Genomic Landscape Comparison of Cardiac versus Extra-Cardiac Angiosarcomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coding Transcriptome Sequencing
2.2. Bioinformatic Analysis
2.3. Transfection of the POTEH Gene Transcript
2.4. Real-Time PCR Analysis
3. Results
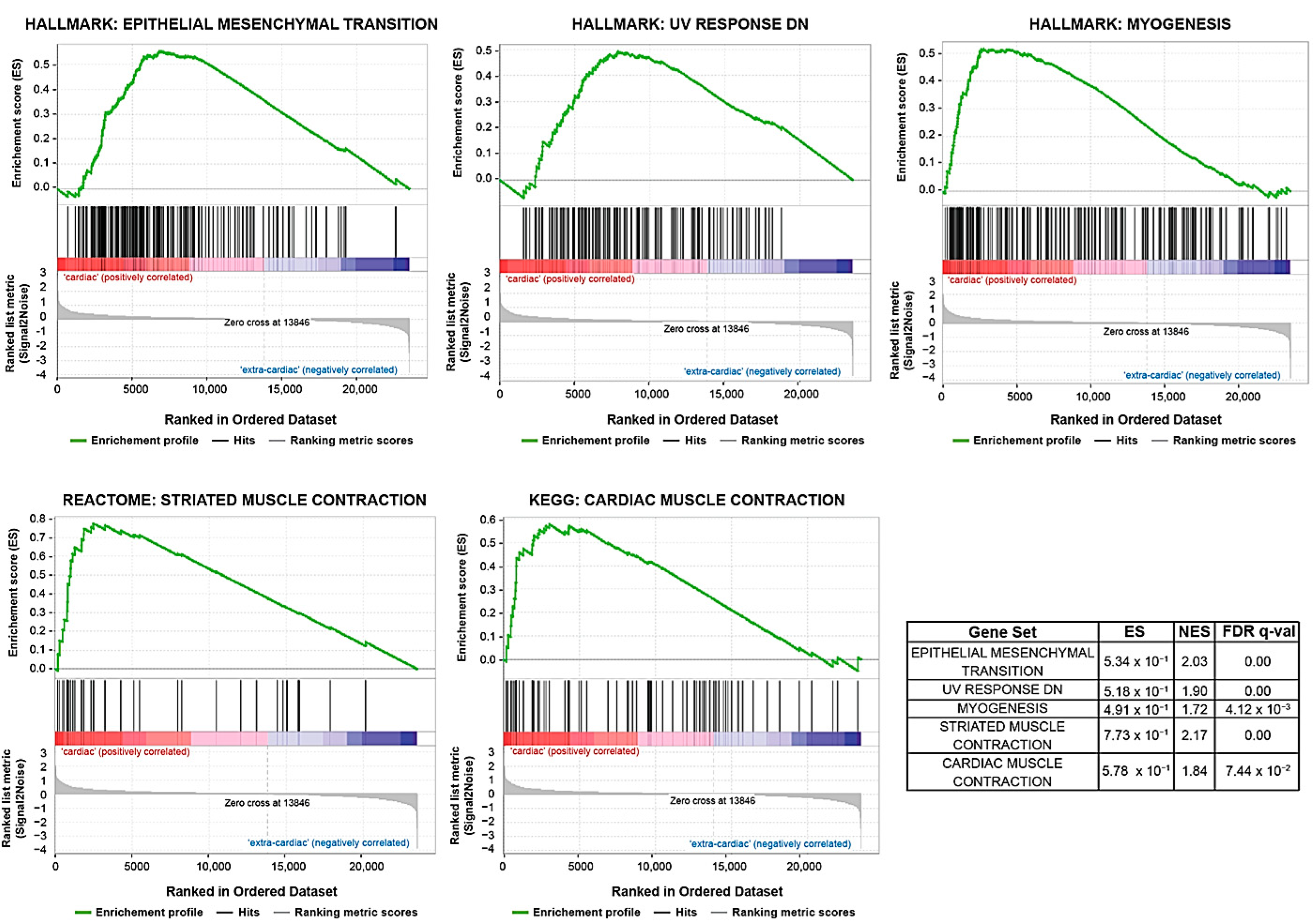
3.1. Gene Expression Analysis
3.2. POTEH Overexpression
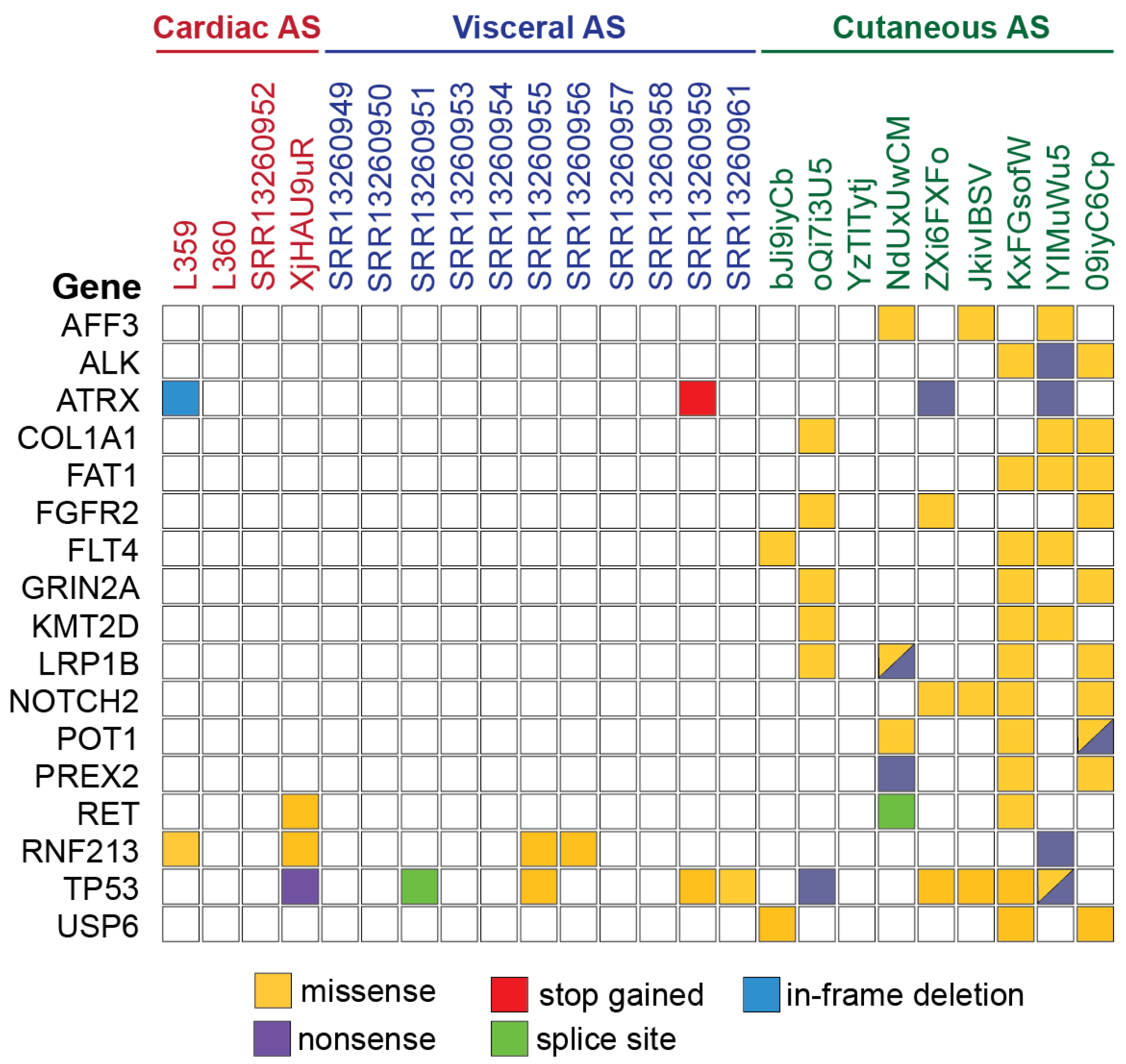
3.3. Gene Alteration Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayette, J.; Martin, E.; Piperno-Neumann, S.; le Cesne, A.; Robert, C.; Bonvalot, S.; Ranchère, D.; Pouillart, P.; Coindre, J.M.; Blay, J.Y. Angiosarcomas, a Heterogeneous Group of Sarcomas with Specific Behavior Depending on Primary Site: A Retrospective Study of 161 Cases. Ann. Oncol. 2007, 18, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Weidema, M.E.; Versleijen-Jonkers, Y.M.H.; Flucke, U.E.; Desar, I.M.E.; van der Graaf, W.T.A. Targeting Angiosarcomas of the Soft Tissues: A Challenging Effort in a Heterogeneous and Rare Disease. Crit. Rev. Oncol. Hematol. 2019, 138, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Lim, J.Q.; Yeong, J.; Ravi, V.; Guan, P.; Boot, A.; Tay, T.K.Y.; Selvarajan, S.; Md Nasir, N.D.; Loh, J.H.; et al. Multiomic Analysis and Immunoprofiling Reveal Distinct Subtypes of Human Angiosarcoma. J. Clin. Investig. 2020, 130, 5833–5846. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Robin, A.; Young, J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, A.H.; Jensen, C.T.; Palmquist, S.; Pickhardt, P.J.; Duran, A.; Broering, G.; Elsayes, K.M. Angiosarcoma: Clinical and Imaging Features from Head to Toe. Br. J. Radiol. 2017, 90, 20170039. [Google Scholar] [CrossRef]
- Sturm, E.C.; Marasco, I.S.; Katz, S.C. Multidisciplinary Management of Angiosarcoma—A Review. J. Surg. Res. 2021, 257, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Urbini, M.; Astolfi, A.; Indio, V.; Nannini, M.; Pizzi, C.; Paolisso, P.; Tarantino, G.; Pantaleo, M.A.; Saponara, M. Genetic Aberrations and Molecular Biology of Cardiac Sarcoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920918492. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Chiara Sergi, M.; Cameli, M.; Miglioranza, M.H.; Ciccone, M.M.; Gentile, M.; Porta, C.; Tucci, M. Primary Soft Tissue Sarcoma of the Heart: An Emerging Chapter in Cardio-Oncology. Biomedicines 2021, 9, 774. [Google Scholar] [CrossRef]
- Patel, S.D.; Peterson, A.; Bartczak, A.; Lee, S.; Chojnowski, S.; Gajewski, P.; Loukas, M. Primary Cardiac Angiosarcoma—A Review. Med. Sci. Monit. 2014, 20, 103–109. [Google Scholar] [CrossRef]
- Gozzellino, L.; Nannini, M.; Pizzi, C.; Leone, O.; Corti, B.; Indio, V.; Baldovini, C.; Paolisso, P.; Foà, A.; Pacini, D.; et al. Genomic Characterization of Rare Primary Cardiac Sarcoma Entities. Diagnostics 2023, 13, 214. [Google Scholar] [CrossRef]
- Saponara, M.; Indio, V.; Pizzi, C.; Serban, E.D.; Urbini, M.; Astolfi, A.; Paolisso, P.; Suarez, S.M.; Nannini, M.; Pacini, D.; et al. Successful Multidisciplinary Clinical Approach and Molecular Characterization by Whole Transcriptome Sequencing of a Cardiac Myxofibrosarcoma: A Case Report. World J. Clin. Cases 2019, 7, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Megquier, K.; Thomas, R.; Sarver, A.L.; Song, J.M.; Kim, Y.T.; Cheng, N.; Schulte, A.J.; Linden, M.A.; Murugan, P.; et al. Genomically Complex Human Angiosarcoma and Canine Hemangiosarcoma Establish Convergent Angiogenic Transcriptional Programs Driven by Novel Gene Fusions. Mol. Cancer Res. 2021, 19, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 7 October 2022).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Painter, C.A.; Jain, E.; Tomson, B.N.; Dunphy, M.; Stoddard, R.E.; Thomas, B.S.; Damon, A.L.; Shah, S.; Kim, D.; Gómez Tejeda Zañudo, J.; et al. The Angiosarcoma Project: Enabling Genomic and Clinical Discoveries in a Rare Cancer through Patient-Partnered Research. Nat. Med. 2020, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve Years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing High-Throughput Sequencing Data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Sigg, C.D.; Buhmann, J.M. Expectation-Maximization for Sparse and Non-Negative PCA. In Proceedings of the Twenty-Fifth International Conference on Machine Learning, Helsinki, Finland, 5–9 June 2008. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Fertig, E.J.; Jaffe, A.E.; Zhang, Y.; Storey, J.D.; Torres, L.C. Package “sva”: Surrogate Variable Analysis; Bioconductor: Boston, MA, USA, 2022. [Google Scholar]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 2, 15545–15550. [Google Scholar] [CrossRef]
- Garcia, J.M.; Gonzalez, R.; Silva, J.M.; Dominguez, G.; Vegazo, I.S.; Gamallo, C.; Provencio, M.; España, P.; Bonilla, F. Mutational Status of K-Ras and TP53 Genes in Primary Sarcomas of the Heart. Br. J. Cancer 2000, 82, 1183–1185. [Google Scholar] [CrossRef]
- Zhrebker, L.; Cherni, I.; Gross, L.M.; Hinshelwood, M.M.; Reese, M.; Aldrich, J.; Guileyardo, J.M.; Roberts, W.C.; Craig, D.; von Hoff, D.D.; et al. Case Report: Whole Exome Sequencing of Primary Cardiac Angiosarcoma Highlights Potential for Targeted Therapies. BMC Cancer 2017, 17, 17. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S.; Sheldon, H.; Martincorena, I.; van Loo, P.; Gundem, G.; Wedge, D.C.; Ramakrishna, M.; Cooke, S.L.; Pillay, N.; et al. Recurrent PTPRB and PLCG1 Mutations in Angiosarcoma. Nat. Genet. 2014, 46, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Chandramohan, R.; Möller, I.; Scholz, S.L.; Berger, M.; Huberman, K.; Viale, A.; Socci, N.D.; Bouvier, N.; Bauer, S.; et al. Targeted Massively Parallel Sequencing of Angiosarcomas Reveals Frequent Activation of the Mitogen Activated Protein Kinase Pathway. Oncotarget 2015, 6, 36041–36052. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Freire, A.P.; Elliott, A.; Rosenberg, A.; Costa, P.A.; Barreto-Coelho, P.; Jonczak, E.; D’amato, G.; Subhawong, T.; Arshad, J.; Diaz-Perez, J.A.; et al. Genomic Landscape of Angiosarcoma: A Targeted and Immunotherapy Biomarker Analysis. Cancers 2021, 13, 4816. [Google Scholar] [CrossRef] [PubMed]
- Naka, N.; Tomita, Y.; Nakamshi, H.; Araki, N.; Hongyo, T.; Ochi, T.; Aozasa, K. Mutations of P53 Tumor-Suppressor Gene in Angiosarcoma. Int. J. Cancer 1997, 71, 952–955. [Google Scholar] [CrossRef]
- Jiang, N.; Zhou, J.; Zhang, W.; Li, P.; Liu, Y.; Shi, H.; Zhang, C.; Wang, Y.; Zhou, C.; Peng, C.; et al. RNF213 Gene Mutation in Circulating Tumor DNA Detected by Targeted Next-Generation Sequencing in the Assisted Discrimination of Early-Stage Lung Cancer from Pulmonary Nodules. Thorac. Cancer 2021, 12, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Pollaci, G.; Gorla, G.; Potenza, A.; Carrozzini, T.; Canavero, I.; Bersano, A.; Gatti, L. Novel Multifaceted Roles for RNF213 Protein. Int. J. Mol. Sci. 2022, 23, 4492. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Zhang, L.; Sung, Y.S.; Chen, C.L.; Kao, Y.C.; Agaram, N.P.; Singer, S.; Tap, W.D.; D’Angelo, S.; Antonescu, C.R. Recurrent CIC Gene Abnormalities in Angiosarcomas: A Molecular Study of 120 Cases with Concurrent Investigation of PLCG1, KDR, MYC, and FLT4 Gene Alterations. Am. J. Surg. Pathol. 2016, 40, 645–655. [Google Scholar] [CrossRef]
- Kunze, K.; Spieker, T.; Gamerdinger, U.; Nau, K.; Berger, J.; Dreyer, T.; Sindermann, J.R.; Hoffmeier, A.; Gattenlöhner, S.; Bräuninger, A. A Recurrent Activating PLCG1 Mutation in Cardiac Angiosarcomas Increases Apoptosis Resistance and Invasiveness of Endothelial Cells. Cancer Res. 2014, 74, 6173–6183. [Google Scholar] [CrossRef]
- Singh, D.K.; Gholamalamdari, O.; Jadaliha, M.; Li, X.L.; Lin, Y.C.; Zhang, Y.; Guang, S.; Hashemikhabir, S.; Tiwari, S.; Zhu, Y.J.; et al. PSIP1/P75 Promotes Tumorigenicity in Breast Cancer Cells by Promoting the Transcription of Cell Cycle Genes. Carcinogenesis 2017, 38, 966–975. [Google Scholar] [CrossRef]
- Liedtke, V.; Schröder, C.; Roggenbuck, D.; Weiss, R.; Stohwasser, R.; Schierack, P.; Rödiger, S.; Schenk, L. LEDGF/P75 Is Required for an Efficient Dna Damage Response. Int. J. Mol. Sci. 2021, 22, 5866. [Google Scholar] [CrossRef]
- Setton, J.; Selenica, P.; Mukherjee, S.; Shah, R.; Pecorari, I.; McMillan, B.; Pei, I.X.; Kemel, Y.; Ceyhan-Birsoy, O.; Sheehan, M.; et al. Germline RAD51B Variants Confer Susceptibility to Breast and Ovarian Cancers Deficient in Homologous Recombination. NPJ Breast Cancer 2021, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, C.B.; Fine, A.D.; André, F.; Ganesan, S.; Heinemann, V.; Rouleau, E.; Turnbull, C.; Palacios, L.G.; Lopez, J.A.; Sokol, E.S.; et al. Pan-Cancer Analysis of Homologous Recombination Repair-Associated Gene Alterations and Genome-Wide Loss-of-Heterozygosity Score. Clin. Cancer Res. 2022, 28, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Pishvaian, M.J.; Lynce, F.; Xiu, J.; Brody, J.R.; Chen, W.J.; Baker, T.M.; Marshall, J.L.; Isaacs, C. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis. Oncol. 2018, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Liu, L.; Ashby, J.M.; Wu, D.; Chen, Y.; O’Neill, S.S.; Huang, S.; Wang, J.; Wang, G.; Cheng, D.; et al. Recruitment of KMT2C/MLL3 to DNA Damage Sites Mediates DNA Damage Responses and Regulates PARP Inhibitor Sensitivity in Cancer. Cancer Res. 2021, 81, 3358–3373. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.; Amato, R.; Sgura, A.; Antoccia, A.; Berardinelli, F. The Multiple Facets of Atrx Protein. Cancers 2021, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Perner, F.; Perner, C.; Ernst, T.; Heidel, F.H. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Ernst, P.; Schnöder, T.M.; Huber, N.; Perner, F.; Jayavelu, A.K.; Eifert, T.; Hsu, C.J.; Tubío-Santamaría, N.; Crodel, C.C.; Ungelenk, M.; et al. Histone Demethylase KDM4C Is a Functional Dependency in JAK2-Mutated Neoplasms. Leukemia 2022, 36, 1843–1849. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Bera, T.K.; Zimonjic, D.B.; Popescu, N.C.; Sathyanarayana, B.K.; Kumar, V.; Lee, B.; Pastan, I. POTE, a Highly Homologous Gene Family Located on Numerous Chromosomes and Expressed in Prostate, Ovary, Testis, Placenta, and Prostate Cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 16975–16980. [Google Scholar] [CrossRef]
- Sharma, A.; Albahrani, M.; Zhang, W.; Kufel, C.N.; James, S.R.; Odunsi, K.; Klinkebiel, D.; Karpf, A.R. Epigenetic Activation of POTE Genes in Ovarian Cancer. Epigenetics 2019, 14, 185–197. [Google Scholar] [CrossRef]
- Barger, C.J.; Zhang, W.; Sharma, A.; Chee, L.; James, S.R.; Kufel, C.N.; Miller, A.; Meza, J.; Drapkin, R.; Odunsi, K.; et al. Expression of the POTE Gene Family in Human Ovarian Cancer. Sci. Rep. 2018, 8, 17136. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, F.A.M.; Mercuri, L.; Antonacci, F.; Anaclerio, F.; Calabrese, F.M.; Lorusso, N.; L’abbate, A.; Sorensen, M.; Giannuzzi, G.; Eichler, E.E.; et al. Evolutionary Dynamics of the POTE Gene Family in Human and Nonhuman Primates. Genes 2020, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Fleur, A.S.; Lee, Y.; Kydd, A.; Hahn, Y.; Popescu, N.C.; Zimonjic, D.B.; Lee, B.; Pastan, I. POTE Paralogs Are Induced and Differentially Expressed in Many Cancers. Cancer Res. 2006, 66, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Calvete, O.; Garcia-Pavia, P.; Domínguez, F.; Bougeard, G.; Kunze, K.; Braeuninger, A.; Teule, A.; Lasa, A.; Ramón, Y.; Cajal, T.; et al. The Wide Spectrum of POT1 Gene Variants Correlates with Multiple Cancer Types. Eur. J. Hum. Genet. 2017, 25, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Calvete, O.; Garcia-Pavia, P.; Domınguez, F.; Mosteiro, L.; Perez-Cabornero, L.; Cantalapiedra, D.; Zorio, E.; Ramon, Y.; Cajal, T.; Crespo-Leiro, M.G.; et al. Pot1 and Damage Response Malfunction Trigger Acquisition of Somatic Activating Mutations in the Vegf Pathway in Cardiac Angiosarcomas. J. Am. Heart Assoc. 2019, 8, e012875. [Google Scholar] [CrossRef] [PubMed]
- Motaparthi, K.; Lauer, S.R.; Patel, R.M.; Vidal, C.I.; Linos, K. MYC Gene Amplification by Fluorescence in Situ Hybridization and MYC Protein Expression by Immunohistochemistry in the Diagnosis of Cutaneous Angiosarcoma: Systematic Review and Appropriate Use Criteria. J. Cutan. Pathol. 2021, 48, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Wagner, M.J.; Othus, M.; Patel, S.P.; Ryan, C.; Sangal, A.; Powers, B.; Budd, G.T.; Victor, A.I.; Hsueh, C.T.; Chugh, R.; et al. Multicenter Phase II Trial (SWOG S1609, Cohort 51) of Ipilimumab and Nivolumab in Metastatic or Unresectable Angiosarcoma: A Substudy of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART). J. Immunother. Cancer 2021, 9, e002990. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Antonescu, C.R.; Smith, S.; Bradic, M.; Kashani, D.; Richards, A.L.; Donoghue, M.; Kelly, C.M.; Nacev, B.; Chan, J.E.; et al. Clinical, Genomic, and Transcriptomic Correlates of Response to Immune Checkpoint Blockade-Based Therapy in a Cohort of Patients with Angiosarcoma Treated at a Single Center. J. Immunother. Cancer 2022, 10, e004149. [Google Scholar] [CrossRef]
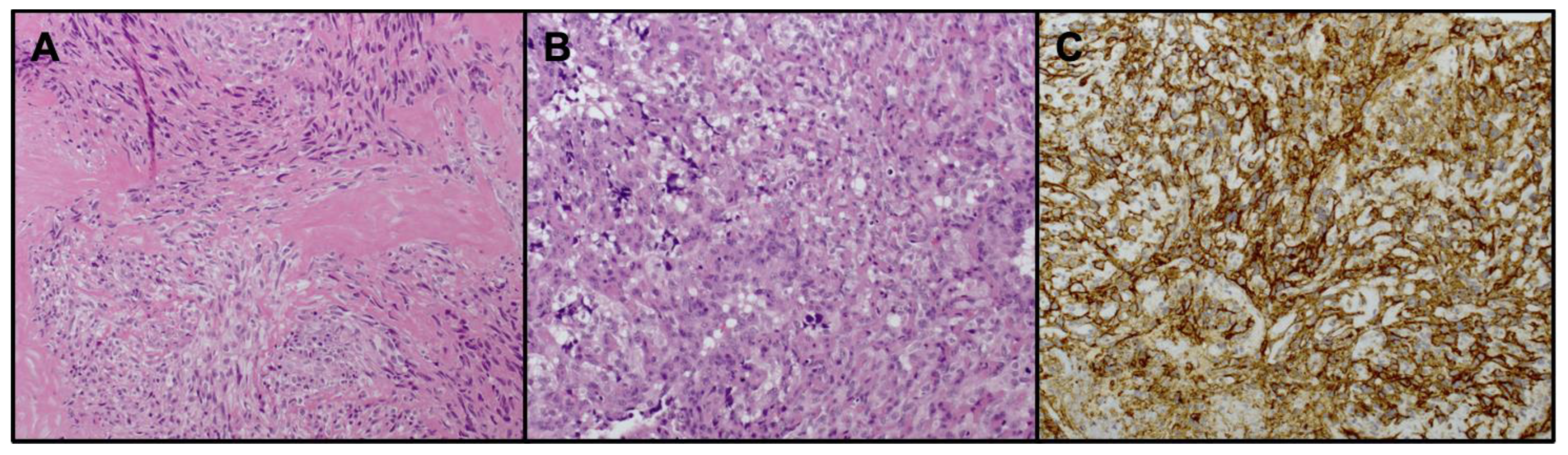



| Sample | Sex | Age | Histotype | Localization |
|---|---|---|---|---|
| L359 | male | 74 | angiosarcoma | heart |
| L360 | male | 39 | angiosarcoma | heart |
| SRR13260949 | female | 69 | angiosarcoma | omentum |
| SRR13260950 | male | 45 | angiosarcoma | lung |
| SRR13260951 | female | 39 | angiosarcoma | perihepatic tissue |
| SRR13260952 | male | 62 | angiosarcoma | heart |
| SRR13260953 | female | 64 | angiosarcoma | breast |
| SRR13260954 | unknown | unknown | angiosarcoma | adrenal gland |
| SRR13260955 | female | 40 | angiosarcoma | ovary |
| SRR13260956 | female | 73 | angiosarcoma | left chest wall |
| SRR13260957 | male | 47 | angiosarcoma | lung |
| SRR13260958 | female | 65 | angiosarcoma | breast |
| SRR13260959 | male | 85 | angiosarcoma | liver |
| SRR13260961 | male | 73 | angiosarcoma | soft tissue |
| Sample | Gene | Chr | cDNA | Protein | Type | COSMIC | ClinVar | Varsome | Gnom- AD Freq.1 | Alt Allele Depth | Total Read Depth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L359 | RAD51B | 14 | c.728A>G | p.(Lys243Arg) | missense | pathogenic | lik. Benign 2 | benign | 0.00679 | 11 | 19 |
| L359 | RNF213 | 17 | c.9952A>G | p.(Ile3318Val) | missense | NA 3 | benign | benign | 0.00198 | 14 | 21 |
| L359 | PSIP1 | 9 | c.651_654del | p.(Ser217Argfs Ter47) | frameshift | NA 3 | NA 3 | NA 3 | NA 3 | 43 | 64 |
| L359 | ATRX | 23 | c.6792_6794del | p.(Glu2265del) | inframe | NA 3 | NA 3 | NA 3 | NA 3 | 142 | 168 |
| SRR13260952 | KDR | 4 | c.2267-1_2267insTTTACATGTT | p.(Gly756Valfs Ter38) | frameshift | NA 3 | NA 3 | lik. Pathog. 4 | NA 3 | 178 | 213 |
| SRR13260952 | JAK2 | 9 | c.2840G>A | p.(Arg947Gln) | missense | pathogenic | NA 3 | uncertain | 0.000019 | 40 | 76 |
| SRR13260950 | KMT2C | 7 | c.10763C>T | p.(Ser3588Leu) | missense | pathogenic | benign | uncertain | 0.00281 | 15 | 29 |
| SRR13260950 | TSC2 | 16 | c.2653A>G | p.(Ile885Val) | missense | NA 3 | uncertain | uncertain | NA 3 | 56 | 96 |
| SRR13260951 | TP53 | 17 | c.920-1G>A | NA 3 | splice acceptor | NA 3 | pathogenic | uncertain | NA 3 | 32 | 34 |
| SRR13260953 | HRAS | 11 | c.182A>T | p.(Gln61Leu) | missense | pathogenic | uncertain | uncertain | NA 3 | 135 | 332 |
| SRR13260955 | TP53 | 17 | c.743G>A | p.(Arg248Gln) | missense | pathogenic | pathogenic | pathogenic | 0.000019 | 68 | 120 |
| SRR13260955 | RNF213 | 17 | c.5114C>A | p.(Thr1705Lys) | missense | NA 3 | benign | benign | 0.0045 | 101 | 131 |
| SRR13260955 | NBN | 8 | c.758C>T | p.(Thr253Ile) | missense | NA 3 | benign | benign | 0.000092 | 71 | 130 |
| SRR13260956 | RNF213 | 17 | c.6821C>T | p.(Pro2274Leu) | missense | NA 3 | NA 3 | benign | 0.000019 | 34 | 63 |
| SRR13260956 | NRAS | 1 | c.182A>T | p.(Gln61Leu) | missense | pathogenic | pathogenic | pathogenic | NA 3 | 23 | 126 |
| SRR13260957 | FANCE | 6 | c.1095A>C | p.(Arg365Ser) | missense | NA 3 | uncertain | benign | 0.000526 | 20 | 36 |
| SRR13260959 | TP53 | 17 | c.659A>G | p.(Tyr220Cys) | missense | pathogenic | pathogenic | pathogenic | 0.000007 | 54 | 143 |
| SRR13260959 | ATRX | 23 | c.4675A>T | p.(Lys1559Ter) | stop gain | NA 3 | NA 3 | lik. Pathog. 4 | NA 3 | 22 | 41 |
| SRR13260961 | TP53 | 17 | c.749C>T | p.(Pro250Leu) | missense | pathogenic | uncertain | pathogenic | NA 3 | 26 | 68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozzellino, L.; Nannini, M.; Urbini, M.; Pizzi, C.; Leone, O.; Corti, B.; Baldovini, C.; Angeli, F.; Foà, A.; Pacini, D.; et al. Genomic Landscape Comparison of Cardiac versus Extra-Cardiac Angiosarcomas. Biomedicines 2023, 11, 3290. https://doi.org/10.3390/biomedicines11123290
Gozzellino L, Nannini M, Urbini M, Pizzi C, Leone O, Corti B, Baldovini C, Angeli F, Foà A, Pacini D, et al. Genomic Landscape Comparison of Cardiac versus Extra-Cardiac Angiosarcomas. Biomedicines. 2023; 11(12):3290. https://doi.org/10.3390/biomedicines11123290
Chicago/Turabian StyleGozzellino, Livia, Margherita Nannini, Milena Urbini, Carmine Pizzi, Ornella Leone, Barbara Corti, Chiara Baldovini, Francesco Angeli, Alberto Foà, Davide Pacini, and et al. 2023. "Genomic Landscape Comparison of Cardiac versus Extra-Cardiac Angiosarcomas" Biomedicines 11, no. 12: 3290. https://doi.org/10.3390/biomedicines11123290






