Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies
Abstract
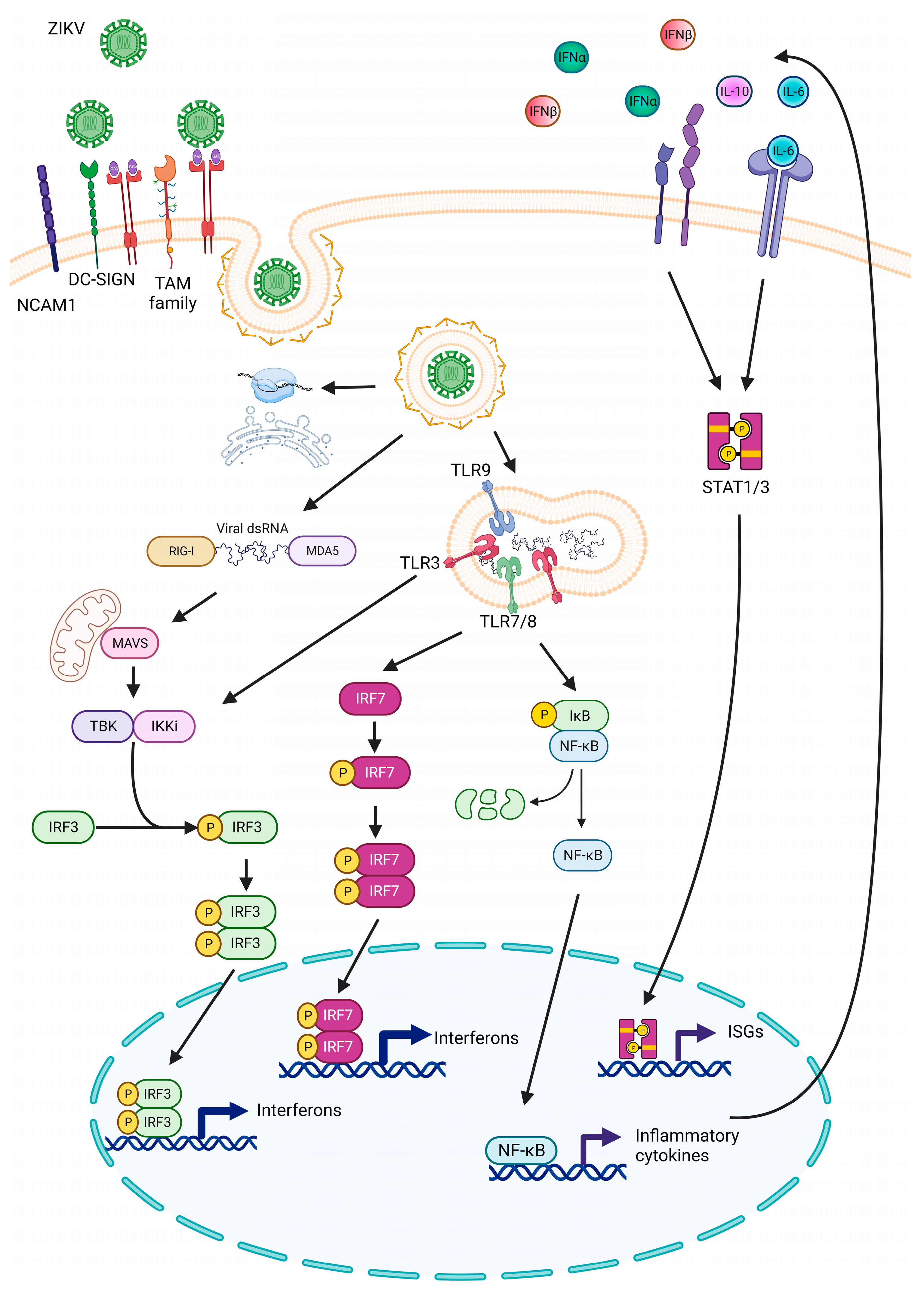
:1. Introduction
2. Clinical Manifestations of ZIKV Infection
3. Microcephaly Associated with ZIKV
4. ZIKV Interactions with the Host Cell
4.1. ZIKV Dissemination Strategy
4.2. ZIKV Entry
4.3. Antiviral Response against ZIKV Infection
4.4. Viral Mechanism to Avoid Cell Antiviral Response
4.5. IFN Pathway Suppression
4.6. Interference with IFN Signaling
4.7. NLRP3 Inflammasome Activation Suppression
4.8. Interaction with Cellular Proteins
4.9. Maternal Inflammation
4.10. Signaling Pathways Regulated via ZIKV Associated with Microcephaly
4.11. Unfolded Protein Response (UPR) and Autophagy during Zika Infection
4.12. miRNAs and Musashi 1 Interaction
5. Therapeutic Strategies versus Zika Virus
5.1. ZIKV Vaccines Development
5.2. Antivirals
5.3. Phytocompounds
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Song, B.-H.; Yun, S.-I.; Woolley, M.; Lee, Y.-M. Zika Virus: History, Epidemiology, Transmission, and Clinical Presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Zika: A Silent Virus Requiring Enhanced Surveillance and Control. Available online: https://www.paho.org/en/news/1-9-2023-zika-silent-virus-requiring-enhanced-surveillance-and-control (accessed on 27 September 2023).
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika Outbreak of the 21st Century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Jibat, T.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global Risk Mapping for Major Diseases Transmitted by Aedes aegypti and Aedes albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Magalhaes, T.; Viridiana Laredo-Tiscareño, S.; Foy, B.D. Sexual Transmission of Arboviruses: A Systematic Review. Viruses 2020, 12, 933. [Google Scholar] [CrossRef]
- Gregory, C.J.; Oduyebo, T.; Brault, A.C.; Brooks, J.T.; Chung, K.W.; Hills, S.; Kuehnert, M.J.; Mead, P.; Meaney-Delman, D.; Rabe, I.; et al. Modes of Transmission of Zika Virus. J. Infect. Dis. 2017, 216, S875–S883. [Google Scholar] [CrossRef]
- WHO. Zika Virus Microcephaly and Guillain-Barré Syndrome; WHO: Geneva, Switzerland, 2016; pp. 1–13. [Google Scholar]
- de Oliveira, W.K.; Cortez-Escalante, J.; de Oliveira, W.T.G.H.; do Carmo, G.M.I.; Henriques, C.M.P.; Coelho, G.E.; Araújo de França, G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Rudolph, K.E.; Lessler, J.; Moloney, R.M.; Kmush, B.; Cummings, D.A. Review Article: Incubation Periods of Mosquito-Borne Viral Infections: A Systematic Review. Am. J. Trop. Med. Hyg. 2014, 90, 882–891. [Google Scholar] [CrossRef]
- Dub, T.; Fontanet, A. Zika Virus and Guillain–Barré Syndrome. Rev. Neurol. 2017, 173, 361–363. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastãre, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika Virus Infection Complicated by Guillain-Barré Syndrome—Case Report, French Polynesia, December 2013. Eurosurveill 2014, 19, 7–9. [Google Scholar] [CrossRef]
- Nascimento, O.J.M.; Da Silva, I.R.F. Guillain-Barré Syndrome and Zika Virus Outbreaks. Curr. Opin. Neurol. 2017, 30, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; Wakimoto, M.D. Congenital Zika Syndrome: A Systematic Review. PLoS ONE 2020, 15, e0242367. [Google Scholar] [CrossRef] [PubMed]
- De Barros Miranda-Filho, D.; Martelli, C.M.T.; De Alencar Ximenes, R.A.; Araújo, T.V.B.; Rocha, M.A.W.; Ramos, R.C.F.; Dhalia, R.; De Oliveira Franca, R.F.; De Azevedo Marques Junior, E.T.; Rodrigues, L.C. Initial Description of the Presumed Congenital Zika Syndrome. Am. J. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef]
- Arraes de Alencar Ximenes, R.; de Barros Miranda-Filho, D.; Brickley, E.B.; Barreto de Araújo, T.V.; Montarroyos, U.R.; Abtibol-Bernardino, M.R.; Mussi-Pinhata, M.M.; Duarte, G.; Coutinho, C.M.; Biason de Moura Negrini, S.F.; et al. Risk of Adverse Outcomes in Offspring with RT-PCR Confirmed Prenatal Zika Virus Exposure: An Individual Participant Data Meta-Analysis of 13 Cohorts in the Zika Brazilian Cohorts Consortium. Lancet Reg. Health-Am. 2023, 17, 100395. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Guerrini, R.; Kuzniecky, R.I.; Jackson, G.D.; Dobyns, W.B. A Developmental and Genetic Classification for Malformations of Cortical Development: Update 2012. Brain 2012, 135, 1348–1369. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Lazear, H.M. Zika Virus-Reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- de Oliveira, W.K.; de França, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-Related Microcephaly after the 2015 and 2016 Zika Virus Outbreaks in Brazil: A Surveillance-Based Analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef]
- Brady, O.J.; Osgood-Zimmerman, A.; Kassebaum, N.J.; Ray, S.E.; De Araùjo, V.E.M.; Da Nóbrega, A.A.; Frutuoso, L.C.V.; Lecca, R.C.R.; Stevens, A.; De Oliveira, B.Z.; et al. The Association between Zika Virus Infectionand Microcephaly in Brazil 2015–2017: Anobservational Analysis of over 4 Million Births. PLoS Med. 2019, 16, e1002755. [Google Scholar] [CrossRef]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.; Tufa, A.J.; Taulung, L.; Bhatnagar, J.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—US Territories and Freely Associated States, 2018. Morb. Mortal. Wkly. Report 2018, 67, 858–867. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.; Borges da Fonseca, E.; Marquez-Ribeiro, E.; Arena, F.; Rasmussen, S.A. Congenital Zika Syndrome: Characterizing the Pattern of Anomalies for Pediatric Healthcare Providers. HHS Public. Access Author 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Satterfield-Nash, A.; Kotzky, K.; Allen, J.; Bertolli, J.; Moore, C.A.; Pereira, I.O.; Pessoa, A.; Melo, F.; Faria e Silva Santelli, A.C.; Boyle, C.A.; et al. Health and Development at Age 19–24 Months of 19 Children Who Were Born with Microcephaly and Laboratory Evidence of Congenital Zika Virus Infection During the 2015 Zika Virus Outbreak—Brazil, 2017. Morb. Mortal. Wkly. Rep. 2017, 66, 1347–1351. [Google Scholar] [CrossRef]
- Bertolli, J.; Attell, J.E.; Rose, C.; Moore, C.A.; Melo, F.; Staples, J.E.; Kotzky, K.; Krishna, N.; Satterfield-Nash, A.; Pereira, I.O.; et al. Functional Outcomes among a Cohort of Children in Northeastern Brazil Meeting Criteria for Follow-up of Congenital Zika Virus Infection. Am. J. Trop. Med. Hyg. 2020, 102, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.I.; Abdullah, M.; Ali, S.; Naqvi, I.H.; Ahmed, A.; Parveen, S. Zika Virus-Induced Microcephaly and Its Possible Molecular Mechanism. Intervirology 2017, 59, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.J. Full-Length Sequencing and Genomic Characterization of Bagaza, Kedougou, and Zika Viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.-C.; Vázquez-Calvo, A.; Blázquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martin-Acebes, M.A. Zika Virus: The Latest Newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.; Lambert, A.; Holodniy, M.; Saavedra, S.; Castillo, C. Phylogeny of Zika Virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Anfasa, F.; Siegers, J.; van der Kroeg, M.; Mumtaz, N.; Stalin Raj, V.; de Vrij, F.; Widagdo, W.; Gabriel, G.; Salinas, S.; Simonin, Y.; et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere 2017, 2, e00292-17. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Zhu, Y.N.; Yang, M.; Goodfellow, F.; Stice, S.L.; Qi, X.P.; Brindley, M.A.; Chen, J.F. The African Zika Virus Mr-766 Is More Virulent and Causes More Severe Brain Damage than Current Asian Lineage and Dengue Virus. Dev. 2017, 144, 4114–4124. [Google Scholar] [CrossRef]
- Simonin, Y.; van Riel, D.; van de Perre, P.; Rockx, B.; Salinas, S. Differential Virulence between Asian and African Lineages of Zika Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Shrestha, B.; Mehlhop, E.; Sitati, E.; Engle, M. Innate and Adaptive Immune Responses Determine Protection against Disseminated Infection by West Nile Encephalitis Virus. Viral Immunol. 2003, 16, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal Brain Lesions after Subcutaneous Inoculation of Zika Virus in a Pregnant Nonhuman Primate. Nat. Med. 2016, 22, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Gorchakov, R.; Carlson, A.R.; Berry, R.; Lai, L.; Natrajan, M.; Garcia, M.N.; Correa, A.; Patel, S.M.; Aagaard, K.; et al. Prolonged Detection of Zika Virus in Vaginal Secretions and Whole Blood. Emerg. Infect. Dis. 2017, 23, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Nicastri, E.; Castilletti, C.; Liuzzi, G.; Iannetta, M.; Capobianchi, M.R.; Ippolito, G. Persistent Detection of Zika Virus RNA in Semen for Six Months after Symptom Onset in a Traveller Returning from Haiti to Italy, February 2016. Eurosurveillance 2016, 21, 30314. [Google Scholar] [CrossRef]
- Srivastava, M.; Zhang, Y.; Chen, J.; Sirohi, D.; Miller, A.; Zhang, Y.; Chen, Z.; Lu, H.; Xu, J.; Kuhn, R.J.; et al. Chemical Proteomics Tracks Virus Entry and Uncovers NCAM1 as Zika Virus Receptor. Nat. Commun. 2020, 11, 3896. [Google Scholar] [CrossRef]
- Carbaugh, D.L.; Baric, R.S.; Lazear, H.M. Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis. J. Virol. 2019, 93, e00113-19. [Google Scholar] [CrossRef]
- Giraldo, M.I.; Xia, H.; Aguilera-Aguirre, L.; Hage, A.; van Tol, S.; Shan, C.; Xie, X.; Studervant, G.L.; Robertson, S.J.; Mc Nally, K.L.; et al. Envelope Protein Ubiquitination Drives Zika Virus Entry and Pathogenesis. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guajardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika Virus Targets Different Primary Human Placental Cells Suggesting Tow Routes for Vertical Transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. ZIKA Virus Entry Mechanisms in Human Cells. Infect. Genet. Evol. 2019, 69, 22–29. [Google Scholar] [CrossRef]
- Sun, X.; Huang, S.; Chen, H.-R.; Ouyang, Z.; Einkauf, K.; Tse, S.; Ard, K.; Ciaranello, A.; Yawets, S.; Sax, P.; et al. Transcriptional Changes during Naturally-Acquired Zika Virus Infection Render Dendritic Cells Highly Conducive to Viral Replication. Cell Rep. 2017, 21, 3471–3482. [Google Scholar] [CrossRef]
- Mesci, P.; Macia, A.; LaRock, C.N.; Tejwani, L.; Fernandes, I.R.; Suarez, N.A.; Zanotto, P.M.d.A.; Beltrão-Braga, P.C.B.; Nizet, V.; Muotri, A.R. Modeling Neuro-Immune Interactions during Zika Virus Infection. Hum. Mol. Genet. 2018, 27, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Yockey, L.J.; Jagger, B.W.; Hwang, J.; Uraki, R.; Gaitsch, H.F.; Parnell, L.A.; Cao, B.; Mysorekar, I.U.; Rothlin, C.V.; et al. TAM Receptors Are Not Required for Zika Virus Infection in Mice. Cell Rep. 2017, 19, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.M.A.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM Receptor Tyrosine Kinases: Biologic Functions, Signaling, and Potential Therapeutic Targeting in Human Cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, J.H.M.; Van Der Poll, T.; Van’t Veer, C. TAM Receptors, Gas6, and Protein S: Roles in Inflammation and Hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Shi, Y.; Li, S.; Wu, Q.; Sun, L.; Zhang, J.; Pan, N.; Wang, Q.; Bi, Y.; An, J.; Lu, X.; et al. Vertical Transmission of the Zika Virus Causes Neurological Disorders in Mouse Offspring. Sci. Rep. 2018, 8, 3541. [Google Scholar] [CrossRef]
- Wells, M.F.; Salick, M.R.; Wiskow, O.; Ho, D.J.; Worringer, K.A.; Ihry, R.J.; Kommineni, S.; Bilican, B.; Klim, J.R.; Hill, E.J.; et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 2016, 19, 703–708. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, Z.; Zhen, Z.D.; Feng, K.H.; Guo, J.; Gao, N.; Fan, D.Y.; Han, D.S.; Wang, P.G.; An, J. Axl Is Not an Indispensable Factor for Zika Virus Infection in Mice. J. Gen. Virol. 2017, 98, 2061–2068. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and Characterization of MAVS, a Mitochondrial Antiviral Signaling Protein That Activates NF-ΚB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential Roles of MDA5 and RIG-I Helicases in the Recognition of RNA Viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Reikine, S.; Nguyen, J.B.; Modis, Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front. Immunol. 2014, 5, 342. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Torres de Azeved MAques Jr, E.; Cherry, S.; Sadovsky, Y.; Coyne, C. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection Against Zika Virus Infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, Y.; Yi, P.; Xu, L.; Hawkins, H.K.; Rossi, S.L.; Soong, L.; Cai, J.; Menon, R.; Sun, J. Outcomes of Congenital Zika Disease Depend on Timing of Infection and Maternal-Fetal Interferon Action. Cell Rep. 2017, 21, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A.; Scheaffer, S.M.; Arora, N.; Zaitsev, K.; Artyomov, M.N.; Coyne, C.B.; Moley, K.H.; Diamond, M.S. Interferon Lambda Protects the Female Reproductive Tract against Zika Virus Infection. Nat. Commun. 2019, 10, 280. [Google Scholar] [CrossRef]
- Duan, X.; Liao, X.; Li, S.; Li, Y.; Xu, M.; Wang, Y.; Ye, H.; Zhao, H.; Yang, C.; Zhu, X.; et al. Transmembrane Protein 2 Inhibits Zika Virus Replication through Activation of the Janus Kinase/Signal Transducers and Activators of Transcription Signaling Pathway. Future Virol. 2019, 14, 9–19. [Google Scholar] [CrossRef]
- Li, L.; Zhao, H.; Liu, P.; Li, C.; Quanquin, N.; Ji, X.; Sun, N.; Du, P.; Qin, C.F.; Lu, N.; et al. PARP12 Suppresses Zika Virus Infection through PARP-Dependent Degradation of NS1 and NS3 Viral Proteins. Sci. Signal. 2019, 11, eaas9332. [Google Scholar] [CrossRef]
- Rolfe, A.J.; Bosco, D.B.; Wang, J.; Nowakowski, R.S.; Fan, J.; Ren, Y. Bioinformatic Analysis Reveals the Expression of Unique Transcriptomic Signatures in Zika Virus Infected Human Neural Stem Cells. Cell Biosci. 2016, 6, 42. [Google Scholar] [CrossRef]
- Ojha, C.R.; Rodriguez, M.; Karuppan, M.K.M.; Lapierre, J.; Kashanchi, F.; El-Hage, N. Toll-like Receptor 3 Regulates Zika Virus Infection and Associated Host Inflammatory Response in Primary Human Astrocytes. PLoS ONE 2019, 14, e0208543. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis HHS Public Access. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.; Vidyadaran, S. Role of Microglia in Embryonic Neurogenesis. Exp. Biol. Med. 2016, 241, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Ziv, Y.; Schwartz, A.; Landa, G.; Talpalar, A.E.; Pluchino, S.; Martino, G.; Schwartz, M. Microglia Activated by IL-4 or IFN-γ Differentially Induce Neurogenesis and Oligodendrogenesis from Adult Stem/Progenitor Cells. Mol. Cell. Neurosci. 2006, 31, 149–160. [Google Scholar] [CrossRef]
- Cacci, E.; Ajmone-Cat, M.A.; Anelly, T.; Biagioni, S.; Minchetti, L. In Vitro Neuronal and Glial Differentiation from Embryonic or Adult Neural Precursor Cells Are Differently Affected by Chronic or Acute Activation of Microglia. Glia 2008, 56, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Shan, C.; Dunn, T.J.; Xie, X.; Xia, H.; Gao, J.; Labastida, J.A.; Zou, J.; Villarreal, P.P.; Schlagal, C.R.; et al. Role of Microglia in the Dissemination of Zika Virus from Mother to Fetal Brain. PLoS Negl. Trop. Dis. 2020, 14, e0009344. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.M.; Low, D.K.S.; Fan, Y.; Tan, J.J.L.; Lee, B.; Chan, J.K.Y.; Rénia, L.; Ginhoux, F.; Ng, L.F.P. Zika Virus Infects Human Fetal Brain Microglia and Induces Inflammation. Clin. Infect. Dis. 2017, 64, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Gama, L.; Krasemann, S.; Chesnut, M.; Odwin-Dacosta, S.; Hogberg, H.T.; Hartung, T.; Pamies, D. Microglia Increase Inflammatory Responses in IPSC-Derived Human BrainSpheres. Front. Microbiol. 2018, 9, 2766. [Google Scholar] [CrossRef]
- Gim, E.; Shim, D.W.; Hwang, I.; Shin, O.S.; Yu, J.W. Zika Virus Impairs Host NLRP3-Mediated Inflammasome Activation in an NS3-Dependent Manner. Immune Netw. 2019, 19, e40. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, H.; Liu, H.; Ha, Y.; Mays, E.R.; Ryan, E.; Winkelmann, E.; Barrett, A.D.; Smith, S.B.; Wang, M. P38MAPK Plays a Critical Role in Induction of a Pro-Inflammatory Phenotype of Retinal Müller Cells Following Zika Virus Infection. Antivir. Res. 2017, 145, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Cheng, Y.; Rolfe, A.; Hammack, C.; Vera, D.; Kyle, K.; Wang, J.; Meissner, T.B.; Ren, Y.; Cowan, C.; et al. An HPSC-Derived Tissue-Resident Macrophage Model Reveals Differential Responses of Macrophages to ZIKV and DENV Infection. Stem Cell Reports 2018, 11, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, G.; De, W.; Luo, Z.; Pan, P.; Tian, M.; Wang, Y.; Xiao, F.; Li, A.; Wu, K.; et al. Zika Virus Infection Induces Host Inflammatory Responses by Facilitating NLRP3 Inflammasome Assembly and Interleukin-1β Secretion. Nat. Commun. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Ngan, N.T.T.; Kim, S.J.; Lee, J.Y.; Myoung, J. Zika Virus Proteins NS2A and NS4A Are Major Antagonists That Reduce IFN-β Promoter Activity Induced by the MDA5/RIG-I Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, W.; Wang, Y.; Chen, K.; Xiao, F.; Hu, D.; Hui, L.; Liu, W.; Feng, Y.; Li, G.; et al. NS5 Conservative Site Is Required for Zika Virus to Restrict the RIG-I Signaling. Front. Immunol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika Virus Evades Interferon-Mediated Antiviral Response through the Co-Operation of Multiple Nonstructural Proteins in Vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.; Vasconcelos, P.F.C.; et al. An Evolutionary NS1 Mutation Enhances Zika Virus Evasion of Host Interferon Induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Dai, S.; Hou, G.; Guo, K.; Chen, X.; Yi, C.; Liu, W.; Deng, F.; Wu, Y.; et al. Zika Virus Circumvents Host Innate Immunity by Targeting the Adaptor Proteins MAVS and MITA. FASEB J. 2019, 33, 9929–9944. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, J.; Dias Junior, A.G.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a Brazilian Isolate of Zika Virus Generates RIG-I Stimulatory RNA and the Viral NS5 Protein Blocks Type I IFN Induction and Signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Q.; Wu, Y.; Ma, L.; Zhang, Z.; Liu, T.; Jin, S.; She, Y.; Li, Y.; Cui, J. Zika Virus Elicits Inflammation to Evade Antiviral Response by Cleaving CGAS via NS 1-caspase-1 Axis. EMBO J. 2018, 37, e99347. [Google Scholar] [CrossRef]
- Shah, P.S.; Link, N.; Jang, G.M.; Sharp, P.P.; Zhu, T.; Swaney, D.L.; Johnson, J.R.; Von Dollen, J.; Ramage, H.R.; Satkamp, L.; et al. Comparative Flavivirus-Host Protein Interaction Mapping Reveals Mechanisms of Dengue and Zika Virus Pathogenesis. Cell 2018, 175, 1931–1945. [Google Scholar] [CrossRef]
- Slawinski, B.L.; Talge, N.; Ingersoll, B.; Smith, A.; Glazier, A.; Kerver, J.; Paneth, N.; Racicot, K. Maternal Cytomegalovirus Sero-Positivity and Autism Symptoms in Children. Am. J. Reprod. Immunol. 2018, 79, e12840. [Google Scholar] [CrossRef]
- Choudhury, Z.; Lennox, B. Maternal Immune Activation and Schizophrenia–Evidence for an Immune Priming Disorder. Front. Psychiatry 2021, 12, 585742. [Google Scholar] [CrossRef] [PubMed]
- Oskvig, D.; Elkahloun, A.; Johnson, K.R.; Phillips, T.M.; Herkenham, M. Maternal Immune Activation by LPS Selectively Alters Specific Gene Expression Profiles of Interneuron Migration and Oxidative Stress in the Fetus without Triggering a Fetal Immune Response. Bone 2012, 2326, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, G.; Chou, S.; Jung, Y.; Coiro, P.; Spartz, E.; Padmashri, R.; Li, M.; Dunaevsky, A. Maternal Immune Activation Causes Behavioral Impairments and Altered Cerebellar Cytokine and Synaptic Protein Expression. Neuropsychopharmacology 2017, 42, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, N.; Servatius, R.; Beck, K.; Marzouk, A.; Kreider, T. Cytokine Levels during Pregnancy Influence Immunological Profiles and Neurobehavioral Patterns of the Offspring. Ann. N. Y. Acad. Sci. 2007, 1107, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Rees, S.; Harding, R. Brain Development during Fetal Life: Influences of the Intra-Uterine Environment. Neurosci. Lett. 2004, 361, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gouilly, J.; Ferrat, Y.J.; Espino, A.; Glaziou, Q.; Cartron, G.; El Costa, H.; Al-Daccak, R.; Jabrane-Ferrat, N. Metabolic Reprogramming by Zika Virus Provokes Inflammation in Human Placenta. Nat. Commun. 2020, 11, 2967. [Google Scholar] [CrossRef]
- Gurung, S.; Reuter, N.; Preno, A.; Dubaut, J.; Nadeau, H.; Hyatt, K.; Singleton, K.; Martin, A.; Parks, W.T.; Papin, J.F.; et al. Zika Virus Infection at Mid-Gestation Results in Fetal Cerebral Cortical Injury and Fetal Death in the Olive Baboon. PLoS Pathog. 2019, 15, e1007507. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.S.; Chen, W.; Chan, Y.; Lee, W.S.; Lee, S.A.; Cheng, G.; Nielsen-Saines, K.; Brasil, P.; Jung, J.U. Biomarkers and Immunoprofiles Associated with Fetal Abnormalities of ZIKV-Positive Pregnancies. JCI insight 2018, 3, e124152. [Google Scholar] [CrossRef]
- Familiar-Macedo, D.; Amancio Paiva, I.; Badolato-Corrêa da Silva, J.; de Carvalho, F.R.; Dias, H.G.; Pauvolid-Corrêa, A.; Dos Santos, C.F.; Gandini, M.; Silva, A.A.; Baeta Cavalcanti, S.M.; et al. Evaluation of the Expression of CCR5 and CX3CR1 Receptors and Correlation with the Functionality of T Cells in Women Infected with ZIKV during Pregnancy. Viruses 2021, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 305, 1760–1765. [Google Scholar] [CrossRef]
- Valliéres, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced Hippocampal Neurogenesis in Adult Transgenic Mice with Chronic Astrocytic Production of Interleukin-6. J. Neurosci. 2002, 22, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Driggers, R.W.; Ho, C.-Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef]
- Barrozo, E.R.; Seferovic, M.D.; Hamilton, M.P.; Moorshead, D.N.; Jochum, M.D.; Do, T.; O’Neil, D.S.; Suter, M.A.; Aagaard, K.M. Zika Virus Co-Opts MiRNA Networks to Persist in Placental Niches Detected by Spatial Transcriptomics. Am. J. Obstet. Gynecol. 2023, 1–17. [Google Scholar] [CrossRef]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika Virus Infection Disrupts Neurovascular Development and Results in Postnatal Microcephaly with Brain Damage. Dev. 2016, 143, 4127–4136. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural Stem Cell Niche Heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.-F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef]
- Bhagat, R.; Prajapati, B.; Narwal, S.; Agnihotri, N.; Adlakha, Y.K.; Sen, J.; Mani, S.; Seth, P. Zika Virus E Protein Alters the Properties of Human Fetal Neural Stem Cells by Modulating MicroRNA Circuitry. Cell Death Differ. 2018, 25, 1837–1854. [Google Scholar] [CrossRef]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Precursors Attenuates Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef]
- Devhare, P.; Meyer, K.; Steele, R.; Ray, R.B.; Ray, R. Zika Virus Infection Dysregulates Human Neural Stem Cell Growth and Inhibits Differentiation into Neuroprogenitor Cells. Cell Death Dis. 2017, 8, e3106. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, X.; Li, S.; Hui, Y.; Feng, H.; Du, Y.; Jin, G.; Zhou, X.; Zhang, X. Protection of ZIKV Infection-Induced Neuropathy by Abrogation of Acute Antiviral Response in Human Neural Progenitors. Cell Death Differ. 2019, 26, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Lossia, O.V.; Conway, M.J.; Tree, M.O.; Williams, R.J.; Goldthorpe, S.C.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. Zika Virus Induces Astrocyte Differentiation in Neural Stem Cells. J. Neurovirol. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Jiang, Y.; Ye, Q.; Xu, D.; Gao, F.; Xu, J.W.; Wang, R.; Zhu, X.; Shi, L.; et al. Disruption of Glial Cell Development by Zika Virus Contributes to Severe Microcephalic Newborn Mice. Cell Discov. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika Virus Infection as a Silent Pathology With Loss of Neurogenic Output in the Fetal Brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-J.; Song, G.; Qian, X.; Pan, J.; Xu, D.; Rho, H.-S.; Kim, N.-S.; Habela, C.; Zheng, L.; Jacob, F.; et al. Zika Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins. Cell Stem Cell 2017, 21, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huo, Y.; Yang, L.; Chen, G.; Luo, M.; Yang, J.; Zhou, J. ZIKV Infection Effects Changes in Gene Splicing, Isoform Composition and LncRNA Expression in Human Neural Progenitor Cells. Virol. J. 2017, 14, 217. [Google Scholar] [CrossRef]
- Laguesse, S.; Creppe, C.; Nedialkova, D.D.; Prévot, P.P.; Borgs, L.; Huysseune, S.; Franco, B.; Duysens, G.; Krusy, N.; Lee, G.; et al. A Dynamic Unfolded Protein Response Contributes to the Control of Cortical Neurogenesis. Dev. Cell 2015, 35, 553–567. [Google Scholar] [CrossRef]
- Gladwyn-Ng, I.; Cordón-Barris, L.; Alfano, C.; Creppe, C.; Couderc, T.; Morelli, G.; Thelen, N.; America, M.; Bessières, B.; Encha-Razavi, F.; et al. Stress-Induced Unfolded Protein Response Contributes to Zika Virus-Associated Microcephaly. Nat. Neurosci. 2018, 21, 63–73. [Google Scholar] [CrossRef]
- Thepparit, C.; Khakpoor, A.; Khongwichit, S.; Wikan, N.; Fongsaran, C.; Chingsuwanrote, P.; Panraksa, P.; Smith, D.R. Dengue 2 Infection of HepG2 Liver Cells Results in Endoplasmic Reticulum Stress and Induction of Multiple Pathways of Cell Death. BMC Res. Notes. 2013, 6, 372. [Google Scholar] [CrossRef] [PubMed]
- Beaufrère, A.; Bessières, B.; Bonnière, M.; Driessen, M.; Alfano, C.; Couderc, T.; Thiry, M.; Thelen, N.; Lecuit, M.; Attié-Bitach, T.; et al. A Clinical and Histopathological Study of Malformations Observed in Fetuses Infected by the Zika Virus. Brain Pathol. 2019, 29, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, W.; Sun, J.; Fu, Z.; Ke, X.; Zheng, C.; Zhang, Y.; Li, P.; Liu, Y.; Hu, Q.; et al. ZIKV Infection Activates the IRE1-XBP1 and ATF6 Pathways of Unfolded Protein Response in Neural Cells. J. Neuroinflammation 2018, 15, 275. [Google Scholar] [CrossRef]
- Kozak, R.A.; Majer, A.; Biondi, M.J.; Medina, S.J.; Goneau, L.W.; Sajesh, B.V.; Slota, J.A.; Zubach, V.; Severini, A.; Safronetz, D.; et al. MicroRNA and MRNA Dysregulation in Astrocytes Infected with Zika Virus. Viruses 2017, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Taguwa, S.; Yeh, M.-T.; Rainbolt, T.K.; Nayak, A.; Shao, H.; Gestwicki, J.E.; Andino, R.; Frydman, J. Zika Virus Dependence on Host Hsp70 Provides a Protective Strategy against Infection and Disease. Cell Rep. 2019, 26, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hammack, C.; Ogden, S.C.; Cheng, Y.; Lee, E.M.; Wen, Z.; Qian, X.; Nguyen, H.N.; Li, Y.; Yao, B.; et al. Molecular Signatures Associated with ZIKV Exposure in Human Cortical Neural Progenitors. Nucleic Acids Res. 2016, 44, 8610–8620. [Google Scholar] [CrossRef]
- Caires-Júnior, L.C.; Goulart, E.; Melo, U.S.; Araujo, B.S.H.; Alvizi, L.; Soares-Schanoski, A.; De Oliveira, D.F.; Kobayashi, G.S.; Griesi-Oliveira, K.; Musso, C.M.; et al. Discordant Congenital Zika Syndrome Twins Show Differential in Vitro Viral Susceptibility of Neural Progenitor Cells. Nat. Commun. 2018, 9, 475. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-MTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, Y.; Zhang, L.; Kim, S.N.; Otaegi, G.; Zhang, Z.; Nie, Y.; Mubarak, T.; Li, C.; Qin, C.F.; et al. Upregulation of MicroRNA MiR-9 Is Associated with Microcephaly and Zika Virus Infection in Mice. Mol. Neurobiol. 2019, 56, 4072–4085. [Google Scholar] [CrossRef]
- Suzuki, I.K.; Gacquer, D.; Van Heurck, R.; Kumar, D.; Wojno, M.; Bilheu, A.; Herpoel, A.; Lambert, N.; Cheron, J.; Polleux, F.; et al. Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell 2018, 173, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, I.T.; Lodewijk, G.A.; Mooring, M.; Bosworth, C.M.; Edwing, A.; Mantalas, G.L.; Novak, A.M.; van den Bout, A.; Bishara, A.; Rosenkrantz, J.L.; et al. Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2019, 173, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.W.; Tiwari, S.K.; Qin, Y.; Rana, T.M. Genome-Wide Integrative Analysis of Zika-Virus-Infected Neuronal Stem Cells Reveals Roles for MicroRNAs in Cell Cycle and Stemness. Cell Rep. 2019, 27, 3618–3628. [Google Scholar] [CrossRef] [PubMed]
- Azouz, F.; Arora, K.; Krause, K.; Nerurkar, V.R.; Kumar, M. Integrated MicroRNA and MRNA Profiling in Zika Virus-Infected Neurons. Viruses 2019, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.L.; Geddes, V.E.; Monteiro, F.L.; Gonçalves, R.M.; Campanati, L.; Pezzuto, P.; Paquin-Proulx, D.; Schamber-Reis, B.L.; Azevedo, G.S.; Gonçalves, A.L.; et al. MicroRNAs 145 and 148a Are Upregulated During Congenital Zika Virus Infection. ASN Neuro 2019, 11, 1759091419850983. [Google Scholar] [CrossRef]
- Glazer, R.I.; Vo, D.T.; Penalva, L.O.F. Musashi1: An RBP with Versatile Functions in Normal and Cancer Stem Cells Robert. Front. Biosci. 2012, 17, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, S.-i.; Nakamura, Y.; Yoshida, T.; Shibata, S.; Koike, M.; Takano, H.; Ueda, S.; Uchiyama, Y.; Noda, T.; Okano, H. RNA-Binding Protein Musashi Family: Roles for CNS Stem Cells and a Subpopulation of Ependymal Cells Revealed by Targeted Disruption and Antisense Ablation. Proc. Natl. Acad. Sci. USA 2002, 99, 15194–15199. [Google Scholar] [CrossRef]
- Jadhav, S.; Ajay, A.K.; Trivedi, P.; Seematti, J.; Pellegrin, K.; Craciun, F.; Vaidya, V.S. RNA-Binding Protein Musashi Homologue 1 Regulates Kidney Fibrosis by Translational Inhibition of P21 and Numb MRNA. J. Biol. Chem. 2016, 291, 14085–14094. [Google Scholar] [CrossRef]
- de Bernardi Schneider, A.; Wolfinger, M.T. Musashi Binding Elements in Zika and Related Flavivirus 3′UTRs: A Comparative Study in Silico. Sci. Rep. 2019, 9, 6911. [Google Scholar] [CrossRef]
- Chavali, P.L.; Stojic, L.; Meredith, L.W.; Joseph, N.; Nahorski, M.S.; Sanford, T.J.; Sweeney, T.R.; Krishna, B.A.; Hosmillo, M.; Firth, A.E.; et al. Neurodevelopmental Protein Musashi-1 Interacts with the Zika Genome and Promotes Viral Replication Pavithra. Science 2017, 357, 83–88. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Xu, Z.; Cheng, M.L.; Ma, Q.Q.; Li, R.T.; Wang, Z.J.; Zhao, H.; Zuo, X.; Li, X.F.; et al. Zika Virus RNA Structure Controls Its Unique Neurotropism by Bipartite Binding to Musashi-1. Nat. Commun. 2023, 14, 1134. [Google Scholar] [CrossRef] [PubMed]
- Gioia, U.; Di Carlo, V.; Caramanica, P.; Toselli, C.; Cinquino, A.; Marchioni, M.; Laneve, P.; Biagioni, S.; Bozzoni, I.; Cacci, E.; et al. Mir-23a and Mir-125b Regulate Neural Stem/Progenitor Cell Proliferation by Targeting Musashi1. RNA Biol. 2014, 11, 1105–1112. [Google Scholar] [CrossRef]
- Guan, A.; Wang, H.; Li, X.; Xie, H.; Wang, R.; Zhu, Y.; Li, R. MiR-330-3p Inhibits Gastric Cancer Progression through Targeting MSI1. Am. J. Transl. Res. 2016, 8, 4802–4811. [Google Scholar] [PubMed]
- Velasco, M.X.; Kosti, A.; Guardia, G.D.A.; Santos, M.C.; Tegge, A.; Qiao, M.; Correa, B.R.; Hernández, G.; Kokovay, E.; Galante, P.A.; et al. Antagonism between the RNA-Binding Protein Musashi1 and MiR-137 and Its Potential Impact on Neurogenesis and Glioblastoma Development. RNA 2019, 27, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Marquez, R.T.; Tsao, W.C.; Pathak, S.; Roy, A.; Ping, J.; Wilkerson, B.; Lan, L.; Meng, W.; Neufeld, K.L.; et al. Tumor Suppressive MicroRNA-137 Negatively Regulates Musashi-1 and Colorectal Cancer Progression. Oncotarget 2015, 6, 12558–12573. [Google Scholar] [CrossRef]
- Lin, H.H.; Yip, B.S.; Huang, L.M.; Wu, S.C. Zika Virus Structural Biology and Progress in Vaccine Development. Biotechnol. Adv. 2018, 36, 47–53. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Zika Vaccine Development: Current Status. Mayo Clin. Proc. 2019, 94, 2572–2586. [Google Scholar] [CrossRef]
- Hassert, M.; Brien, J.D.; Pinto, A.K. Mouse Models of Heterologous Flavivirus Immunity: A Role for Cross-Reactive T Cells. Front. Immunol. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Wen, J.; Shresta, S. ADE-Ing and Abetting Zika. Cell Host Micro 2017, 21, 557–558. [Google Scholar] [CrossRef]
- Fierz, W.; Walz, B. Antibody Dependent Enhancement Due to Original Antigenic Sin and the Development of SARS. Front. Immunol. 2020, 11, 1120. [Google Scholar] [CrossRef]
- Bardina, S.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Kriysztof, D.; Tortorella, D.; et al. Enhancement of Zika Virus Pathogenesis by Preexisting Antiflavivirus Immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef]
- Barrows, N.J.; Campos, R.K.; Powell, S.; Prasanth, K.R.; Schott-lerner, G.; Soto-acosta, R.; Galarza-muñoz, G.; Erica, L.; Urrabaz-garza, R.; Gao, J.; et al. A Screen of FDA-Approved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2017, 20, 259–270. [Google Scholar] [CrossRef]
- Chan, J.F.; Zhu, Z.; Chu, H.; Yuan, S.; Chik, K.K.; Chan, C.C.; Poon, V.K.; Yip, C.C. The Celecoxib Derivative Kinase Inhibitor AR-12 (OSU-03012) Inhibits Zika Virus via down-Regulation of the PI3K/Akt Pathway and Protects Zika Virus- Infected A129 Mice: A Host-Targeting Treatment Strategy. Antivir. Res. 2018, 160, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; De Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; del Ángel, R.M. The Antiviral Effect of Metformin on Zika and Dengue Virus Infection. Sci. Rep. 2021, 11, 8743. [Google Scholar] [CrossRef]
- Sacramento, C.Q.; De Melo, G.R.; De Freitas, C.S.; Rocha, N.; Hoelz, L.V.B.; Miranda, M.; Fintelman-Rodrigues, N.; Marttorelli, A.; Ferreira, A.C.; Barbosa-Lima, G.; et al. The Clinically Approved Antiviral Drug Sofosbuvir Inhibits Zika Virus Replication. Sci. Rep. 2017, 7, 40920. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.C.M.; da Silva, P.G.; Dantas, W.M.; da Silva, S.J.R.; da Silva, C.T.A.; Chaves, E.J.F.; de Araújo, D.A.M.; de Oliveira, R.N.; Rodrigues-Mascarenhas, S.; Pena, L.J. Antiviral Activity of Ouabain against a Brazilian Zika Virus Strain. Sci. Rep. 2022, 12, 12598. [Google Scholar] [CrossRef]
- Choudhry, H.; Alzahrani, F.A.; Hassan, M.A.; Alghamdi, A.; Abdulaal, W.H.; Bakhrebah, M.A.; Zamzami, M.A.; Helmi, N.; Bokhari, F.F.; Zeyadi, M.; et al. Zika Virus Targeting by Screening Inhibitors against NS2B/NS3 Protease. Biomed. Res. Int. 2019, 2019, 3947245. [Google Scholar] [CrossRef] [PubMed]
- Riva, L.; Goellner, S.; Biering, S.B.; Huang, C.-T.; Rubanov, A.N.; Haselmann, U.; Warnes, C.M.; De Jesus, P.D.; Martin-Sancho, L.; Terskikh, A.V.; et al. The Compound SBI-0090799 Inhibits Zika Virus Infection by Blocking De Novo Formation of the Membranous Replication Compartment. J. Virol. 2021, 95, e00996-21. [Google Scholar] [CrossRef]
- Sabino, C.; Basic, M.; Bender, D.; Elgner, F.; Himmelsbach, K.; Hildt, E. Bafilomycin A1 and U18666A Efficiently Impair ZIKV Infection. Viruses 2019, 11, 524. [Google Scholar] [CrossRef]
- de Castro Barbosa, E.; Alves, T.M.A.; Kohlhoff, M.; Jangola, S.T.G.; Pires, D.E.V.; Figueiredo, A.C.C.; Alves, É.A.R.; Calzavara-Silva, C.E.; Sobral, M.; Kroon, E.G.; et al. Searching for Plant-Derived Antivirals against Dengue Virus and Zika Virus. Virol. J. 2022, 19, 31. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Persaud, M.; Chavez, M.P.; Zhang, H.; Rong, L.; Liu, S.; Wang, T.T.; Sarafianos, S.G.; Diaz-Griffero, F. Glycosylated Diphyllin as a Broad-Spectrum Antiviral Agent against Zika Virus. eBioMedicine 2019, 47, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Kumar, N.S.; Hemalatha, S. Antiviral Phytocompounds Target Envelop Protein to Control Zika Virus. Comput. Biol. Chem. 2018, 77, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocatechin Gallate, an Active Green Tea Compound Inhibits the Zika Virus Entry into Host Cells via Binding the Envelope Protein. Int. J. Biol. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Yadav, R.; Selvaraj, C.; Aarthy, M.; Kumar, P.; Kumar, A.; Singh, S.K.; Giri, R. Investigating into the Molecular Interactions of Flavonoids Targeting NS2B-NS3 Protease from ZIKA Virus through in-Silico Approaches. J. Biomol. Struct. Dyn. 2021, 39, 272–284. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.; et al. The Citrus Flavonoid Naringenin Impairs the in Vitro Infection of Human Cells by Zika Virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, N.; Aarthy, M.; Singh, S.K.; Giri, R. Mechanistic Insights into Zika Virus NS3 Helicase Inhibition by Epigallocatechin-3-Gallate. ACS Omega 2020, 5, 11217–11226. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid.-Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic Acid Profiling, Antioxidant, and Anti-Inflammatory Activities, and MiRNA Regulation in the Polyphenols of 16 Blueberry Samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef]
- Feng, M.; Luo, X.; Gu, C.; Li, Y.; Zhu, X.; Fei, J. Systematic Analysis of Berberine-Induced Signaling Pathway between MiRNA Clusters and MRNAs and Identification of Mir-99a~125b Cluster Function by Seed-Targeting Inhibitors in Multiple Myeloma Cells. RNA Biol. 2015, 12, 82–91. [Google Scholar] [CrossRef]
- Tunca, B.; Tezcan, G.; Cecener, G.; Egeli, U.; Ak, S.; Malyer, H.; Tumen, G.; Bilir, A. Olea Europaea Leaf Extract Alters MicroRNA Expression in Human Glioblastoma Cells. J. Cancer Res. Clin. Oncol. 2012, 138, 1831–1844. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Xing, N.; Han, D.; Kuang, H.; Ge, P. American Ginseng Regulates Gene Expression to Protect against Premature Ovarian Failure in Rats. Biomed. Res. Int. 2015, 2015, 767124. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Concha, N.; Santana-Román, M.E.; Sánchez, N.C.; Velasco, I.; Pando-Robles, V.; Pedraza-Alva, G.; Pérez-Martínez, L. Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies. Biomedicines 2023, 11, 3316. https://doi.org/10.3390/biomedicines11123316
Camacho-Concha N, Santana-Román ME, Sánchez NC, Velasco I, Pando-Robles V, Pedraza-Alva G, Pérez-Martínez L. Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies. Biomedicines. 2023; 11(12):3316. https://doi.org/10.3390/biomedicines11123316
Chicago/Turabian StyleCamacho-Concha, Nohemi, María E. Santana-Román, Nilda C. Sánchez, Iván Velasco, Victoria Pando-Robles, Gustavo Pedraza-Alva, and Leonor Pérez-Martínez. 2023. "Insights into Zika Virus Pathogenesis and Potential Therapeutic Strategies" Biomedicines 11, no. 12: 3316. https://doi.org/10.3390/biomedicines11123316




