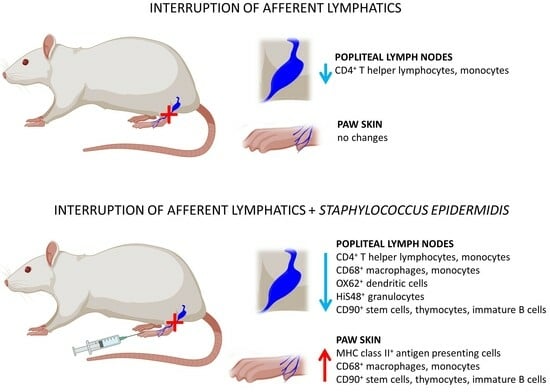
Interruption of Lymph Flow Worsens the Skin Inflammation Caused by Saprophytic Staphylococcus epidermidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interruption of Afferent Lymphatics
2.2. Cell Isolation and Flow Cytometry
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Lymph Node Mass and Cell Number
3.2. Quantitative Changes in Cell Subpopulations in the Lymph Node
3.3. Quantitative Changes in Cell Subpopulations in the Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Engelhard, V.H. Lymphatic endothelial cells—Key players in regulation of tolerance and immunity. Front. Immunol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Teijeira, A.; Russo, E.; Halin, C. Taking the lymphatic route: Dendritic cell migration to draining lymph nodes. Semin. Immunopathol. 2014, 36, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Teijeira, A.; Halin, C. T cell trafficking through lymphatic vessels. Front. Immunol. 2016, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Baik, J.E.; Park, H.J.; Wiser, I.; Rehal, S.; Shin, J.Y.; Mehrara, B.J. Regulation of immune function by the lymphatic system in lymphedema. Front. Immunol. 2019, 10, 470. [Google Scholar] [CrossRef]
- Corthésy-Theulaz, I.E.; Hopkins, S.; Bachmann, D.; Saldinger, P.F.; Porta, N.; Haas, R.; Zheng-Xin, Y.; Meyer, T.; Bouzourène, H.; Blum, A.L.; et al. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect. Immun. 1998, 66, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Lynskey, N.N.; Banerji, S.; Johnson, L.A.; Holder, K.A.; Reglinski, M.; Wing, P.A.; Rigby, D.; Jackson, D.G.; Sriskandan, S. Rapid lymphatic dissemination of encapsulated group A Streptococci via lymphatic vessel endothelial receptor-1 interaction. PLoS Pathog. 2015, 11, e1005137. [Google Scholar] [CrossRef] [PubMed]
- Von Bargen, K.; Gagnaire, A.; Arce-Gorvel, V.; de Bovis, B.; Baudimont, F.; Chasson, L.; Bosilkovski, M.; Papadopoulos, A.; Martirosyan, A.; Henri, S.; et al. Cervical lymph nodes as a selective niche for Brucella during oral infections. PLoS ONE. 2015, 10, e0121790. [Google Scholar] [CrossRef]
- Timm, C.M.; Loomis, K.; Stone, W.; Mehoke, T.; Brensinger, B.; Pellicore, M.; Staniczenko, P.P.A.; Charles, C.; Nayak, S.; Karig, D.K. Isolation and characterization of diverse microbial representatives from the human skin microbiome. Microbiome 2020, 8, 58. [Google Scholar] [CrossRef]
- Olszewski, W.L.; Zaleska, M.T. Inflammation. In Peripheral Lymphedema; Liu, N., Ed.; Springer: Singapore, 2021; pp. 119–130. [Google Scholar] [CrossRef]
- Cąkała-Jakimowicz, M.; Puzianowska-Kuznicka, M. Towards understanding the lymph node response to skin infection with saprophytic Staphylococcus epidermidis. Biomedicines 2022, 10, 1021. [Google Scholar] [CrossRef]
- Hayes, S.C.; Janda, M.; Cornish, B.H.; Battistutta, D.; Newman, B. Lymphedema secondary to breast cancer: How choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology 2008, 41, 18–28. [Google Scholar]
- Waltham, M. Lymphoedema—Principles, genetics and pathophysiology. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 497–509. [Google Scholar]
- Oropallo, A.; Donis-Garcia, M.; Ahn, S.; Rao, A. Current concepts in the diagnosis and management of lymphedema. Adv. Skin. Wound Care 2020, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.S.; Rockson, S.G. New developments in clinical aspects of lymphatic disease. Clin. Investig. 2014, 124, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.L.; Zaleska, M.T. Diagnosis and management of infection in lymphedema. In Lymphedema; Lee, B.B., Rockson, S., Bergan, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 465–480. [Google Scholar] [CrossRef]
- Hara, H.; Mihara, M. Bacterial flora in the genital area of patients with lower limb lymphedema. Lymphat. Res. Biol. 2020, 18, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.G. Cellulitis: Definition, etiology, and clinical features. Am. J. Med. 2011, 124, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.L.; Zaleska, M.; Stelmach, E.; Swoboda-Kopec, E.; Jain, P.; Agrawal, K.; Gogia, S.; Gogia, A.; Andziak, P.; Durlik, M. Cryptic bacteria of lower limb deep tissues as a possible cause of inflammatory and necrotic changes in ischemia, venous stasis and varices, and lymphedema. Surg. Infect. 2015, 16, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.R.; Hsieh, F.; Huang, C.T.; Tsai, T.J.; Chen, C.; Cheng, M.H. Clinical features, microbiological epidemiology and recommendations for management of cellulitis in extremity lymphedema. J. Surg. Oncol. 2020, 121, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Inflammatory manifestations of lymphedema. Int. J. Mol. Sci. 2017, 18, 171. [Google Scholar] [CrossRef]
- Galkowska, H.; Olszewski, W.L. Cellular composition of lymph in experimental lymphedema. Lymphology 1986, 19, 139–145. [Google Scholar]
- Wireko, S.; Asiedu, S.; Kini, P.; Aglomasa, B.; Amewu, E.; Asiedu, E.; Osei-Akoto, F.; Boahen, K.; Obiri-Yeboah, D.; Amato, K.; et al. Prevalence of methicillin-resistant Staphylococcus species among filarial lymphedema patients in Ahanta West District of Ghana. Front. Tropical Dis. 2021, 2, 786378. [Google Scholar] [CrossRef]
- Olszewski, W.L.; Jamal, S.; Manokaran, G.; Pani, S.; Kumaraswami, V.; Kubicka, U.; Lukomska, B.; Dworczynski, A.; Swoboda, E.; Meisel-Mikolajczyk, F. Bacteriologic studies of skin, tissue fluid, lymph, and lymph nodes in patients with filarial lymphedema. Am. J. Trop. Med. Hyg. 1997, 57, 7–15. [Google Scholar] [CrossRef]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Masamatti, S.S.; Narasimha, A.; Janardhan, J.V.; Chowdappa, V. Lymph node fibrosis in a case of primary lymphoedema—A report of two cases. J. Clin. Diag Res. 2016, 10, ED08–ED09. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Eskin-Schwartz, M.; Mimouni, D.; Ray, S.; Days, W.; Hodak, E.; Leibovici, L.; Paul, M. Interventions for the prevention of recurrent erysipelas and cellulitis. Cochrane Database Syst. Rev. 2017, 6, CD009758. [Google Scholar] [CrossRef] [PubMed]
- Collazos, J.; de la Fuente, B.; García, A.; Gómez, H.; Menéndez, C.; Enríquez, H.; Sánchez, P.; Alonso, M.; López-Cruz, I.; Martín-Regidor, M.; et al. Cellulitis in adult patients: A large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS ONE 2018, 13, e0204036. [Google Scholar] [CrossRef]
- Olszewski, W.L.; Zaleska, M.T. Long-term benzathine penicillin prophylaxis lasting for years effectively prevents recurrence of dermato-lymphangio-adenitis (cellulitis) in limb lymphedema. Lymphat. Res. Biol. 2021, 19, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Chaniotakis, I.; Ioannis, D.B. The first disease episode: A strategic treatment target to prevent cellulitis relapses. Dermatology 2021, 237, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Cąkała, M.; Olszewski, W.L. The response of the lymphatic system to the human skin resident bacteria. Ann. Transplant. 2002, 7, 30–35. [Google Scholar]
- García Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4+ T cells are activated in region al lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef]
- Zampell, J.C.; Avraham, T.; Yoder, N.; Fort, N.; Yan, A.; Weitman, E.S.; Mehrara, B.J. Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti-lymphangiogenic cytokines. Am. J. Physiol. Cell Physiol. 2012, 302, C392–C404. [Google Scholar] [CrossRef] [PubMed]
- Künzli, M.; Masopust, D. CD4+ T cell memory. Nat. Immunol. 2023, 24, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, S.; Ding, X.; Li, S.; Luo, X.; Cao, Y.; Gao, F.; Zou, M. Research progress on the mechanism by which skin macrophage dysfunction mediates chronic inflammatory injury in diabetic skin. Front. Endocrinol. 2022, 13, 960551. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, R.; Forde, A.J.; Lohrmann, F.; Kolter, J.; Ramirez, N.J.; Zimmermann, J.; Gomez de Agüero, M.; Henneke, P. Resident macrophages acquire innate immune memory instaphylococcal skin infection. eLife 2020, 9, e55602. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W.L.; Engeset, A.; Romaniuk, A.; Grzelak, I.; Ziolkowska, A. Immune cells in peripheral lymph and skin of patients with obstructive lymphedema. Lymphology 1990, 23, 23–33. [Google Scholar]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Moya, M.; Johannes, J.; Goldman, J.; Swartz, M.A. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc. Res. 2006, 72, 161–171. [Google Scholar] [CrossRef]
- Hampton, H.R.; Bailey, J.; Tomura, M.; Brink, R.; Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat. Commun. 2015, 6, 7139. [Google Scholar] [CrossRef]
- Tzani, I.; Tsichlaki, M.; Zerva, E.; Papathanasiou, G.; Dimakakos, E. Physiotherapeutic rehabilitation of lymphedema: State-of-the-art. Lymphology 2018, 51, 1–12. [Google Scholar] [PubMed]











| Experimental Group | Procedure |
|---|---|
| Interruption of lymphatics n = 6 rats (12 lymph nodes, 4 paw skin biopsies) |
|
| Interruption of lymphatics + S. epidermidis infection n = 6 rats (12 lymph nodes, 4 paw skin biopsies) |
|
| Cell Type | Antigen (Rat Clone) |
|---|---|
| T lymphocytes | CD43+ (W3/13+) |
| T helper lymphocytes, monocytes | CD4+ (W3/25+) |
| T cytotoxic lymphocytes | CD8+ (OX8+) |
| B lymphocytes | CD19+ (OX12+) |
| Dendritic cells | OX62+ |
| Macrophages, monocytes | CD68+ (ED1+) |
| Stem cells, thymocytes, immature B lymphocytes | CD90+ (OX7+) |
| Granulocytes | HiS48+ |
| Activated antigen-presenting cells | MHC class II+ (OX6+) |
| ICAM-1, dendritic cells, endothelial cells | CD54+ |
| Activated T lymphocytes | CD43+ MHC class II+ |
| Activated helper T lymphocytes | CD4+ MHC class II+ |
| Activated cytotoxic T lymphocytes | CD8+ MHC class II+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cąkała-Jakimowicz, M.; Domaszewska-Szostek, A.; Puzianowska-Kuznicka, M. Interruption of Lymph Flow Worsens the Skin Inflammation Caused by Saprophytic Staphylococcus epidermidis. Biomedicines 2023, 11, 3234. https://doi.org/10.3390/biomedicines11123234
Cąkała-Jakimowicz M, Domaszewska-Szostek A, Puzianowska-Kuznicka M. Interruption of Lymph Flow Worsens the Skin Inflammation Caused by Saprophytic Staphylococcus epidermidis. Biomedicines. 2023; 11(12):3234. https://doi.org/10.3390/biomedicines11123234
Chicago/Turabian StyleCąkała-Jakimowicz, Marta, Anna Domaszewska-Szostek, and Monika Puzianowska-Kuznicka. 2023. "Interruption of Lymph Flow Worsens the Skin Inflammation Caused by Saprophytic Staphylococcus epidermidis" Biomedicines 11, no. 12: 3234. https://doi.org/10.3390/biomedicines11123234
APA StyleCąkała-Jakimowicz, M., Domaszewska-Szostek, A., & Puzianowska-Kuznicka, M. (2023). Interruption of Lymph Flow Worsens the Skin Inflammation Caused by Saprophytic Staphylococcus epidermidis. Biomedicines, 11(12), 3234. https://doi.org/10.3390/biomedicines11123234