Risk Assessment of Psychotropic Drugs on Mitochondrial Function Using In Vitro Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Isolation of Rat Liver Mitochondria
2.4. Measurement of Mitochondrial Toxicity Using Seahorse XFe96 Extracellular Flux Analyzer—Acute Extracellular Flux (AEF) Assay in Intact HepG2 Cells
2.5. Assessing Mitochondrial Respiratory Complexes Using the Seahorse XF Analyzer—Permeabilized HepG2 Cells
2.6. Assessment of Mitochondrial Toxicity Using Glucose and Galactose Media Conditions (Glu/Gal Assay)
2.7. High-Resolution Respirometry and Mitochondrial Membrane Potential (MMP)
3. Results
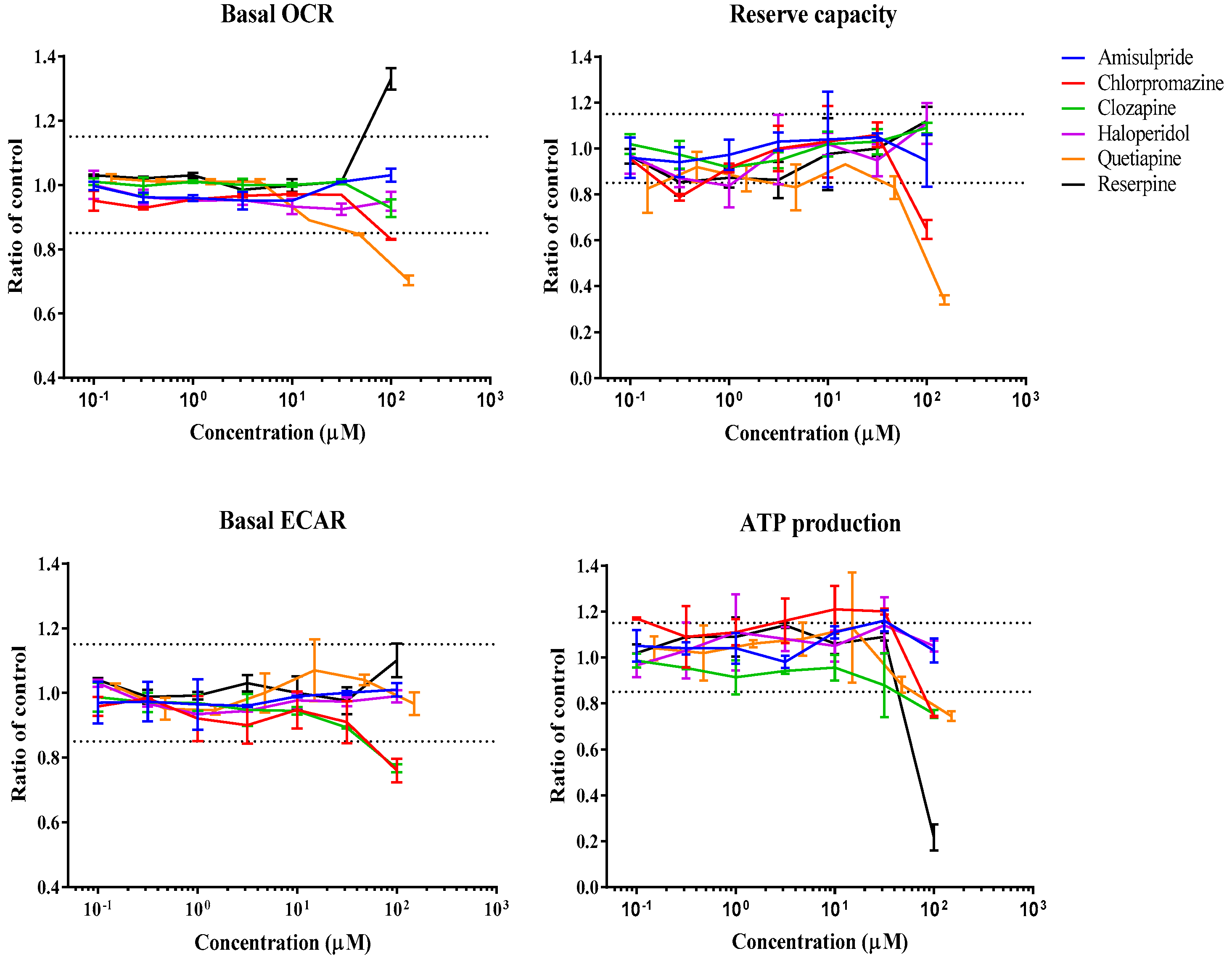
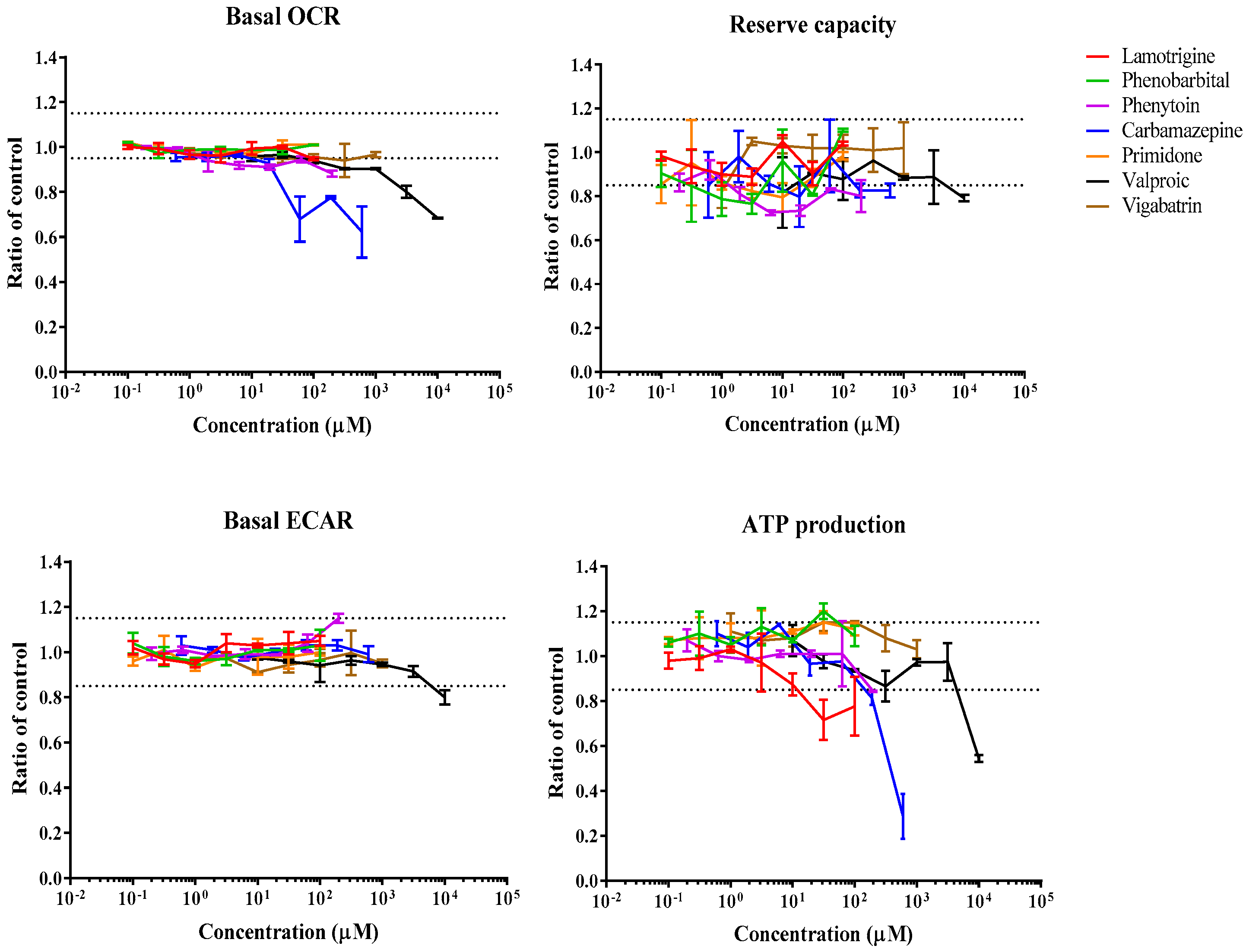
3.1. Real-Time Effects of Drugs on Mitochondrial Respiration
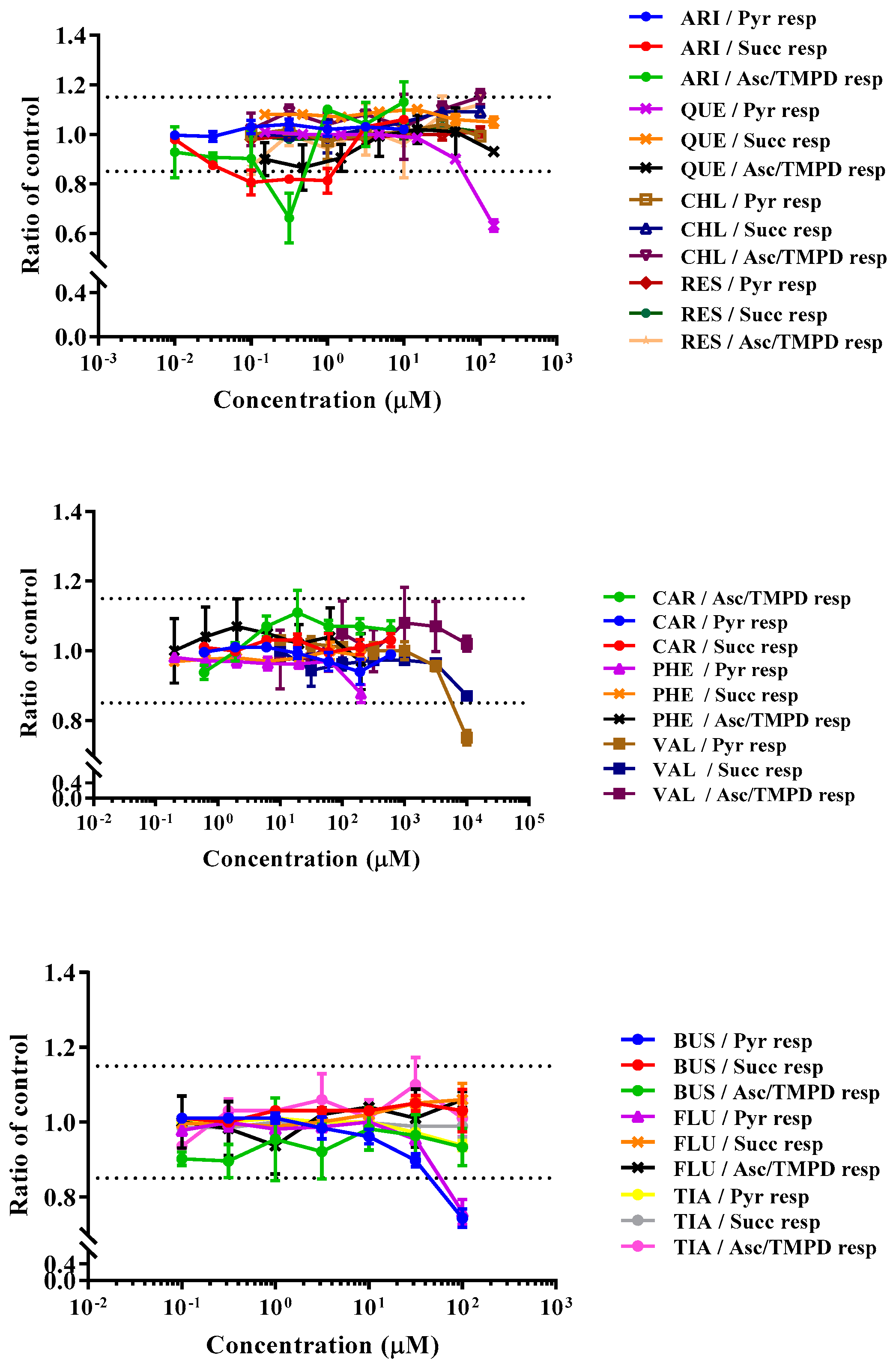
3.2. Drugs’ Effects on Respiratory Activity of Permeabilized HepG2 Cells
3.3. Assessment of Mitochondrial Toxicity Using Selective Media Conditions (Glu/Gal Assay)
3.4. High-Resolution Respirometry Readings
4. Discussion
4.1. Antipsychotics
4.2. Anticonvulsants
4.3. Antidepressants and Anxiolytic Drugs
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef]
- Rana, P.; Aleo, M.D.; Wen, X.; Kogut, S. Hepatotoxicity reports in the FDA adverse event reporting system database: A comparison of drugs that cause injury via mitochondrial or other mechanisms. Acta Pharm. Sin. B 2021, 11, 3857–3868. [Google Scholar] [CrossRef]
- Dykens, J.A.; Marroquin, L.D.; Will, Y. Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev. Mol. Diagn. 2007, 7, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.; Billingham, L.K.; Ramaswamy, M.; Siegel, R.M. Chapter Seven—Extracellular Flux Analysis to Monitor Glycolytic Rates and Mitochondrial Oxygen Consumption, in Methods in Enzymology; Galluzzi, L., Kroemer, G., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 125–149. [Google Scholar]
- Wills, L.P. The use of high-throughput screening techniques to evaluate mitochondrial toxicity. Toxicology 2017, 391, 34–41. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Jastroch, M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat. Met. 2022, 4, 978–994. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: Isolation, structure and function. J. Physiol. 2011, 589, 4413–4421. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Rogers, G.W.; Murphy, A.N. Measuring Mitochondrial Function in Permeabilized Cells Using the Seahorse XF Analyzer or a Clark-Type Oxygen Electrode. Curr. Prot. Toxicol. 2014, 60, 25.2.1–25.2.16. [Google Scholar] [CrossRef]
- Luvsannyam, E.; Jain, M.S.; Pormento, M.K.L.; Siddiqui, H.; Balagtas, A.R.A.; Emuze, B.O.; Poprawski, T. Neurobiology of Schizophrenia: A Comprehensive Review. Cureus 2022, 14, e23959. [Google Scholar] [CrossRef]
- Dazzan, P.; Murray, R.M. Neurological soft signs in first-episode psychosis: A systematic review. Br. J. Psychiatry Suppl. 2002, 43, s50–s57. [Google Scholar] [CrossRef]
- Li, P.; Snyder, G.L.; Vanover, K.E. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403. [Google Scholar] [CrossRef]
- Seeman, M.V. History of the dopamine hypothesis of antipsychotic action. World J. Psychiat. 2021, 11, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.; Barnes, T.R.; Davies, L.; Dunn, G.; Lloyd, H.; Hayhurst, K.P.; Murray, R.M.; Markwick, A.; Lewis, S.W. Randomized controlled trial of the effect on Quality of Life of second- vs. first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch. Gen. Psychiatry 2006, 63, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Mittal, K.; Gonçalves, V.F.; Harripaul, R.; Cuperfain, A.B.; Rollins, B.; Tiwari, A.K.; Zai, C.C.; Maciukiewicz, M.; Müller, D.J.; Vawter, M.P.; et al. A comprehensive analysis of mitochondrial genes variants and their association with antipsychotic-induced weight gain. Schizophr. Res. 2017, 187, 67–73. [Google Scholar] [CrossRef]
- Scaini, G.; Quevedo, J.; Velligan, D.; Roberts, D.L.; Raventos, H.; Walss-Bass, C. Second generation antipsychotic-induced mitochondrial alterations: Implications for increased risk of metabolic syndrome in patients with schizophrenia. Eur. Neuropsychopharmacol. 2018, 28, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Al-Ghafari, A.; Aggour, A.M.; Mosad, S.M.; Khan, R.; Amer, S. Effect of antipsychotics on mitochondrial bioenergetics of rat ovarian theca cells. Toxicol. Lett. 2017, 272, 94–100. [Google Scholar] [CrossRef]
- Serretti, A.; Chiesa, A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int. Clin. Psychopharmacol. 2011, 26, 130–140. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Muench, J.; Hamer, A.M. Adverse effects of antipsychotic medications. Am. Fam. Phys. 2010, 81, 617–622. [Google Scholar]
- Holt, R.I.G. Association Between Antipsychotic Medication Use and Diabetes. Curr. Diab. Rep. 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.M.; Quinones, M.; Marballi, K.; Gao, X.; Valdez, C.; Ahuja, S.S.; Velligan, D.; Walss-Bass, C. Metabolomic profiling of schizophrenia patients at risk for metabolic syndrome. Int. J. Neuropsychopharmacol. 2014, 17, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Lucenteforte, E.; Battini, V.; Mazhar, F.; Fornili, M.; Invernizzi, E.; Mosini, G.; Gringeri, M.; Capuano, A.; Scavone, C.; et al. Association between the glyco-metabolic adverse effects of antipsychotic drugs and their chemical and pharmacological profile: A network meta-analysis and regression. Psychol. Med. 2022, 52, 3508–3520. [Google Scholar] [CrossRef]
- Tschoner, A.; Engl, J.; Rettenbacher, M.; Edlinger, M.; Kaser, S.; Tatarczyk, T.; Effenberger, M.; Patsch, J.R.; Fleischhacker, W.W.; Ebenbichler, C.F. Effects of six second generation antipsychotics on body weight and metabolism—Risk assessment and results from a prospective study. Pharmacopsychiatry 2009, 42, 29–34. [Google Scholar] [CrossRef]
- Rummel-Kluge, C.; Komossa, K.; Schwarz, S.; Hunger, H.; Schmid, F.; Kissling, W.; Davis, J.M.; Leucht, S. Second-generation antipsychotic drugs and extrapyramidal side effects: A systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 2012, 38, 167–177. [Google Scholar] [CrossRef]
- Carbon, M.; Hsieh, C.H.; Kane, J.M.; Correll, C.U. Tardive Dyskinesia Prevalence in the Period of Second-Generation Antipsychotic Use: A Meta-Analysis. J. Clin. Psychiatry 2017, 78, e264–e278. [Google Scholar] [CrossRef]
- Siafis, S.; Wu, H.; Wang, D.; Burschinski, A.; Nomura, N.; Takeuchi, H.; Schneider-Thoma, J.; Davis, J.M.; Leucht, S. Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: A systematic review and dose-response meta-analysis. Mol. Psychiatry 2023, 28, 3267–3277. [Google Scholar] [CrossRef]
- Roberts, R.C. Postmortem studies on mitochondria in schizophrenia. Schizophr. Res. 2017, 187, 17–25. [Google Scholar] [CrossRef]
- Chan, S.T.; McCarthy, M.J.; Vawter, M.P. Psychiatric drugs impact mitochondrial function in brain and other tissues. Schizophr. Res. 2020, 217, 136–147. [Google Scholar] [CrossRef]
- WHO. Epilepsy. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 1 December 2022).
- Ahmed, S.N.; Siddiqi, Z.A. Antiepileptic drugs and liver disease. Seizure 2006, 15, 156–164. [Google Scholar] [CrossRef]
- Abou-Khalil, B.W. Update on antiepileptic drugs 2019. Contin. Lifelong Learn. Neurol. 2019, 25, 508–536. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, B.; Seizure Medications. StatPearls 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482269/ (accessed on 1 December 2022).
- Hanada, T. The AMPA receptor as a therapeutic target in epilepsy: Preclinical and clinical evidence. J. Recept. Ligand Channel Res. 2014, 7, 39–50. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Expert Opin. Drug Metab. Toxicol. 2012, 8, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A. Effects of antiepileptic drugs on mitochondrial functions, morphology, kinetics, biogenesis, and survival. Epilepsy Res. 2017, 136, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kudin, A.P.; Mawasi, H.; Eisenkraft, A.; Elger, C.E.; Bialer, M.; Kunz, W.S. Mitochondrial Liver Toxicity of Valproic Acid and Its Acid Derivatives Is Related to Inhibition of α-Lipoamide Dehydrogenase. Int. J. Mol. Sci. 2017, 18, 1912. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.G.; Medina, W.S.G.; Martins, N.M.; Mingatto, F.E.; Curti, C.; Santos, A.C.D. Aromatic antiepileptic drugs and mitochondrial toxicity: Effects on mitochondria isolated from rat liver. Toxicol. In Vitro 2008, 22, 1143–1152. [Google Scholar] [CrossRef]
- Boelsterli, U.A.; Lim, P.L. Mitochondrial abnormalities—A link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 2007, 220, 92–107. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Cikánková, T.; Fišar, Z.; Hroudová, J. In vitro effects of antidepressants and mood-stabilizing drugs on cell energy metabolism. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 797–811. [Google Scholar] [CrossRef]
- Abdel-Razaq, W.; Kendall, D.A.; Bates, T.E. The Effects of Antidepressants on Mitochondrial Function in a Model Cell System and Isolated Mitochondria. Neurochemical. Res. 2011, 36, 327–338. [Google Scholar] [CrossRef]
- Ghafourian, T.; Baricicova, M. Prediction of drug-induced liver injury. Toxicol. Lett. 2018, 295, S105. [Google Scholar] [CrossRef]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree Effect: Replacing Media Glucose with Galactose Increases Susceptibility of HepG2 Cells to Mitochondrial Toxicants. Toxicol. Sci. 2007, 97, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, F.; Zhao, J.; Zhang, W.; Chen, L.; Wang, X.; Yang, P.; Tang, J.; Chi, Y. Unraveling mitochondria-targeting reactive oxygen species modulation and their implementations in cancer therapy by nanomaterials. Exploration 2023, 3, 20220115. [Google Scholar] [CrossRef]
- Gnaiger, E. Polarographic oxygen sensors, the oxygraph and high-resolution respirometry to assess mitochondrial function. In Drug-Induced Mitochondrial Dysfunction; Dykens, J., Will, Y., Eds.; John Wiley & Son: Hoboken, NJ, USA, 2008; pp. 327–352. [Google Scholar]
- Krumschnabel, G.; Eigentler, A.; Fasching, M.; Gnaiger, E. Chapter Nine—Use of Safranin for the Assessment of Mitochondrial Membrane Potential by High-Resolution Respirometry and Fluorometry, in Methods in Enzymology; Galluzzi, L., Kroemer, G., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 163–181. [Google Scholar]
- Cikánková, T.; Fišar, Z.; Bakhouche, Y.; Ľupták, M.; Hroudová, J. In vitro effects of antipsychotics on mitochondrial respiration. N-S Arch. Pharmacol. 2019, 392, 1209–1223. [Google Scholar] [CrossRef]
- Dykens, J.A.; Jamieson, J.D.; Marroquin, L.D.; Nadanaciva, S.; Xu, J.J.; Dunn, M.C.; Smith, A.R.; Will, Y. In vitro Assessment of Mitochondrial Dysfunction and Cytotoxicity of Nefazodone, Trazodone, and Buspirone. Toxicol. Sci. 2008, 103, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Segal, I.; Shmueli, D.; Saada, A. The effect of antiepileptic drugs on mitochondrial activity: A pilot study. J. Child Neurol. 2010, 25, 541–545. [Google Scholar] [CrossRef]
- Hroudova, J.; Fisar, Z. Activities of respiratory chain complexes and citrate synthase influenced by pharmacologically different antidepressants and mood stabilizers. Neuro Endocrinol. Lett. 2010, 31, 336–342. [Google Scholar]
- Xia, Z.; Lundgren, B.; Bergstrand, A.; DePierre, J.W.; Nässberger, L. Changes in the generation of reactive oxygen species and in mitochondrial membrane potential during apoptosis induced by the antidepressants imipramine, clomipramine, and citalopram and the effects on these changes by Bcl-2 and Bcl-X(L). Biochem. Pharmacol. 1999, 57, 1199–1208. [Google Scholar] [CrossRef]
- Modica-Napolitano, J.S.; Lagace, C.J.; Brennan, W.A.; Aprille, J.R. Differential effects of typical and atypical neuroleptics on mitochondrial function in vitro. Arch. Pharm. Res. 2003, 26, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Mingattao, F.E.; Polizello, A.C.; Galastri, L.O.; Uyemura, S.A.; Santos, A.C. Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol. Cell. Biochem. 1999, 199, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.E.J.; Polizello, A.C.M.; Uyemura, S.A.; Castro-Silva, O., Jr.; Curti, C. Effect of fluoxetine on rat liver mitochondria. Biochem. Pharmacol. 1994, 48, 535–541. [Google Scholar] [CrossRef]
- Hroudová, J.; Fišar, Z. In vitro inhibition of mitochondrial respiratory rate by antidepressants. Toxicol. Lett. 2012, 213, 345–352. [Google Scholar] [CrossRef]
- Bogdanov, G.N.; Mishchenko, D.V.; Kotel’nikova, R.A.; Frog, E.S.; Faĭngol’d, I.I.; Tat’ianenko, L.V.; Dorokhotova, O.V.; IaR, N. Anticonvulsants as bioantioxidants under stress conditions. Biomed. Khim. 2009, 55, 519–524. [Google Scholar] [PubMed]
- Maina, G. Reserpine as an uncoupling agent. Biochim. Biophys. Acta BBA Bioenerg. 1974, 333, 481–486. [Google Scholar] [CrossRef]
- Fromenty, B.; Freneaux, E.; Labbe, G.; Deschamps, D.; Larrey, D.; Letteron, P.; Pessayre, D. Tianeptine, a new tricyclic antidepressant metabolized by β-oxidation of its heptanoic side chain, inhibits the mitochondrial oxidation of medium and short chain fatty acids in mice. Biochem. Pharmacol. 1989, 38, 3743–3751. [Google Scholar] [CrossRef]
- Cheah, K.S.; Waring, J.C. Effect of trifluoperazine on skeletal muscle mitochondrial respiration. Biochim. Biophys. Acta 1983, 723, 45–51. [Google Scholar] [CrossRef]
- Ruben, L.; Rasmussen, H. Phenothiazines and related compounds disrupt mitochondrial energy production by a calmodulin-independent reaction. Biochim. Biophys. Acta BBA Bioenerg. 1981, 637, 415–422. [Google Scholar] [CrossRef]
- Silva, M.F.; Ruiter, J.P.; IJlst, L.; Jakobs, C.; Duran, M.; de Almeida, I.T.; Wanders, R.J. Differential effect of valproate and its Delta2- and Delta4-unsaturated metabolites, on the beta-oxidation rate of long-chain and medium-chain fatty acids. Chem. Biol. Interact. 2001, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Massart, J.; Robin, M.A.; Borgne-Sanchez, A.; Fromenty, B. Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011, 54, 773–794. [Google Scholar] [CrossRef]
- Komulainen, T.; Lodge, T.; Hinttala, R.; Bolszak, M.; Pietilä, M.; Koivunen, P.; Hakkola, J.; Poulton, J.; Morten, K.J.; Uusimaa, J. Sodium valproate induces mitochondrial respiration dysfunction in HepG2 in vitro cell model. Toxicology 2015, 331, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Mogili, R.; Kanala, K.; Challa, B.R.; Chandu, B.R.; Bannoth, C.K. Development and validation of amisulpride in human plasma by HPLC coupled with tandem mass spectrometry and its application to a pharmacokinetic study. Sci. Pharm. 2011, 79, 583–599. [Google Scholar] [CrossRef]
- Mallikaarjun, S.; Kane, J.M.; Bricmont, P.; McQuade, R.; Carson, W.; Sanchez, R.; Forbes, R.A.; Fleischhacker, W.W. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: An open-label, parallel-arm, multiple-dose study. Schizophr. Res. 2013, 150, 281–288. [Google Scholar] [CrossRef]
- Nadanaciva, S.; Bernal, A.; Aggeler, R.; Capaldi, R.; Will, Y. Target identification of drug induced mitochondrial toxicity using immunocapture based OXPHOS activity assays. Toxicol. In Vitro 2007, 21, 902–911. [Google Scholar] [CrossRef]
- Chang, W.H.; Augustin, B.; Lane, H.Y.; ZumBrunnen, T.; Liu, H.C.; Kazmi, Y.; Jann, M.W. In-vitro and in-vivo evaluation of the drug-drug interaction between fluvoxamine and clozapine. Psychopharmacology 1999, 145, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kunka, R.L.; Metz, A.; Lloyd, T.; Rudolph, G.; Perel, J.M. Effect of alosetron (a new 5-HT3 receptor antagonist) on the pharmacokinetics of haloperidol in schizophrenic patients. J. Clin. Pharmacol. 1995, 35, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Gossen, D.; De Suray, J.M.; Vandenhende, F.; Onkelinx, C.; Gangji, D. Influence of fluoxetine on olanzapine pharmacokinetics. AAPS PharmSci. 2002, 4, E11. [Google Scholar] [CrossRef]
- Darwish, M.; Bond, M.; Hellriegel, E.T.; Youakim, J.M.; Yang, R.; Jr, P.R. Investigation of a possible interaction between quetiapine and armodafinil in patients with schizophrenia: An open-label, multiple-dose study. J. Clin. Pharmacol. 2012, 52, 1399–1409. [Google Scholar] [CrossRef]
- El-Din, M.M.S.; Nassar, M.W.; Attia, K.A.; El Demellawy, M.A.; Kaddah, M.M. Validated liquid chromatography-tandem mass spectrometry method for simultaneous determination of clopamide, reserpine and dihydroergotoxine: Application to pharmacokinetics in human plasma. J. Pharm. Biomed. Anal. 2016, 125, 236–244. [Google Scholar] [CrossRef]
- Midha, K.K.; Korchinski, E.D.; Roscoe, R.M.H.; Hawes, E.M.; Cooper, J.K.; McKay, G. Relative bioavailability of a commercial trifluoperazine tablet formulation using a radioimmunoassay technique. J. Pharm. Sci. 1984, 73, 261–263. [Google Scholar] [CrossRef]
- Darwish, M.; Bond, M.; Yang, R.; Hellriegel, E.T.; Robertson, P. Evaluation of the potential for a pharmacokinetic drug-drug interaction between armodafinil and ziprasidone in healthy adults. Clin. Drug Investig. 2014, 34, 691–699. [Google Scholar] [CrossRef]
- Darwish, M.; Bond, M.; Yang, R.; Hellriegel, E.T.; Robertson, P., Jr. Evaluation of the potential for pharmacokinetic drug-drug interaction between armodafinil and carbamazepine in healthy adults. Clin. Ther. 2015, 37, 325–337. [Google Scholar] [CrossRef]
- Burger, D.M.; Huisman, A.; Van Ewijk, N.; Neisingh, H.; Van Uden, P.; Rongen, G.A.; Koopmans, P.; Bertz, R.J. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin. Pharmacol. Ther. 2008, 84, 698–703. [Google Scholar] [CrossRef]
- Dalmora, S.L.; Sangoi, M.D.S.; Nogueira, D.R.; D’Avila, F.B.; Moreno, R.A.; Sverdloff, C.E.; Oliveira, R.A.D.; Borges, N.C. Determination of phenobarbital in human plasma by a specific liquid chromatography method: Application to a bioequivalence study. Quim. Nova 2010, 33, 124–129. [Google Scholar] [CrossRef]
- Inoue, Y.; Usui, N.; Hiroki, T.; Shimizu, K.; Kobayashi, S.; Shimasaki, S. Bioavailability of intravenous fosphenytoin sodium in healthy Japanese volunteers. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Huh, W.; Jung, J.A.; Yoo, H.M.; Ko, J.W.; Kim, J.R. Effects of amoxicillin/clavulanic acid on the pharmacokinetics of valproic acid. Drug Des. Dev. Ther. 2015, 9, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- He, Y.L.; Zhao, C.X.; Wang, Y.; Campestrini, J.; Prasad, P.; Marion, A.; Ligueros-Saylan, M. Lack of pharmacokinetic interaction between vildagliptin and amlodipine in healthy volunteers. J. Clin. Pharmacol. 2005, 45, 1084. [Google Scholar]
- Gammans, R.E.; Mayol, R.F.; LaBudde, J.A. Metabolism and disposition of buspirone. Am. J. Med. 1986, 80, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Blin, O.; Jacquet, A.; Callamand, S.; Jouve, E.; Habib, M.; Gayraud, D.; Durand, A.; Bruguerolle, B.; Pisano, P. Pharmacokinetic-pharmacodynamic analysis of mnesic effects of lorazepam in healthy volunteers. Br. J. Clin. Pharmacol. 1999, 48, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Baumann, P. Pharmacology and pharmacokinetics of citalopram and other SSRIs. Int. Clin. Psychopharmacol. 1996, 11 (Suppl. 1), 5–11. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.C.; Mazure, C.M.; Bowers, M.B.; Jatlow, P.I. A preliminary, open study of the combination of fluoxetine and desipramine for rapid treatment of major depression. Arch. Gen. Psychiatry 1991, 48, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Kim, B.H. Pharmacokinetic and bioequivalence assessment of two formulations of tianeptine sodium in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 2014, 52, 817–823. [Google Scholar] [CrossRef]
- Rossjohn, J.; Polekhina, G.; Feil, S.C.; Morton, C.J.; Tweten, R.K.; Parker, M.W. Structures of perfringolysin O suggest a pathway for activation of cholesterol-dependent cytolysins. J. Mol. Biol. 2007, 367, 1227–1236. [Google Scholar] [CrossRef]
- Eakins, J.; Bauch, C.; Woodhouse, H.; Park, B.; Bevan, S.; Dilworth, C.; Walker, P. A combined in vitro approach to improve the prediction of mitochondrial toxicants. Toxicol. In Vitro 2016, 34, 161–170. [Google Scholar] [CrossRef]
- Emmerzaal, T.L.; Nijkamp, G.; Veldic, M.; Rahman, S.; Andreazza, A.C.; Morava, E.; Rodenburg, R.J.; Kozicz, T. Effect of neuropsychiatric medications on mitochondrial function: For better or for worse. Neurosci. Biobehav. Rev. 2021, 127, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, C.; Kelly, J.P.; Lim, Y.H.; Filley, C.M.; Parker Jr, W.D. Neuroleptic medications inhibit complex I of the electron transport chain. Ann. Neurol. 1993, 33, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Holper, L.; Ben-Shachar, D.; Mann, J.J. Psychotropic and neurological medication effects on mitochondrial complex I and IV in rodent models. Eur. Neuropsychopharmacol. 2019, 29, 986–1002. [Google Scholar] [CrossRef]
- Casademont, J.; Garrabou, G.; Miró, Ò.; López, S.; Pons, A.; Bernardo, M.; Cardellach, F. Neuroleptic treatment effect on mitochondrial electron transport chain: Peripheral blood mononuclear cells analysis in psychotic patients. J. Clin. Psychopharmacol. 2007, 27, 284–288. [Google Scholar] [CrossRef]
- Balijepalli, S.; Boyd, M.R.; Ravindranath, V. Inhibition of mitochondrial complex I by haloperidol: The role of thiol oxidation. Neuropharmacology 1999, 38, 567–577. [Google Scholar] [CrossRef]
- Maurer, I.; Moller, H.J. Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol. Cell Biochem. 1997, 174, 255–259. [Google Scholar] [CrossRef]
- Dunn, P.P.; Slabas, A.R.; Cottingham, I.R.; Moore, A.L. Trifluoperazine inhibition of electron transport and adenosine triphosphatase in plant mitochondria. Arch. Biochem. Biophys. 1984, 229, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, H.A.; Elmorsy, E.; Mahmoud, E.H.M.; Aggour, A.M.; Amer, S.A. The role of oxidative stress in ovarian toxicity induced by haloperidol and clozapine-a histological and biochemical study in albino rats. Cell Tissue Res. 2019, 378, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Williams, D.; Madden, S.; Templeton, E.; Park, B.K. Metabolism and bioactivation of clozapine by human liver in vitro. J. Pharmacol. Exp. Ther. 1995, 272, 984–990. [Google Scholar] [PubMed]
- Dragovic, S.; Gunness, P.; Ingelman-Sundberg, M.; Vermeulen, N.P.; Commandeur, J.N. Characterization of Human Cytochrome P450s Involved in the Bioactivation of Clozapine. Drug Metab. Dispos. 2013, 41, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Engqvist, H.; Biermann, J.; Werner Rönnerman, E.; Forssell-Aronsson, E.; Kovács, A.; Karlsson, P.; Helou, K.; Parris, T.Z. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 2020, 10, 5798. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.J.; Grime, K.; Weaver, R. Time-dependent CYP inhibition. Expert Opin. Drug Metab. Toxicol. 2007, 3, 51–66. [Google Scholar] [CrossRef]
- Beedham, C.; Miceli, J.J.; Obach, R.S. Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J. Clin. Psychopharmacol. 2003, 23, 229–232. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsson, R. Suspected drug-induced liver fatalities reported to the WHO database. Digest. Liver Dis. 2006, 38, 33–38. [Google Scholar] [CrossRef]
- Chabrol, B.; Mancini, J.; Chretien, D.; Rustin, P.; Munnich, A.; Pinsard, N. Valproate-induced hepatic failure in a case of cytochrome c oxidase deficiency. Eur. J. Pediatr. 1994, 153, 133–135. [Google Scholar] [CrossRef]
- Krähenbühl, S.; Brandner, S.; Kleinle, S.; Liechti, S.; Straumann, D. Mitochondrial diseases represent a risk factor for valproate-induced fulminant liver failure. Liver 2000, 20, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Pronicka, E.; Węglewska-Jurkiewicz, A.; Pronicki, M.; Sykut-Cegielska, J.; Kowalski, P.; Pajdowska, M.; Jankowska, I.; Kotulska, K.; Kaliciński, P.; Jakobkiewicz-Banecka, J.; et al. Drug-resistant epilepsia and fulminant valproate liver toxicity. Alpers-Huttenlocher syndrome in two children confirmed post mortem by identification of p.W748S mutation in POLG gene. Med. Sci. Monit. 2011, 17, Cr203–Cr209. [Google Scholar] [CrossRef]
- Saneto, R.P.; Lee, I.C.; Koenig, M.K.; Bao, X.; Weng, S.W.; Naviaux, R.K.; Wong, L.J.C. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure 2010, 19, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ponchaut, S.; van Hoof, F.; Veitch, K. Cytochrome aa3 depletion is the cause of the deficient mitochondrial respiration induced by chronic valproate administration. Biochem. Pharmacol. 1992, 43, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Ormrod, D.; Spencer, C.M. Tianeptine: A review of its use in depressive disorders. CNS Drugs 2001, 15, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Eftekhari, A.; Fard, J.K.; Babaei, H.; Nayebi, A.M.; Mohammadnejad, D.; Eghbal, M.A. In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch. Pharmacol. Res. 2017, 40, 1296–1313. [Google Scholar] [CrossRef]
- Brambilla, P.; Cipriani, A.; Hotopf, M.; Barbui, C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: A meta-analysis of clinical trial data. Pharmacopsychiatry 2005, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Suzuki, A.; Thakkar, S.; Yu, K.; Hu, C.; Tong, W. DILIrank: The largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov. Today 2016, 21, 648–653. [Google Scholar] [CrossRef]
- Jackson, M.C.; Jafarpour, S.; Klehm, J.; Thome-Souza, S.; Coughlin, F.; Kapur, K.; Loddenkemper, T. Effect of vigabatrin on seizure control and safety profile in different subgroups of children with epilepsy. Epilepsia 2017, 58, 1575–1585. [Google Scholar] [CrossRef]
- Canter, J.A.; Haas, D.W.; Kallianpur, A.R.; Ritchie, M.D.; Robbins, G.K.; Shafer, R.W.; Clifford, D.B.; Murdock, D.G.; Hulgan, T. The mitochondrial pharmacogenomics of haplogroup T: MTND2*LHON4917G and antiretroviral therapy-associated peripheral neuropathy. Pharmacogenom. J. 2008, 8, 71–77. [Google Scholar] [CrossRef]
- Kampira, E.; Kumwenda, J.; van Oosterhout, J.J.; Dandara, C. Mitochondrial DNA subhaplogroups L0a2 and L2a modify susceptibility to peripheral neuropathy in malawian adults on stavudine containing highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2013, 63, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, G.; Soriano, À.; Pinós, T.; Casanova-Mollà, J.; Pacheu-Grau, D.; Morén, C.; García-Arumí, E.; Morales, M.; Ruiz-Pesini, E.; Catalán-Garcia, M.; et al. Influence of Mitochondrial Genetics on the Mitochondrial Toxicity of Linezolid in Blood Cells and Skin Nerve Fibers. Antimicrob. Agents Chemother. 2017, 61, e00542-17. [Google Scholar] [CrossRef] [PubMed]
- Pacheu-Grau, D.; Gomez-Durán, A.; Iglesias, E.; Lopez-Gallardo, E.; Montoya, J.; Ruiz-Pesini, E. Mitochondrial antibiograms in personalized medicine. Hum. Mol. Genet. 2012, 22, 1132–1139. [Google Scholar] [CrossRef] [PubMed]




| Compounds | Drug Class | Literature Mechanism | Reference |
|---|---|---|---|
| Amisulpride | Antipsychotic | no reported effects | N/A |
| Aripiprazole | Antipsychotic | CI inhibitor | [51] |
| Buspirone | Anxiolytic | CI inhibitor | [52] |
| Carbamazepine | Anticonvulsant | decreased ATP production | [53] |
| CI and IV inhibitor | [44] | ||
| Chlorpromazine | Antipsychotic | CI and IV inhibitor | [20,51] |
| Citalopram | Antidepressant | CI inhibitor | [54] |
| increased ROS, loss of MMP | [55] | ||
| Clozapine | Antipsychotic | CI, II+III, and IV inhibitor | [20,51,56] |
| Fluoxetine | Antidepressant | F1F0 ATPase inhibitor decreased state 3 respiration and RCR | [57] [58] |
| decreased CI and CII-linked respiration | [59] | ||
| Haloperidol | Antipsychotic | CI inhibitor | [51] |
| Lamotrigine | Anticonvulsant | increased ATP production | [53] |
| decreased CI-linked respiration | [44] | ||
| Lorazepam | Anxiolytic | no reported effects | N/A |
| Olanzapine | Antipsychotic | CI and IV inhibitor | [54] |
| activation of citrate synthase activity | [54] | ||
| Phenobarbital | Anticonvulsant | Decreased CI, II, and IV-linked respiration | [41] |
| Phenytoin (bioactivated) | Anticonvulsant | decreased state-3 respiration and ATP synthesis | [41] |
| Primidone | Anticonvulsant | enhanced SOD activity and decrease in monoamine oxidases | [60] |
| Quetiapine | Antipsychotic | CI inhibitor | [51] |
| Reserpine | Antipsychotic | uncoupler | [61] |
| Tianeptine | Antidepressant | mitochondrial FAO inhibitor | [62] |
| CI and II inhibitor | [54] | ||
| Trifluoperazine | Antipsychotic | ETC inhibitor | [63] |
| ATPase inhibitor | [64] | ||
| Valproic acid | Anticonvulsant | CI and IV inhibitor | [54] |
| mitochondrial FAO inhibitor | [65] | ||
| MPTP opening | [66] | ||
| decreased OCR, MMP, ATP, and increased ROS | [67] | ||
| Vigabatrin | Anticonvulsant | no reported effects | N/A |
| Ziprasidone | Antipsychotic | CII, III, and IV inhibitors | [19,51] |
| Compounds | Cmax µM | Cmax Reference | Conc. Range µM | Direction of Change | Summary Mechanism | ||||
|---|---|---|---|---|---|---|---|---|---|
| OCR | Reserve Capacity | ECAR | ATP | Proton Leak | |||||
| Amisulpride | 2.56 | [68] | 0.1–100 | NR | NR | NR | NR | NR | - |
| Aripiprazole | 0.7 | [69] | 0.01–10 | ↓ | ↓ | ↑ | ↓ | NR | ETC inhibitor |
| Chlorpromazine | 0.9 | [70] | 0.1–100 | ↓ | ↓ | ↓ | ↓ | ↑ | Cytotoxicity |
| Clozapine | 0.22 | [71] | 0.1–100 | NR | NR | ↓ | ↓ | NR | Other |
| Haloperidol | 0.02 | [72] | 0.1–100 | NR | NR | NR | NR | NR | - |
| Olanzapine | 0.02 | [73] | 0.05–50 | NR | NR | NR | NR | NR | - |
| Quetiapine | 1.88 | [74] | 0.15–150 | ↓ | ↓ | NR | ↓ | NR | Substrate inhibitor |
| Reserpine | 0.0004 | [75] | 0.1–100 | ↑ | NR | NR | ↓ | ↑ | Uncoupler |
| Trifluoperazine | 0.005 | [76] | 0.002–2 | NR | ↓ | NR | NR | NR | Other |
| Ziprasidone | 0.11 | [77] | 0.04–40 | NR | NR | NR | ↑ | ↓ | Other |
| Carbamazepine | 6.3 | [78] | 0.6–600 | ↓ | ↓ | NR | ↓ | ↓ | Substrate inhibitor |
| Lamotrigine | 4 | [79] | 0.1–100 | NR | NR | NR | ↓ | NR | Other |
| Phenobarbital | 13.8 | [80] | 0.1–100 | NR | NR | NR | NR | NR | - |
| Phenytoin | 30 | [81] | 0.2–200 | ↓ | ↓ | ↑ | ↓ | NR | ETC inhibitor |
| Primidone | N/A | N/A | 0.1–100 | NR | NR | NR | NR | NR | - |
| Valproic acid | 367 | [82] | 10–10,000 | ↓ | ↓ | ↓ | ↓ | ↑ | Cytotoxicity |
| Vigabatrin | 4 | [83] | 1–1000 | NR | NR | NR | NR | NR | - |
| Buspirone | 0.003 | [84] | 0.1–100 | ↓ | ↓ | NR | ↓ | NR | Substrate inhibitor |
| Lorazepam | 0.1 | [85] | 0.1–100 | ↓ | NR | NR | ↓ | ↑ | Other |
| Citalopram | 0.1 | [86] | 0.01–10 | NR | NR | NR | NR | ↓ | Other |
| Fluoxetine | 0.04 | [87] | 0.1–100 | ↓ | ↓ | ↑ | ↓ | NR | ETC inhibitor |
| Tianeptine | 0.62 | [88] | 0.1–100 | ↓ | ↓ | NR | ↓ | NR | Substrate inhibitor |
| Rotenone | N/A | N/A | 0.003–1 | ↓ | ↓ | ↑ | ↓ | ↓ | ETC inhibitor |
| Compounds | MEC (µM) | AC50 (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OCR | Reserve Capacity | ECAR | ATP Production | Proton Leak | OCR | Reserve Capacity | ECAR | ATP Production | Proton Leak | |
| Amisulpride | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Aripiprazole | 2.06 | 0.344 | 9.20 | 3.49 | NR | >10 | >10 | >10 | >10 | NR |
| Chlorpromazine | 73.4 | 78.4 | 15.6 | 94.2 | 31 | >100 | >100 | >100 | >100 | >100 |
| Clozapine | NR | NR | 20.2 | 37.3 | NR | NR | NR | >100 | >100 | NR |
| Haloperidol | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Olanzapine | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Quetiapine | 19 | 26.1 | NR | 92.3 | NR | >150 | 124 | NR | >150 | NR |
| Reserpine | 60.3 | NR | NR | 59.4 | 30 | >100 | NR | NR | 77.3 | 36.8 |
| Trifluoperazine | NR | <0.002 | NR | NR | NR | NR | 0.157 | NR | NR | NR |
| Ziprasidone | NR | NR | NR | 0.949 | 0.12 | NR | NR | NR | >12.7 | 2.19 |
| Carbamazepine | 13.1 | 137 | NR | 165 | 97 | >600 | >190 | NR | 363 | >600 |
| Lamotrigine | NR | NR | NR | 14 | NR | NR | NR | NR | 75.7 | NR |
| Phenobarbital | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Phenytoin | 90.9 | 1.45 | 138 | 197 | NR | >200 | 26.6 | >200 | >200 | NR |
| Primidone | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Valproic acid | 520 | 3090 | 2600 | 5200 | 1140 | >10,000 | >10,000 | >10,000 | >10,000 | 9180 |
| Vigabatrin | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Buspirone | 4.83 | 8.57 | NR | 80.9 | NR | >100 | 43.7 | NR | >100 | NR |
| Lorazepam | 63.7 | NR | NR | 3.2 | 3.14 | >100 | NR | NR | >100 | 15 |
| Citalopram | NR | NR | NR | NR | 7.17 | NR | NR | NR | NR | >10 |
| Fluoxetine | 8.12 | 25.2 | 67.3 | 68.6 | NR | >100 | 70.4 | >100 | >100 | NR |
| Tianeptine | 5.5 | 0.182 | NR | 40.3 | NR | >100 | 21.6 | NR | >100 | NR |
| Rotenone | 0.0053 | <0.003 | 0.0274 | 0.0159 | 0.0120 | 0.0433 | 0.0106 | >1 | 0.0459 | 0.213 |
| Compounds | rPFO Permeabilized HepG2 Cells | Intact HepG2 Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| ⮁ | Pyruvate Respiration (μM) | Succinate Respiration (μM) | Ascorbate Respiration (μM) | Outcome of AEF Assay on Intact HepG2 Cells | ||||
| AC50 | MEC | AC50 | MEC | AC50 | MEC | |||
| Aripiprazole | NR | NR | NR | NR | NR | NR | ETC inhibitor | |
| Chlorpromazine | NR | NR | NR | NR | NR | NR | Cytotoxicity | |
| Quetiapine | ↓ | >150 | 66.3 | NR | NR | NR | NR | Substrate inhibitor |
| Reserpine | NR | NR | NR | NR | NR | NR | Uncoupler | |
| Carbamazepine | NR | NR | NR | NR | NR | NR | Substrate inhibitor | |
| Phenytoin | NR | NR | NR | NR | NR | NR | ETC inhibitor | |
| Valproic acid | ↓ | >10000 | 6730 | NR | NR | NR | NR | Cytotoxicity |
| Buspirone | ↓ | >100 | 44.5 | NR | NR | NR | NR | Substrate inhibitor |
| Fluoxetine | ↓ | >100 | 72.1 | NR | NR | NR | NR | ETC inhibitor |
| Tianeptine | NR | NR | NR | NR | NR | NR | Substrate inhibitor | |
| Rotenone | ↓ | 0.0071 | 0.0009 | NR | NR | NR | NR | ETC inhibitor |
| Compounds | ⮁ | Glu AC50 (μM) | Glu MEC (μM) | ⮁ | Gal AC50 (μM) | Gal MEC (μM) | AC50 Fold Change | |
|---|---|---|---|---|---|---|---|---|
| Antipsychotics | Amisulpride | NR | NR | NR | NR | NR | ||
| Aripiprazole | NR | NR | ↓ | 9.17 | 5.67 | UD | ||
| Chlorpromazine | ↓ | 15.9 | 10.7 | ↓ | 16.9 | 10.5 | 0.941 | |
| Clozapine | ↓ | 56.7 | 34.1 | ↓ | 60.8 | 44.4 | 0.933 | |
| Haloperidol | ↓ | 57.5 | 26.2 | ↓ | 78.6 | 26.0 | 0.732 | |
| Olanzapine | NR | NR | NR | NR | NR | |||
| Quetiapine | ↓ | 76.7 | 49.3 | ↓ | 79.0 | 57.7 | 0.971 | |
| Reserpine | ↓ | 48.9 | 6.68 | ↓ | 79.0 | 19.0 | 0.619 | |
| Trifluoperazine | ↓ | 1.0 | 0.75 | ↓ | 1.13 | 0.80 | 0.885 | |
| Ziprasidone | NR | NR | ↓ | >40 | 28.3 | UD | ||
| Anticonvulsants | Carbamazepine | NR | NR | ↓ | >600 | 408 | UD | |
| Lamotrigine | NR | NR | NR | NR | NR | |||
| Phenobarbital | NR | NR | NR | NR | NR | |||
| Phenytoin | NR | NR | NR | NR | NR | |||
| Primidone | NR | NR | NR | NR | NR | |||
| Valproic acid | NR | NR | NR | NR | NR | |||
| Vigabatrin | NR | NR | NR | NR | NR | |||
| Antidepressants/ Anxiolytic drugs | Buspirone | NR | NR | ↓ | 99 | 35.7 | UD | |
| Lorazepam | NR | NR | NR | NR | NR | |||
| Citalopram | ↓ | >10 | 4.43 | ↓ | >10 | 7.11 | UD | |
| Fluoxetine | ↓ | 13.3 | 6.89 | ↓ | 17.5 | 12.1 | 0.76 | |
| Tianeptine | NR | NR | ↓ | 57.6 | 41.8 | UD | ||
| CI inhibitor | Rotenone | ↓ | 27.8 | 0.29 | ↓ | 0.00605 | 0.002 | 4600 |
| Assays | Amisulpride | Aripiprazole | Chlorpromazine | Clozapine | Haloperidol | Olanzapine | Quetiapine | Reserpine | Trifluoperazine | Ziprasidone | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute HepG2 Extracellular Flux Assay | OCR (AC50 μM) | NR | >10↓ | >100↓ | NR | NR | NR | >150↓ | >100↑ | NR | NR |
| Reserve capacity (AC50 μM) | NR | >10↓ | >100↓ | NR | NR | NR | 124↓ | NR | 0.157↓ | NR | |
| ECAR (AC50 μM) | NR | >10↑ | >100↓ | >100↓ | NR | NR | NR | NR | NR | NR | |
| ATP production (AC50 μM) | NR | >10↓ | >100↓ | >100↓ | NR | NR | >150↓ | 77.3↓ | NR | >12.7↑ | |
| Proton leak (AC50 μM) | NR | NR | >100↑ | NR | NR | NR | NR | 36.8↑ | NR | 2.19↓ | |
| Summary mechanism | NR | ETC inhibitor | Cytotoxicity | Other | NR | NR | Substrate inhibitor | Uncoupler | Other | Other | |
| Permeabilized HepG2 Extracellular Flux Assay (OCR) | Most sensitive mechanism (AC50 μM) | N/A | NR | NR | N/A | N/A | N/A | Pyruvate respiration↓ >150 | NR | N/A | N/A |
| Glu/Gal assay (cell viability reduction, 24 h incubation treatment) | Glucose (AC50 μM) | NR | NR | 15.9↓ | 56.7↓ | 57.5↓ | NR | 76.7↓ | 48.9↓ | 1↓ | NR |
| Galactose (AC50 μM) | NR | 9.17↓ | 16.9↓ | 60.8↓ | 78.6↓ | NR | 79↓ | 79↓ | 1.13↓ | >40↓ | |
| Fold change | NR | UD | 0.941 | 0.933 | 0.732 | NR | 0.971 | 0.619 | 0.885 | UD | |
| Basal succinate-driven respiration in isolated RLM | % MMP (Conc. μM) | 88 (20), 79 (50), 72 (100) | 88 (20), 69 (50) | 92 (20), 90 (50) | |||||||
| % O2 consumption (Conc. μM) | 111 (20), 125 (50), 131 (100) | 85 (20), 77 (50) | 90 (20), 92 (50) | ||||||||
| Cmax (μM) | 2.56 | 0.7 | 0.9 | 0.22 | 0.02 | 0.02 | 1.88 | 0.0004 | 0.005 | 0.11 |
| Assays | Carbamazepine | Lamotrigine | Phenobarbital | Phenytoin | Primidone | Valproic Acid | Vigabatrin | |
|---|---|---|---|---|---|---|---|---|
| Acute HepG2 Extracellular Flux Assay | OCR (AC50 μM) | >600↓ | NR | NR | >200↓ | NR | >10,000↓ | NR |
| Reserve capacity (AC50 μM) | >190↓ | NR | NR | 26.6↓ | NR | >10,000↓ | NR | |
| ECAR (AC50 μM) | NR | NR | NR | >200↑ | NR | >10,000↓ | NR | |
| ATP production (AC50 μM) | 363↓ | 75.7↓ | NR | >200↓ | NR | >10,000↓ | NR | |
| Proton leak (AC50 μM) | >600↓ | NR | NR | NR | NR | 9180↑ | NR | |
| Summary mechanism | Substrate inhibitor | Other | NR | ETC inhibitor | NR | Cytotoxicity | NR | |
| Permeabilized HepG2 Extracellular Flux Assay (OCR) | Most sensitive mechanism (AC50 μM) | NR | N/A | N/A | NR | N/A | pyruvate respiration↓ >10,000 | N/A |
| Glu/Gal assay (cell viability reduction, 24 h incubation treatment) | Glucose (AC50 μM) | NR | NR | NR | NR | NR | NR | NR |
| Galactose (AC50 μM) | >600↓ | NR | NR | NR | NR | NR | NR | |
| Fold Change | UD | NR | NR | NR | NR | NR | NR | |
| Cmax (μM) | 6 | 4 | 89 | 30 | N/A | 367 | 4 |
| Assays | Buspirone | Lorazepam | Citalopram | Fluoxetine | Tianeptine | |
|---|---|---|---|---|---|---|
| Acute HepG2 Extracellular Flux Assay | OCR (AC50 μM) | >100↓ | >100↓ | NR | >100↓ | >100↓ |
| Reserve capacity (AC50 μM) | 43.7↓ | NR | NR | 70.4↓ | 21.6↓ | |
| ECAR (AC50 μM) | NR | NR | NR | >100↑ | NR | |
| ATP production (AC50 μM) | >100↓ | >100↓ | NR | >100↓ | >100↓ | |
| Proton leak (AC50 μM) | NR | 15↑ | >10↓ | NR | NR | |
| Summary mechanism | Substrate inhibitor | Other | Other | ETC inhibitor | Substrate inhibitor | |
| Permeabilized HepG2 Extracellular Flux Assay (OCR) | Most sensitive mechanism (AC50 μM) | pyruvate respiration↓ >100 | N/A | N/A | pyruvate respiration↓ >100 | NR |
| Glu/Gal assay (cell viability reduction, 24 h incubation treatment) | Glucose (AC50 μM) | NR | NR | >10↓ | 13.3↓ | NR |
| Galactose (AC50 μM) | 99↓ | NR | >10↓ | 17.5↓ | 57.6↓ | |
| Fold Change | UD | NR | UD | 0.76 | UD | |
| Cmax (μM) | 0.003 | 0.1 | 0.1 | 0.04 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosell-Hidalgo, A.; Eakins, J.; Walker, P.; Moore, A.L.; Ghafourian, T. Risk Assessment of Psychotropic Drugs on Mitochondrial Function Using In Vitro Assays. Biomedicines 2023, 11, 3272. https://doi.org/10.3390/biomedicines11123272
Rosell-Hidalgo A, Eakins J, Walker P, Moore AL, Ghafourian T. Risk Assessment of Psychotropic Drugs on Mitochondrial Function Using In Vitro Assays. Biomedicines. 2023; 11(12):3272. https://doi.org/10.3390/biomedicines11123272
Chicago/Turabian StyleRosell-Hidalgo, Alicia, Julie Eakins, Paul Walker, Anthony L. Moore, and Taravat Ghafourian. 2023. "Risk Assessment of Psychotropic Drugs on Mitochondrial Function Using In Vitro Assays" Biomedicines 11, no. 12: 3272. https://doi.org/10.3390/biomedicines11123272
APA StyleRosell-Hidalgo, A., Eakins, J., Walker, P., Moore, A. L., & Ghafourian, T. (2023). Risk Assessment of Psychotropic Drugs on Mitochondrial Function Using In Vitro Assays. Biomedicines, 11(12), 3272. https://doi.org/10.3390/biomedicines11123272






