- ECIBC Guideline Development Group (GDG); Borisch, B.; Broeders, M.; Castells, X.; Daneš, X.; Duffy, S.; Fitzpatrick, P.; Follmann, M.; Giordano, L.; Rossi, P.; et al. Should Digital Breast Tomosynthesis (DBT) (Including Synthesized 2D Images) vs. Digital Mammography Be Used in Organised Screening Programmes for Early Detection of Breast Cancer in Women with High Mammographic Breast Density Detected in Previous Screening Exams? Available online: https://dbep.ec.gdt.evidenceprime.com/profile/Z0nwFlhjPCg (accessed on 21 September 2021).
- Luczyńska, E.; Heinze, S.; Adamczyk, A.; Rys, J.; Mitus, J.W.; Hendrick, E. Comparison of the Mammography, Contrast-Enhanced Spectral Mammography and Ultrasonography in a Group of 116 patients. Anticancer Res. 2016, 36, 4359–4366. [Google Scholar] [PubMed]
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sklair-Levy, M.; Sorin, V.; Yagil, Y.; Shalmon, A.; Samoocha, D. Contrast Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breast. Harefuah 2022, 161, 89–94. [Google Scholar] [PubMed]
- Patel, B.K.; Garza, S.A.; Eversman, S.; Lopez-Alvarez, Y.; Kosiorek, H.; Pockaj, B.A. Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin. Imaging 2017, 46, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hagen, A.I.; Kvistad, K.A.; Maehle, L.; Holmen, M.M.; Aase, H.; Styr, B.; Vabø, A.; Apold, J.; Skaane, P.; Møller, P. Sensitivity of MRI versus conventional screening in the diagnosis of BRCA-associated breast cancer in a national prospective series. Breast 2007, 16, 367–374. [Google Scholar] [CrossRef]
- Hartman, A.R.; Daniel, B.L.; Kurian, A.W.; Mills, M.A.; Nowels, K.W.; Dirbas, F.M.; Kingham, K.E.; Chun, N.M.; Herfkens, R.J.; Ford, J.M.; et al. Breast magnetic resonance image screening and ductal lavage in women at high genetic risk for breast carcinoma. Cancer 2004, 100, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Muller, S.H.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Rutgers, E.J.; de Koning, H.J.; et al. Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition. Cancer 2006, 106, 2318–2326. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.O.; Boggis, C.R.; Dixon, A.K.; Easton, D.F.; Eeles, R.A.; Evans, D.G.; Gilbert, F.J.; Griebsch, I.; Hoff, R.J.; Kessar, P.; et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005, 365, 1769–1778. [Google Scholar]
- Lehman, C.D.; Isaacs, C.; Schnall, M.D.; Pisano, E.D.; Ascher, S.M.; Weatherall, P.T.; Bluemke, D.A.; Bowen, D.J.; Marcom, P.K.; Armstrong, D.K.; et al. Cancer yield of mammography, MR, and US in high-risk women: Prospective multi-institution breast cancer screening study. Radiology 2007, 244, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Podo, F.; D’Agnolo, G.; Verdecchia, A.; Santaquilani, M.; Musumeci, R.; Trecate, G.; Manoukian, S.; Morassut, S.; de Giacomi, C.; et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): Interim results. Radiology 2007, 242, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.; Plewes, D.B.; Hill, K.A.; Causer, P.A.; Zubovits, J.T.; Jong, R.A.; Cutrara, M.R.; DeBoer, G.; Yaffe, M.J.; Messner, S.J.; et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004, 292, 1317–1325. [Google Scholar] [CrossRef]
- Lee, C.H.; Dershaw, D.D.; Kopans, D.; Evans, P.; Monsees, B.; Monticciolo, D.; Brenner, R.J.; Bassett, L.; Berg, W.; Feig, S.; et al. Breast cancer screening with imaging: Recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J. Am. Coll. Radiol. 2010, 7, 18–27. [Google Scholar] [CrossRef]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- Yuan, W.H.; Hsu, H.C.; Chen, Y.Y.; Wu, C.H. Supplemental breast cancer-screening ultrasonography in women with dense breasts: A systematic review and meta-analysis. Br. J. Cancer 2020, 123, 673–688. [Google Scholar] [CrossRef]
- Berg, W.A.; Blume, J.D.; Cormack, J.B.; Mendelson, E.B.; Lehrer, D.; Böhm-Vélez, M.; Pisano, E.D.; Jong, R.A.; Evans, W.P.; Morton, M.J.; et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008, 299, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Vourtsis, A. Three-dimensional automated breast ultrasound: Technical aspects and first results. Diagn. Interv. Imaging 2019, 100, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Karst, I.; Henley, C.; Gottschalk, N.; Floyd, S.; Mendelson, E.B. Three-dimensional Automated Breast US: Facts and Artifacts. Radiographics 2019, 39, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Ferrari, F.; Bozzini, A.C.; Latronico, A.; Trentin, C.; Meneghetti, L.; Pesapane, F.; Pizzamiglio, M.; Balesetreri, N.; Cassano, E. Automatic breast ultrasound: State of the art and future perspectives. Ecancermedicalscience 2020, 14, 1062. [Google Scholar] [CrossRef]
- Freer, P.E. Mammographic breast density: Impact on breast cancer risk and implications for screening. Radiographics 2015, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Lin, X.; Zhou, X.; Yan, H.; Chen, Y.; Liu, P.; Bao, L.; Li, A.; Basu, P.; Qiao, Y.; et al. Diagnostic performance of automated breast ultrasound and handheld ultrasound in women with dense breasts. Breast Cancer Res. Treat. 2020, 181, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Van Zelst, J.C.; Tan, T.; Platel, B.; De Jong, M.; Steenbakkers, A.; Mourits, M.; Grivegnee, A.; Borelli, C.; Karssemeijer, N.; Mann, R.M. Improved cancer detection in automated breast ultrasound by radiologists using Computer Aided Detection. Eur. J. Radiol. 2017, 89, 54–59. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.H.; Kang, B.J.; Kim, Y.J. Interobserver agreement on the interpretation of automated whole breast ultrasonography. Ultrasonography 2014, 33, 252–258. [Google Scholar] [CrossRef]
- Wilczek, B.; Wilczek, H.E.; Rasouliyan, L.; Leifland, K. Adding 3D automated breast ultrasound to mammography screening in women with heterogeneously and extremely dense breasts: Report from a hospital-based, high-volume, single-center breast cancer screening program. Eur. J. Radiol. 2016, 85, 1554–1563. [Google Scholar] [CrossRef]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef]
- Kelly, K.M.; Dean, J.; Comulada, W.S.; Lee, S.J. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur. Radiol. 2010, 20, 734–742. [Google Scholar] [CrossRef]
- Giuliano, V.; Giuliano, C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging 2013, 37, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Giger, M.L.; Inciardi, M.F.; Edwards, A.; Papaioannou, J.; Drukker, K.; Jiang, Y.; Brem, R.; Brown, J.B. Automated Breast Ultrasound in Breast Cancer Screening of Women With Dense Breasts: Reader Study of Mammography-Negative and Mammography-Positive Cancers. Am. J. Roentgenol. 2016, 206, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.K.; Shen, Y.W.; Huang, C.S.; Luo, S.C.; Kuzucan, A.; Chen, J.H.; Chang, R.F. Comparative study of density analysis using automated whole breast ultrasound and MRI. Med. Phys. 2011, 38, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.K.; Lo, C.M.; Chang, J.M.; Bae, M.S.; Kim, W.H.; Huang, C.S.; Chen, J.H.; Kuo, M.H.; Chang, R.F. Rapid breast density analysis of partial volumes of automated breast ultrasound images. Ultrason. Imaging 2013, 35, 333–343. [Google Scholar] [CrossRef]
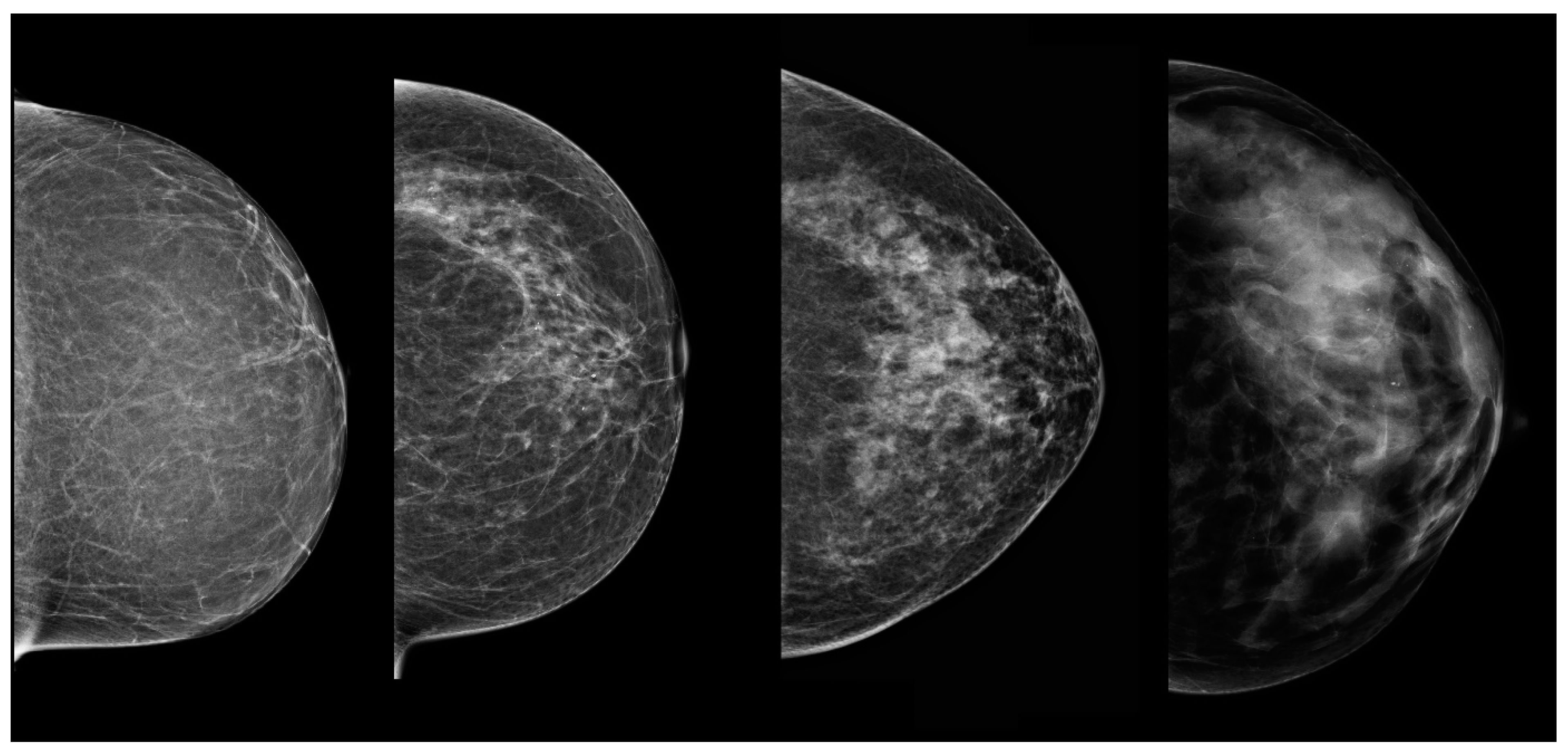

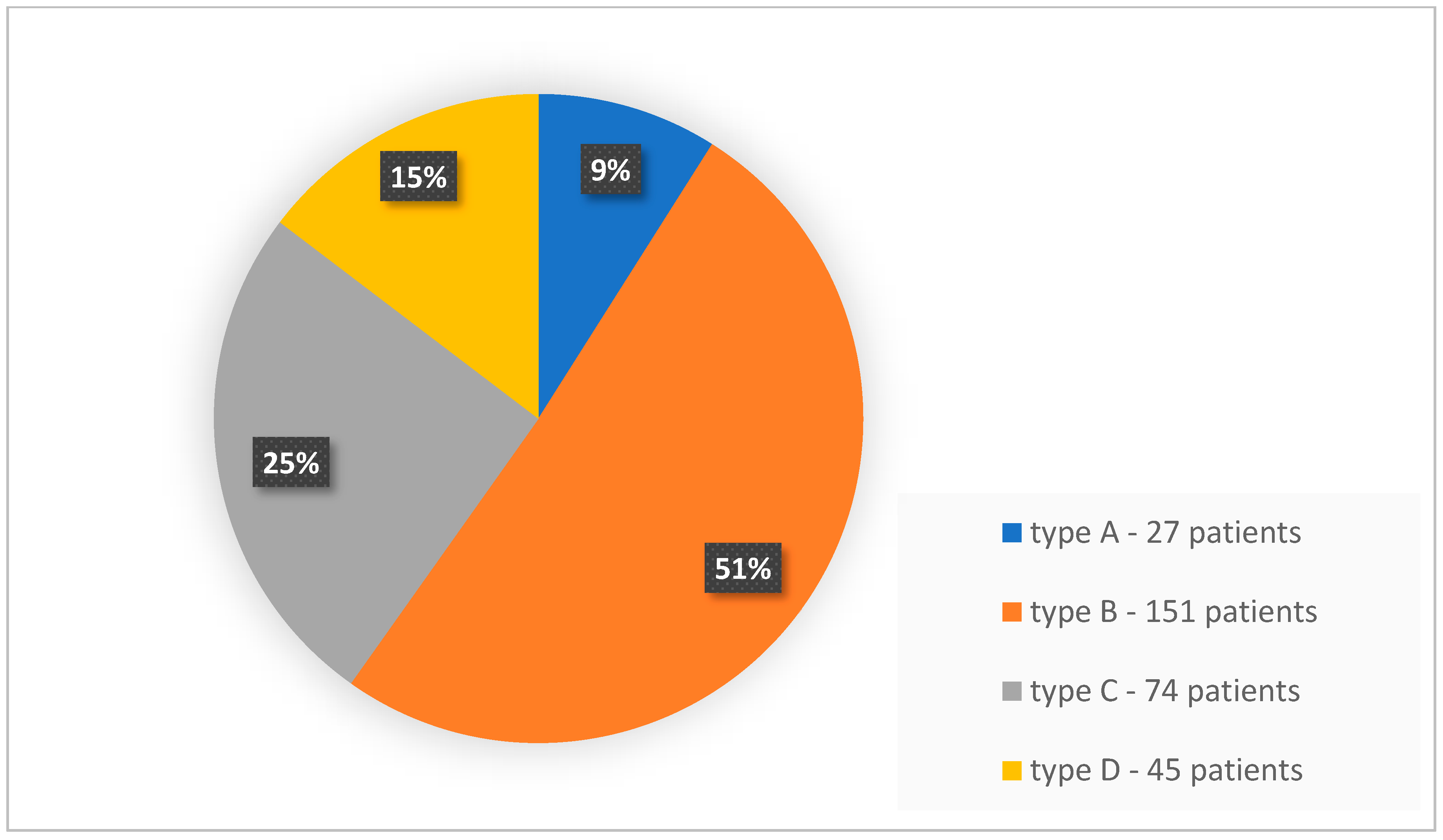
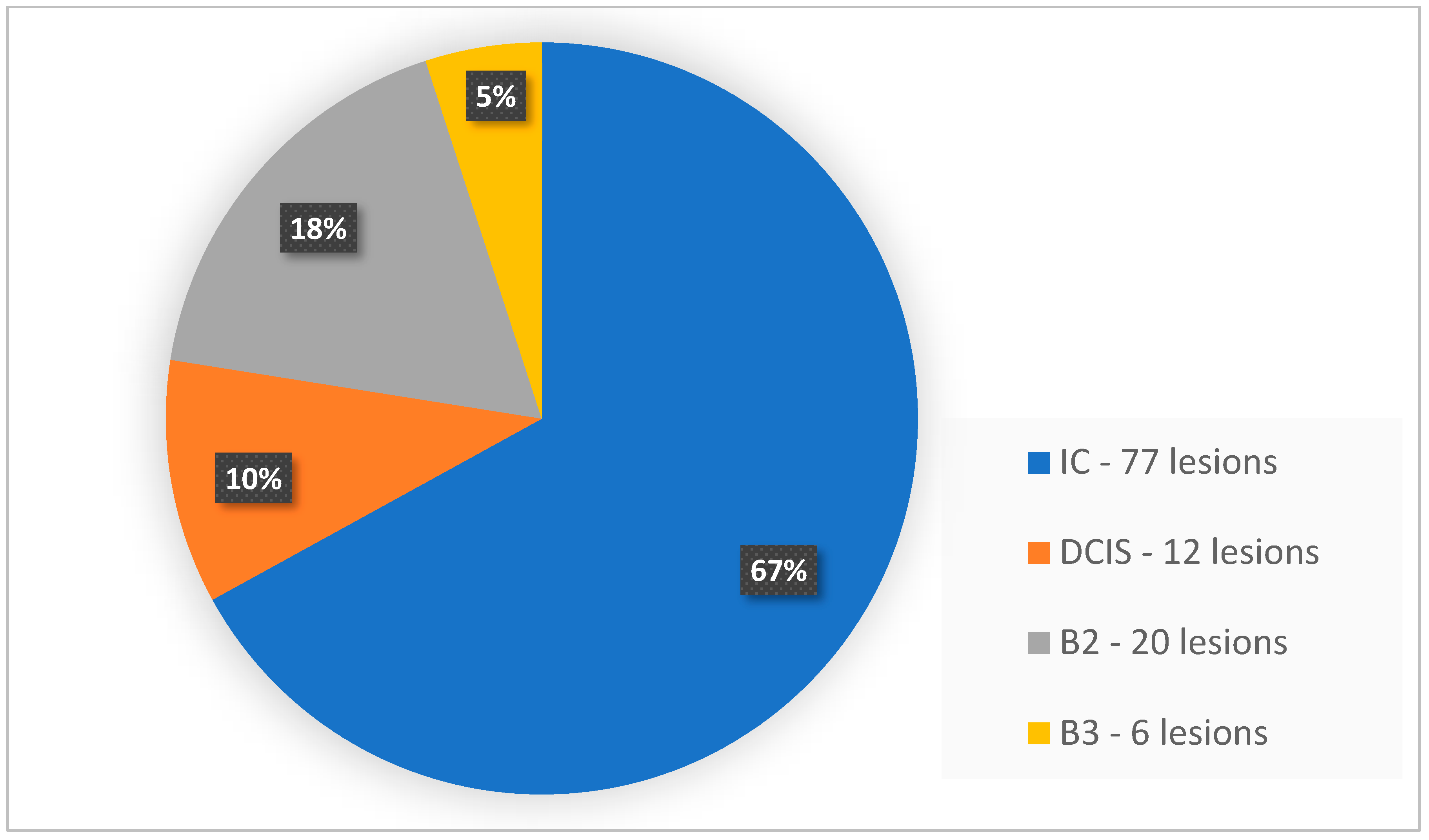
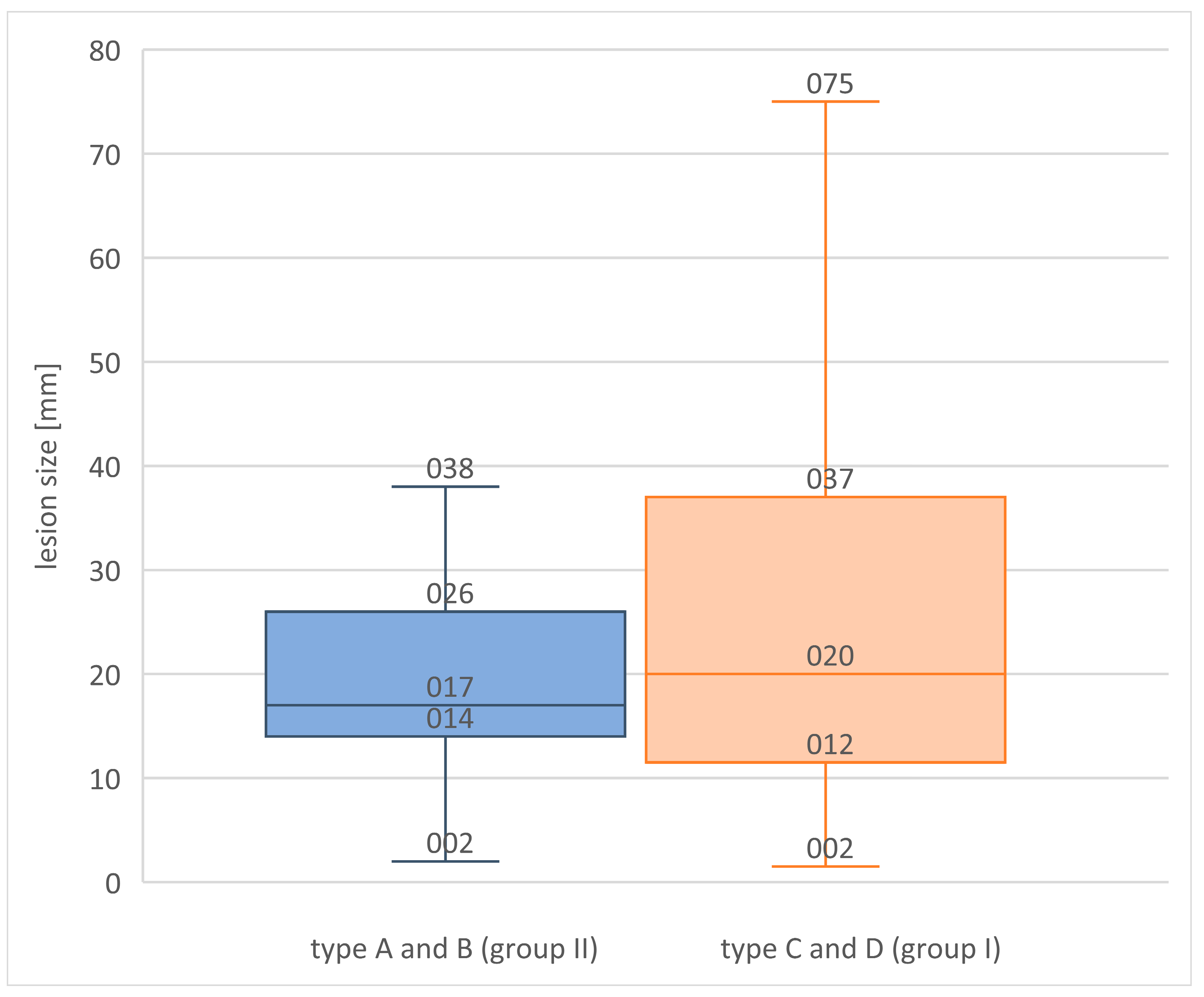

| No. of Lesions in a Patient (Any Method) | No. of Patients | Total No. of Lesions | Lesions Visible with FFDM | Lesions Visible with ABUS | Lesions Visible with CEM |
|---|---|---|---|---|---|
| 2 | 3 | 6 | 2 cases (2 patients with 1 lesion) | 5 cases (including 2 patients with 2 lesions and 1 patient with 1 lesion => 3 patients) | 5 cases (including 1 patient with 2 lesions and 3 patients with 1 lesion => 4 patients) |
| 3 | 4 | 12 | 4 cases (including 2 patients with 2 lesions) | 10 cases (3 patients with 3 lesions and 1 patient with 1 lesion => 4 patients) | 4 cases (including 1 patient with 2 lesions and 2 patients with 1 lesion => 3 patients) |
| 4 | 5 | 20 | 6 cases (including 2 patients with 2 lesions and 2 patients with 1 lesion => 4 patients) | 15 cases (including 2 patients with 4 lesions, 1 patient with 3 lesions and 2 patients with 2 lesions => 5 patients) | 11 cases (including 1 patient with 4 lesions, 2 patients with 3 lesions and 1 patient with 1 lesion => 4 patients) |
| 5 | 2 | 10 | 1 case (1 patient with 1 lesion) | 10 cases (2 patients with 5 lesions) | 1 case (1 patient with 1 lesion) |
| Total | 14 | 48 | 13 cases => 9 patients | 40 cases => 14 patients | 21 cases (12 patients) |
| Specification | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| ABUS | 78.05 [65.38; 90.72] | 40 [15.21; 64.79] | 78.05 [65.38; 90.72] | 40 [15.21; 64.79] | 67.86 [55.63; 80.09] |
| FFDM | 85.37 [74.55; 96.18] | 13.33 [0; 30.54] | 72.92 [60.34; 85.49] | 25 [0; 55.01] | 66.07 [53.67; 78.47] |
| CEM | 92.68 [84.71; 100] | 8.33 [0; 23.97] | 77.55 [65.87; 89.23] | 25 [0; 67.44] | 73.58 [61.72; 85.45] |
| FFDM + ABUS | 100 | 6.67 [0; 19.29] | 74.55 [63.03; 86.06] | 100 | 75 [0; 100] |
| p (ABUS vs. FFDM) | 0.606 | 0.22 | 0.575 | 0.472 | 0.841 |
| p (ABUS vs. CEM) | 0.149 | 0.37 | 0.954 | 0.567 | 0.512 |
| p (FFDM vs. CEM) | 0.248 | 1 | 0.597 | 1 | 0.393 |
| p (FFDM + ABUS vs. CEM) | 0.248 | 0.479 | 0.719 | 0.171 | 0.866 |
| p (FFDM + ABUS vs. FFDM) | 0.04 | 1 | 0.851 | 0.134 | 0.299 |
| Specification | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| ABUS | 81.25 [70.21; 92.29] | 18.18 [0; 40.97] | 81.25 [70.21; 92.29] | 18.18 [0; 40.97] | 69.49 [57.74; 81.24] |
| FFDM | 93.75 [86.9; 100] | 18.18 [0; 40.97] | 83.33 [73.39; 93.27] | 40 [0; 82.94] | 79.66 [69.39; 89.93] |
| CEM | 93.48 [86.34; 100] | 16.67 [0; 46.49] | 89.58 [80.94; 98.23] | 25 [0; 67.44] | 84.62 [74.81; 94.42] |
| FFDM + ABUS | 100 [100; 100] | - | 81.36 [71.42; 91.29] | - | 81.36 [71.42; 91.29] |
| p (ABUS vs. FFDM) | 0.150 | 0.617 | 0.783 | 0.350 | 0.205 |
| p (ABUS vs. CEM) | 0.150 | 0.480 | 0.247 | 0.770 | 0.056 |
| p (FFDM vs. CEM) | 1 | 1 | 0.359 | 0.635 | 0.498 |
| p (FFDM + ABUS vs. CEM) | 0.248 | - | 0.235 | - | 0.816 |
| p (FFDM + ABUS vs. FFDM) | 0.248 | - | 0.783 | - | 0.649 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak, M.E.; Rudnicki, W.; Borkowska, A.; Skubisz, K.; Rydzyk, R.; Łuczyńska, E. Comparative Analysis of Diagnostic Performance of Automatic Breast Ultrasound, Full-Field Digital Mammography and Contrast-Enhanced Mammography in Relation to Breast Composition. Biomedicines 2023, 11, 3226. https://doi.org/10.3390/biomedicines11123226
Pawlak ME, Rudnicki W, Borkowska A, Skubisz K, Rydzyk R, Łuczyńska E. Comparative Analysis of Diagnostic Performance of Automatic Breast Ultrasound, Full-Field Digital Mammography and Contrast-Enhanced Mammography in Relation to Breast Composition. Biomedicines. 2023; 11(12):3226. https://doi.org/10.3390/biomedicines11123226
Chicago/Turabian StylePawlak, Marta Ewa, Wojciech Rudnicki, Anna Borkowska, Karolina Skubisz, Rafał Rydzyk, and Elżbieta Łuczyńska. 2023. "Comparative Analysis of Diagnostic Performance of Automatic Breast Ultrasound, Full-Field Digital Mammography and Contrast-Enhanced Mammography in Relation to Breast Composition" Biomedicines 11, no. 12: 3226. https://doi.org/10.3390/biomedicines11123226
APA StylePawlak, M. E., Rudnicki, W., Borkowska, A., Skubisz, K., Rydzyk, R., & Łuczyńska, E. (2023). Comparative Analysis of Diagnostic Performance of Automatic Breast Ultrasound, Full-Field Digital Mammography and Contrast-Enhanced Mammography in Relation to Breast Composition. Biomedicines, 11(12), 3226. https://doi.org/10.3390/biomedicines11123226







