Cannabidiol Modifies the Glutamate Over-Release in Brain Tissue of Patients and Rats with Epilepsy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Collection of Tissue
2.2. Evaluation of the Effect of CBD on the Evoked Glutamate Release in Synaptic Terminals Obtained from Patients with DRE
2.2.1. Isolation and Purification of Synaptosomes
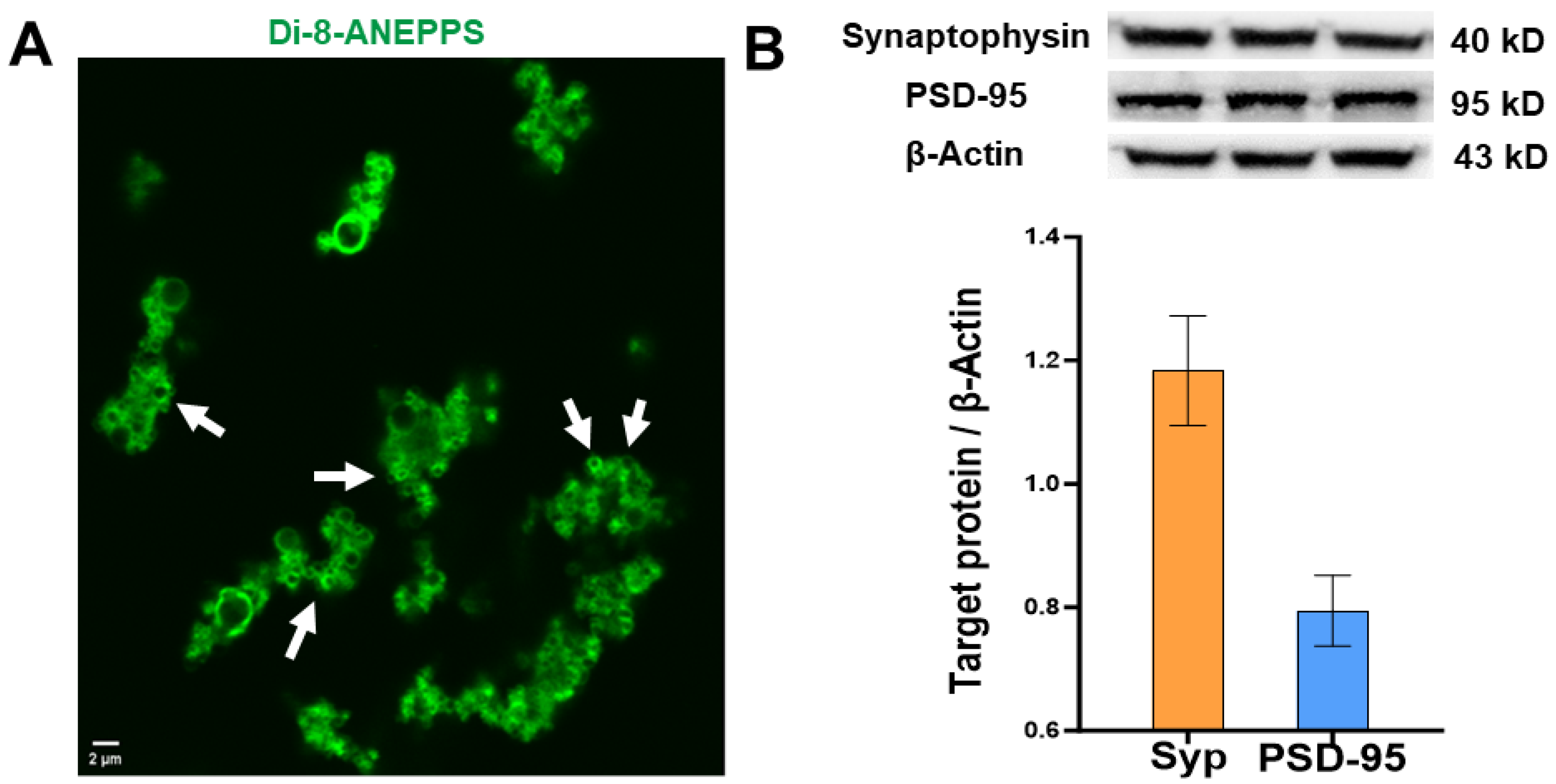
2.2.2. Characterization of Synaptosomes by Confocal Microscopy
2.2.3. Analysis of Protein Expression of Synaptophysin and Postsynaptic Density Protein 95 in Human Synaptosomes
2.2.4. Evaluation of Glutamate Release Evoked by High KCl in Human Synaptosomes
- (a)
- Evoked glutamate release in the presence of CBD. Independent aliquots were incubated with CBD at different concentrations (100 nM, 1 µM, 10 µM, 100 µM, and 1 mM) at 24 °C for 15 min. Then, KCl was added to the preparation to achieve a final concentration of 33 mM and was incubated at 24 °C for 2 min. Afterward, the samples were centrifuged at 21,000× g for 5 min at 4 °C. The supernatant was collected and stored at −80 °C until the analysis of glutamate release by HPLC (see below).
- (b)
- Evoked glutamate release in the absence of CBD. A parallel fraction from the same tissue was handled as indicated previously, except that the incubation was carried out with 50 µL of sodium dodecyl sulfate (DMSO 0.5%, vehicle of CBD) instead of CBD.
- (c)
- Basal glutamate release. An independent aliquot was manipulated similarly to the fraction used in (b), except that it was incubated with 5 µL of ASCF instead of KCl 33 mM.
2.2.5. Quantification of Glutamate by High-Performance Liquid Chromatography (HPLC)
2.3. Evaluation of Viability of the Neocortical Epileptic Tissue by In Vitro Electrophysiology
2.3.1. Whole Cell Recordings
2.3.2. Electrophysiological Evaluation
2.3.3. Extracellular Recordings
2.4. Evaluation of the Effect of Subchronic Treatment with CBD on Glutamate Over-Release in the Hippocampus of Rats with Temporal Lobe Epilepsy
2.4.1. Animals
2.4.2. Induction of Status Epilepticus and Spontaneous Recurrent Seizures by Lithium-Pilocarpine
2.4.3. Experimental Groups
- (a)
- SRS-CBD group (n = 7). Rats received CBD 200 mg/kg, p.o. every 24 h for 7 days. Twenty-four hours after the last administration of CBD, the animals were subjected to microdialysis experiments to determine the interictal extracellular levels of glutamate in the hippocampus. A microdialysis cannula designed according to Maidment et al., 1989 [25], with a 3 mm active part of polyacrylonitrile (40 kDa pore), was inserted through the guide cannula and implanted in the left dorso-ventral hippocampus. The microdialysis cannula was constantly perfused with fresh and sterile ACSF (see Section 2.2.1) at a flow rate of 2 µL/min. Two hours after the probe implantation, recovery of the dialysates was carried out every 30 min for 2 h. Dialysates were processed for glutamate quantification by HPLC as previously described (see Section 2.2.5). Twenty-four hours after the end of the microdialysis experiment, rats were sacrificed with an overdose of pentobarbital (70 mg/kg i.p.), and their brains were used to determine the location of the microdialysis probes by Nissl staining.
- (b)
- SRS-Vh group (n = 7). The animals were handled in a similar way to the SRS-CBD group, with the exception that they received a vehicle (coconut oil 9.52 mL/kg, p.o.) instead of CBD.
- (c)
- Sham-CBD group (n = 7). Rats were handled similarly to the SRS-CBD group, except that saline (1 mL/kg, i.p.) was applied instead of lithium chloride, methyl scopolamine, and pilocarpine.
- (d)
- Sham-Vh group (n = 7). The animals were handled in a similar way to the Sham-CBD group, with the exception that a vehicle (coconut oil 9.52 mL/kg, p.o.) was administered instead of CBD.
2.5. Statistical Analysis
3. Results
3.1. Characterization of Synaptosomes
3.2. Effects of CBD on Glutamate Release from Synaptic Terminals from Patients with DRE
3.2.1. Effects of CBD on Evoked Glutamate Release in Responsive Human Epileptic Neocortex of Patients with DRE
3.2.2. CBD Fails to Modify the Evoked Glutamate Release in Non-Responsive Human Epileptic Neocortex
3.3. Neocortical Tissue of Patients with DRE Keeps Its Viability and Electrophysiological Properties after Surgery
3.3.1. Whole Cell Recording
3.3.2. Extracellular Recordings
3.4. Effect of Subchronic Administration with CBD on Interictal Glutamate Release in the Hippocampus of Rats with Epilepsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE Official Report: A Practical Clinical Definition of Epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Çavuş, I.; Romanyshyn, J.C.; Kennard, J.T.; Farooque, P.; Williamson, A.; Eid, T.; Spencer, S.S.; Duckrow, R.; Dziura, J.; Spencer, D.D. Elevated Basal Glutamate and Unchanged Glutamine and GABA in Refractory Epilepsy: Microdialysis Study of 79 Patients at the Yale Epilepsy Surgery Program. Ann. Neurol. 2016, 80, 35–45. [Google Scholar] [CrossRef]
- During, M.; Spencer, D. Extracellular Hippocampal Glutamate and Spontaneous Seizure in the Conscious Human Brain. Lancet 1993, 341, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Luna-Munguia, H.; Orozco-Suarez, S.; Rocha, L. Effects of High Frequency Electrical Stimulation and R-Verapamil on Seizure Susceptibility and Glutamate and GABA Release in a Model of Phenytoin-Resistant Seizures. Neuropharmacology 2011, 61, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, Neuroprotection and Neuropsychiatric Disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Patra, P.H.; Barker-Haliski, M.; White, H.S.; Whalley, B.J.; Glyn, S.; Sandhu, H.; Jones, N.; Bazelot, M.; Williams, C.M.; McNeish, A.J. Cannabidiol Reduces Seizures and Associated Behavioral Comorbidities in a Range of Animal Seizure and Epilepsy Models. Epilepsia 2019, 60, 303–314. [Google Scholar] [CrossRef]
- Gobira, P.H.; Vilela, L.R.; Gonçalves, B.D.C.; Santos, R.P.M.; de Oliveira, A.C.; Vieira, L.B.; Aguiar, D.C.; Crippa, J.A.; Moreira, F.A. Cannabidiol, a Cannabis Sativa Constituent, Inhibits Cocaine-Induced Seizures in Mice: Possible Role of the MTOR Pathway and Reduction in Glutamate Release. Neurotoxicology 2015, 50, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Castañeda, C.; Huerta de la Cruz, S.; Martínez-Aguirre, C.; Orozco-Suárez, S.A.; Rocha, L. Cannabidiol Reduces Short- and Long-Term High Glutamate Release after Severe Traumatic Brain Injury and Improves Functional Recovery. Pharmaceutics 2022, 14, 1609. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Maa, E.; Figi, P. The Case for Medical Marijuana in Epilepsy. Epilepsia 2014, 55, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.S.; Crippa, A.C.S.; Hallak, J.E.C.; Martín-Santos, R.; Zuardi, A.W. Δ9-THC Intoxication by Cannabidiol-Enriched Cannabis Extract in Two Children with Refractory Epilepsy: Full Remission after Switching to Purified Cannabidiol. Front. Pharmacol. 2016, 7, 359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and Potential Therapeutic Role in Epilepsy and Other Neuropsychiatric Disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef]
- Davis, K.A.; Nanga, R.P.R.; Das, S.; Chen, S.H.; Hadar, P.N.; Pollard, J.R.; Lucas, T.H.; Shinohara, R.T.; Litt, B.; Hariharan, H.; et al. Glutamate Imaging (GluCEST) Lateralizes Epileptic Foci in Nonlesional Temporal Lobe Epilepsy. Sci. Transl. Med. 2015, 7, 309ra161. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A Rapid Percoll Gradient Procedure for Preparation of Synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Porcelli, G.; Bavisotto, C.C.; Nuzzo, D.; Galizzi, G.; Biagio, P.L.S.; Bulone, D.; Di Carlo, M. Synaptosomes: New Vesicles for Neuronal Mitochondrial Transplantation. J. Nanobiotechnol. 2021, 19, 6. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K. Randomized, Dose-Ranging Safety Trial of Cannabidiol in Dravet Syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [PubMed]
- Maidment, N.T.; Brumbaugh, D.R.; Rudolph, V.D.; Erdelyi, E.; Evans, C.J. Microdialysis of Extracellular Endogenous Opioid Peptides from Rat Brain in Vivo. Neuroscience 1989, 33, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.W.; Sun, Z.; Kvajo, M.; Broek, J.A.C.; Fénelon, K.; McKellar, H.; Xiao, L.; Xu, B.; Bahn, S.; O’Donnell, J.M.; et al. Alteration of Neuronal Excitability and Short-Term Synaptic Plasticity in the Prefrontal Cortex of a Mouse Model of Mental Illness. J. Neurosci. 2017, 37, 4158–4180. [Google Scholar] [CrossRef] [PubMed]
- Glien, M.; Brandt, C.; Potschka, H.; Löscher, W. Effects of the Novel Antiepileptic Drug Levetiracetam on Spontaneous Recurrent Seizures in the Rat Pilocarpine Model of Temporal Lobe Epilepsy. Epilepsia 2002, 43, 350–357. [Google Scholar] [CrossRef]
- Löscher, W.; White, H.S. Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells 2023, 12, 1233. [Google Scholar] [CrossRef]
- Cavus, I.; Widi, G.A.; Duckrow, R.B.; Zaveri, H.; Kennard, J.T.; Krystal, J.; Spencer, D.D. 50 Hz Hippocampal Stimulation in Refractory Epilepsy: Higher Level of Basal Glutamate Predicts Greater Release of Glutamate. Epilepsia 2016, 57, 288–297. [Google Scholar] [CrossRef]
- Silm, K.; Yang, J.; Marcott, P.F.; Asensio, C.S.; Eriksen, J.; Guthrie, D.A.; Newman, A.H.; Ford, C.P.; Edwards, R.H. Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties. Neuron 2019, 102, 786–800.e5. [Google Scholar] [CrossRef]
- Lattanzi, S.; Trinka, E.; Striano, P.; Rocchi, C.; Salvemini, S.; Silvestrini, M.; Brigo, F. Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox–Gastaut Syndrome. CNS Drugs 2021, 35, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol Attenuates Seizures and Social Deficits in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.B.; Heckman, L.; Niday, Z.; Jo, S.; Fujita, A.; Shim, J.; Pandey, R.; Al Jandal, H.; Jayakar, S.; Barrett, L.B.; et al. Cannabidiol Activates Neuronal Kv7 Channels. eLife 2022, 11, 73246. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of Recombinant Human T-Type Calcium Channels by Δ9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Smith, S.M. Calcium Dependence of Spontaneous Neurotransmitter Release. J. Neurosci. Res. 2018, 96, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Peres, F.F.; Almeida, V.; Calzavara, M.B.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; Abílio, V.C. Effects of Cannabinoid Drugs on the Deficit of Prepulse Inhibition of Startle in an Animal Model of Schizophrenia: The SHR Strain. Front. Pharmacol. 2014, 5, 10. [Google Scholar] [CrossRef]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol Presents an Inverted U-Shaped Dose-Response Curve in a Simulated Public Speaking Test. Rev. Bras. Psiquiatr. 2019, 41, 9–14. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R.A.; Craib, S.J.; Thomas, A. (−)-Cannabidiol Antagonizes Cannabinoid Receptor Agonists and Noradrenaline in the Mouse Vas Deferens. Eur. J. Pharmacol. 2002, 456, 99–106. [Google Scholar] [CrossRef]
- Kenakin, T. G-Protein/Receptor Inhibitors as Blockers of Receptor Signaling. J. Theor. Biol. 2019, 480, 23–33. [Google Scholar] [CrossRef]
- Chun, C.; Smith, A.S.T.; Kim, H.; Kamenz, D.S.; Lee, J.H.; Lee, J.B.; Mack, D.L.; Bothwell, M.; Clelland, C.D.; Kim, D.H. Astrocyte-Derived Extracellular Vesicles Enhance the Survival and Electrophysiological Function of Human Cortical Neurons in Vitro. Biomaterials 2021, 271, 120700. [Google Scholar] [CrossRef]
- Eugène, E.; Cluzeaud, F.; Cifuentes-Diaz, C.; Fricker, D.; Le Duigou, C.; Clemenceau, S.; Baulac, M.; Poncer, J.C.; Miles, R. An Organotypic Brain Slice Preparation from Adult Patients with Temporal Lobe Epilepsy. J. Neurosci. Methods 2014, 235, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Contreras, S.A.; Schleimer, J.H.; Gulledge, A.T.; Schreiber, S. Activity-Mediated Accumulation of Potassium Induces a Switch in Firing Pattern and Neuronal Excitability Type. PLoS Comput. Biol. 2021, 17, e1008510. [Google Scholar] [CrossRef] [PubMed]
- Palomero-Gallagher, N.; Schleicher, A.; Bidmon, H.J.; Pannek, H.W.; Hans, V.; Gorji, A.; Speckmann, E.J.; Zilles, K. Multireceptor Analysis in Human Neocortex Reveals Complex Alterations of Receptor Ligand Binding in Focal Epilepsies. Epilepsia 2012, 53, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.; Alonso-Vanegas, M.; Martínez-Juarez, I.E.; Orozco-Suárez, S.; Escalante-Santiago, D.; Feria-Romero, I.A.; Zavala-Tecuapetla, C.; Cisneros-Franco, J.M.; Buentello-García, R.M.; Cienfuegos, J. Gabaergic Alterations in Neocortex of Patients with Pharmacoresistant Temporal Lobe Epilepsy Can Explain the Comorbidity of Anxiety and Depression: The Potential Impact of Clinical Factors. Front. Cell Neurosci. 2015, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.; Frías-Soria, C.L.; Ortiz, J.G.; Auzmendi, J.; Lazarowski, A. Is Cannabidiol a Drug Acting on Unconventional Targets to Control Drug-Resistant Epilepsy? Epilepsia Open 2020, 5, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Levental, I.; Veatch, S.L. The Continuing Mystery of Lipid Rafts. J. Mol. Biol. 2016, 428, 4749–4764. [Google Scholar] [CrossRef]
- Bouwman, B.M.; Suffczynski, P.; Lopes Da Silva, F.H.; Maris, E.; van Rijn, C.M. GABAergic Mechanisms in Absence Epilepsy: A Computational Model of Absence Epilepsy Simulating Spike and Wave Discharges after Vigabatrin in WAG/Rij Rats. Eur. J. Neurosci. 2007, 25, 2783–2790. [Google Scholar] [CrossRef]
- Perescis, M.F.J.; van Luijtelaar, G.; van Rijn, C.M. Neonatal Exposure to AY-9944 Increases Typical Spike and Wave Discharges in WAG/Rij and Wistar Rats. Epilepsy Res. 2019, 157, 106184. [Google Scholar] [CrossRef]
- Hou, Q.; Huang, Y.; Amato, S.; Snyder, S.H.; Huganir, R.L.; Man, H.Y. Regulation of AMPA Receptor Localization in Lipid Rafts. Mol. Cell. Neurosci. 2008, 38, 213–223. [Google Scholar] [CrossRef]
- Di Nunno, N.; Esposito, M.; Argo, A.; Salerno, M.; Sessa, F. Pharmacogenetics and Forensic Toxicology: A New Step towards a Multidisciplinary Approach. Toxics 2021, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, S.; Sisodiya, S.M. Pharmacogenomics in Epilepsy. Neurosci. Lett. 2018, 667, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Makowska, M.; Romanowicz, H. Pharmacogenetics of Drug-Resistant Epilepsy (Review of Literature). Int. J. Mol. Sci. 2021, 22, 11696. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Gáll, Z.; Kelemen, K.; Tolokán, A.; Zolcseak, I.; Sável, I.; Bod, R.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Anticonvulsant Action and Long-Term Effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines 2022, 10, 1811. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The Neuroprotective Effect of Cannabidiol in an in Vitro Model of Newborn Hypoxic-Ischemic Brain Damage in Mice Is Mediated by CB2 and Adenosine Receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]
- Potschka, H.; Bhatti, S.F.M.; Tipold, A.; McGrath, S. Cannabidiol in Canine Epilepsy. Vet. J. 2022, 290, 105913. [Google Scholar] [CrossRef]
- Gray, R.A.; Stott, C.G.; Jones, N.A.; Di Marzo, V.; Whalley, B.J.; Whalley, B.J. Anticonvulsive Properties of Cannabidiol in a Model of Generalized Seizure Are Transient Receptor Potential Vanilloid 1 Dependent. Cannabis Cannabinoid Res. 2020, 5, 145–149. [Google Scholar] [CrossRef]
- Aghamiri, H.; Jafari-Sabet, M.; Hoormand, M. Ameliorative Effect of Cannabidiol on Topiramate-Induced Memory Loss: The Role of Hippocampal and Prefrontal Cortical NMDA Receptors and CREB/BDNF Signaling Pathways in Rats. Neurochem. Res. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Banerjee, J.; Banerjee Dixit, A.; Tripathi, M.; Sarkar, C.; Gupta, Y.K.; Chandra, P.S. Enhanced Endogenous Activation of NMDA Receptors in Pyramidal Neurons of Hippocampal Tissues from Patients with Mesial Temporal Lobe Epilepsy: A Mechanism of Hyper Excitation. Epilepsy Res. 2015, 117, 11–16. [Google Scholar] [CrossRef]



| Subject | Sex | Age (Years) | Age of Seizure Onset (Years) | Duration of Epilepsy (Years) | Frequency of the Seizures (per Month) | Location of the Focus | ASMs Administered before Surgery |
|---|---|---|---|---|---|---|---|
| Drug-resistant temporal lobe epilepsy | |||||||
| HUM-202 | F | 29 | 21 | 8 | 7 | Right temporal lobe | LEV, VPA, CPM |
| HUM-203 | M | 29 | 23 | 6 | 10 | Right temporal lobe | LEV |
| HUM-204 | M | 35 | 34 | 1 | 5 | Right temporal lobe | LEV, VPA |
| HUM-205 | F | 33 | 1.5 | 31.5 | 2 | Left temporal lobe | LEV, CBZ, TOP, GAB, VPA, LAM, LAC, ZSM |
| HUM-209 | F | 21 | 10 | 11 | 7 | Left temporal lobe | LEV, OXC, NMP |
| HUM-211 | M | 21 | 13 | 8 | 12 | Right temporal lobe | LEV, VPA, PAL, OXC, ZSM |
| HUM-220 | F | 23 | 0.5 | 22.5 | 30 | Left temporal lobe | PHN, CBZ, VPA, TOP, VIG, LEV, CLZ, LAC |
| HUM-221 | F | 20 | 10 | 10 | 90 | Left temporal lobe | PHN, CBZ, VPA, TOP, VIG, LEV, CLZ, LAC |
| HUM-222 | M | 14 | 3 | 11 | 5 | Right temporal lobe | LEV, CBZ, OXC, CLB |
| HUM-223 | M | 16 | 10 | 6 | 8 | Right temporal lobe | LEV, CBZ, OXC, VPA |
| Drug-resistant extratemporal lobe epilepsy | |||||||
| HUM-206 | F | 11 | 0 | 11 | 5 | Left parietal lobe | LEV, CBZ, OXC |
| HUM-207 | F | 18 | 5 | 13 | 3 | Right frontal lobe | OXC, LEV, VPA, CLB, LAC, ZSM |
| HUM-208 | M | 1.9 | 0 | 1.9 | 150 | Left frontal lobe | CLB, VPA, BRI, LEV, TOP, VIG |
| HUM-210 | F | 30 | 13 | 17 | 20 | Right frontal lobe | OXC, LEV, TOP |
| HUM-212 | M | 19 | 4 | 15 | 7 | Right frontal lobe | LEV, CLB, OXC, CBZ, LAM, VIG, LAC, TOP, BRI, LZM |
| HUM-213 | M | 18 | 2.5 | 15.5 | 4 | Left frontal lobe | LEV, OXC, VPA, LAC, PRG |
| HUM-214 | M | 2.6 | 1.2 | 1.4 | 10 | Right frontal lobe | LEV, LAC |
| HUM-217 | F | 7 | 1.25 | 5.75 | ND | Left parietal lobe | LEV, OXC, BRI, PAL, LAC, VPA, CLB |
| HUM-218 | F | 24 | 16 | 8 | ND | Right frontal lobe | LEV, LAM, VPA |
| HUM-219 | M | 4 | 2.75 | 1.25 | 1 | Right parietal lobe | VPA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Aguirre, C.; Márquez, L.A.; Santiago-Castañeda, C.L.; Carmona-Cruz, F.; Nuñez-Lumbreras, M.d.l.A.; Martínez-Rojas, V.A.; Alonso-Vanegas, M.; Aguado-Carrillo, G.; Gómez-Víquez, N.L.; Galván, E.J.; et al. Cannabidiol Modifies the Glutamate Over-Release in Brain Tissue of Patients and Rats with Epilepsy: A Pilot Study. Biomedicines 2023, 11, 3237. https://doi.org/10.3390/biomedicines11123237
Martínez-Aguirre C, Márquez LA, Santiago-Castañeda CL, Carmona-Cruz F, Nuñez-Lumbreras MdlA, Martínez-Rojas VA, Alonso-Vanegas M, Aguado-Carrillo G, Gómez-Víquez NL, Galván EJ, et al. Cannabidiol Modifies the Glutamate Over-Release in Brain Tissue of Patients and Rats with Epilepsy: A Pilot Study. Biomedicines. 2023; 11(12):3237. https://doi.org/10.3390/biomedicines11123237
Chicago/Turabian StyleMartínez-Aguirre, Christopher, Luis Alfredo Márquez, Cindy Lizbeth Santiago-Castañeda, Francia Carmona-Cruz, Maria de los Angeles Nuñez-Lumbreras, Vladimir A. Martínez-Rojas, Mario Alonso-Vanegas, Gustavo Aguado-Carrillo, Norma L. Gómez-Víquez, Emilio J. Galván, and et al. 2023. "Cannabidiol Modifies the Glutamate Over-Release in Brain Tissue of Patients and Rats with Epilepsy: A Pilot Study" Biomedicines 11, no. 12: 3237. https://doi.org/10.3390/biomedicines11123237
APA StyleMartínez-Aguirre, C., Márquez, L. A., Santiago-Castañeda, C. L., Carmona-Cruz, F., Nuñez-Lumbreras, M. d. l. A., Martínez-Rojas, V. A., Alonso-Vanegas, M., Aguado-Carrillo, G., Gómez-Víquez, N. L., Galván, E. J., Cuéllar-Herrera, M., & Rocha, L. (2023). Cannabidiol Modifies the Glutamate Over-Release in Brain Tissue of Patients and Rats with Epilepsy: A Pilot Study. Biomedicines, 11(12), 3237. https://doi.org/10.3390/biomedicines11123237







