The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19
Abstract
:1. Introduction
2. The Rational of HUD-Based Cardiac POCUS Utilization in COVID-19 Setting
3. HUDs: Probe Technology and Equipment
4. POCUS Usefulness for Echocardiography Assessment in COVID-19 Patients
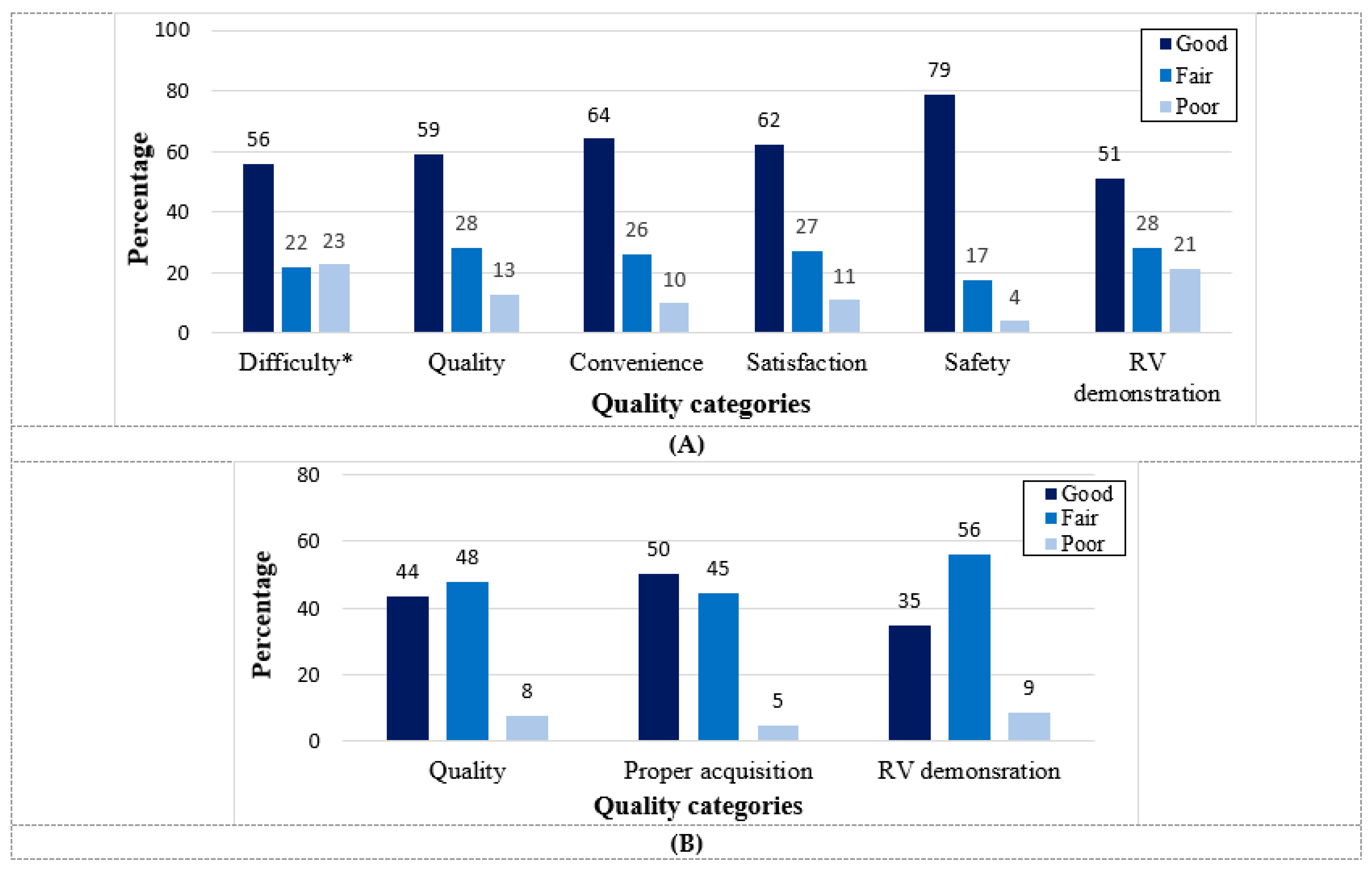
5. HUD Feasibility and Quality for Echocardiographic Assessment in COVID-19 Patients
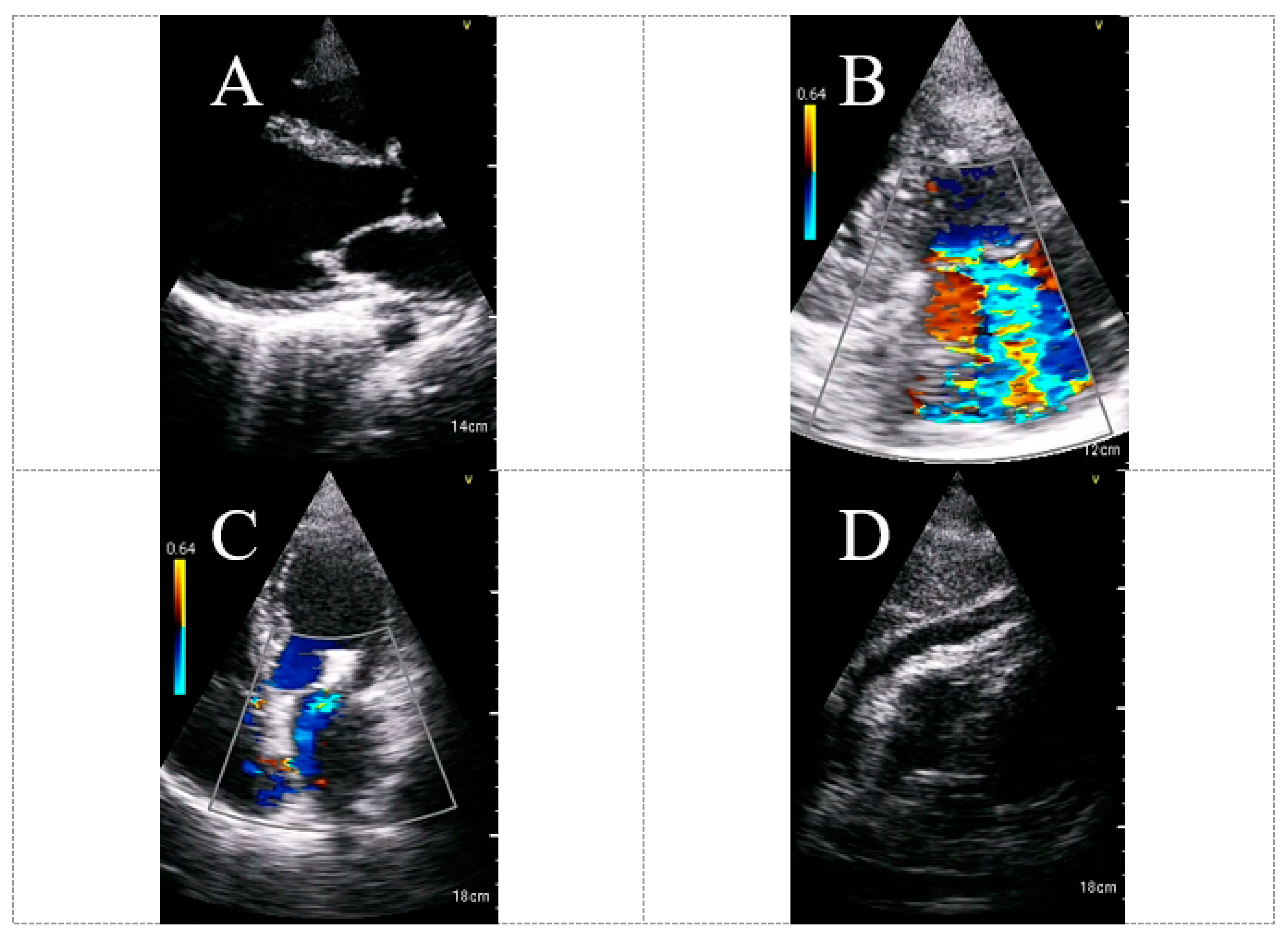
6. The Usefulness and Diagnostic Yield of HUD for Echocardiography Assessment in COVID-19 Patients
7. Evolving Technology and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Sahu, K.K.; George, A.A.; Lal, A. A review of cardiac manifestations and predictors of outcome in patients with COVID-19. Heart Lung 2020, 49, 848–852. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, B.; Zhou, J.; Kirkpatrick, J.; Xie, M.; Johri, A.M. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: The role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Task Force for the management of COVID-19 of the European Society of Cardiology. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1—epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef]
- Kirkpatrick, J.N.; Mitchell, C.; Taub, C.; Kort, S.; Hung, J.; Swaminathan, M. ASE Statement on protection of patients and echocardiography service providers during the 2019 Novel coronavirus outbreak: Endorsed by the American College of Cardiology. J. Am. Soc. Echocardiogr. 2020, 33, 648–653. [Google Scholar] [CrossRef]
- Skulstad, H.; Cosyns, B.; Popescu, B.A.; Galderisi, M.; Salvo, G.D.; Donal, E.; Petersen, S.; Gimelli, A.; Haugaa, K.H.; Muraru, D.; et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 592–598. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-de-Toledo, J.; Brierley, J.; Colunga, J.M.; Raffaj, D.; et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- McCormick, T.J.; Miller, E.C.; Chen, R.; Naik, V.N. Acquiring and maintaining point-of-care ultrasound (POCUS) competence for anesthesiologists. Can. J. Anaesth. 2018, 65, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Lewis, D.; Rang, L.; Kim, D.; Robichaud, L.; Kwan, C.; Pham, C.; Shefrin, A.; Ritcey, B.; Atkinson, P.; Woo, M.; et al. Recommendations for the use of point-of-care ultrasound (POCUS) by emergency physicians in Canada. CJEM 2019, 21, 721–726. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Current concepts: Point-of-care ultrasonography. N. Engl. J. Med. 2011, 364, 749:57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagra, A.; Tierney, D.M.; Sekiguchi, H.; Soni, N.J. Point-of-Care Ultrasonography for primary care physicians and general internists. Mayo Clin. Proc. 2016, 91, 1811–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardim, N.; Dalen, H.; Voigt, J.U.; Ionescu, A.; Price, S.; Neskovic, A.N.; Edvardsen, T.; Galderisi, M.; Sicari, R.; Donal, E.; et al. The use of handheld ultrasound devices: A position statement of the European Association of Cardiovascular Imaging (2018 update). Eur. Heart J. Cardiovasc. Imaging 2019, 20, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ma, I.W.; Somayaji, R.; Rennert-May, E.; Minardi, J.; Walsh, M.H.; Wiskar, K.; Smyth, L.M.; Burgoyne, S.; Chan, B.; Haroon, B.A.; et al. Canadian Internal Medicine Ultrasound (CIMUS) recommendations regarding internal medicine point-of-care ultrasound (POCUS) use during Coronavirus (COVID-19) pandemic. Can. J. Gen. Intern. Med. 2020, 15, 8–11. [Google Scholar] [CrossRef]
- Khanji, M.Y.; Ricci, F.; Patel, R.S.; Chahal, A.A.; Bhattacharyya, S.; Galusko, V.; Narula, J.; Ionescu, A. The role of hand-held ultrasound for cardiopulmonary assessment during a pandemic. Prog. Cardiovasc. Dis. 2020, 63, 690–695. [Google Scholar] [CrossRef]
- Naderi, H.; Robinson, S.; Swaans, M.J.; Bual, N.; Cheung, W.S.; Reid, L.; Shun-Shin, M.; Asaria, P.; Pabari, P.; Cole, G.; et al. Adapting the role of handheld echocardiography during the COVID-19 pandemic: A practical guide. Perfusion 2021, 36, 547–558. [Google Scholar] [CrossRef]
- Savino, K.; Ambrosio, G. Handheld ultrasound and focused cardiovascular echography: Use and information. Medicina 2019, 55, 423. [Google Scholar] [CrossRef] [Green Version]
- Baribeau, Y.; Sharkey, A.; Chaudhary, O.; Krumm, S.; Fatima, H.; Mahmood, F.; Matyal, R. Handheld point-of-care ultrasound probes: The new generation of POCUS. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3139–3145. [Google Scholar] [CrossRef]
- D’Ardes, D.; Tana, C.; Salzmann, A.; Ricci, F.; Guagnano, M.T.; Giamberardino, M.A.; Cipollone, F. Ultrasound assessment of SARS-CoV-2 pneumonia: A literature review for the primary care physician. Ann. Med. 2022, 54, 1140–1149. [Google Scholar] [CrossRef]
- Yamada, H.; Ito, H.; Fujiwara, M. Cardiac and vascular point-of-care ultrasound: Current situation, problems, and future prospects. J. Med. Ultrason. 2022, 2001, 601–608. [Google Scholar] [CrossRef]
- Solomon, S.D.; Saldana, F. Point-of-care ultrasound in medical education—Stop listening and look. N. Engl. J. Med. 2014, 370, 1083–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonsenso, D.; Pata, D.; Chiaretti, A. COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir. Med. 2020, 8, e27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkan, B.; Ünlüer, E.E.; Akyol, P.Y.; Karagöz, A.; Bayata, M.S.; Akoğlu, H.; Oyar, O.; Dalli, A.; Topal, F.E. Stethoscope versus point-of-care ultrasound in the differential diagnosis of dyspnea: A randomized trial. Eur. J. Emerg. Med. 2015, 22, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Kimura, B.J. Point-of-care cardiac ultrasound techniques in the physical examination: Better at the bedside. Heart 2017, 103, 987–994. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, M.; Zhang, L.; Xie, M. The prevalence, risk factors and outcome of cardiac dysfunction in hospitalized patients with COVID-19. Intensive Care Med. 2020, 46, 2096–2098. [Google Scholar] [CrossRef]
- Jain, S.S.; Liu, Q.; Raikhelkar, J.; Fried, J.; Elias, P.; Poterucha, T.J.; DeFilippis, E.M.; Rosenblum, H.; Wang, E.Y.; Redfors, B.; et al. Indications for and findings on transthoracic echocardiography in COVID-19. J. Am. Soc. Echocardiogr. 2020, 33, 1278–1284. [Google Scholar] [CrossRef]
- Moody, W.E.; Mahmoud-Elsayed, H.M.; Senior, J.; Gul, U.; Khan-Kheil, A.M.; Horne, S.; Banerjee, A.; Bradlow, W.M.; Huggett, R.; Hothi, S.S.; et al. Impact of right ventricular dysfunction on mortality in patients hospitalized with COVID-19, according to race. CJC Open 2021, 3, 91–100. [Google Scholar] [CrossRef]
- van den Heuvel, F.M.A.; Vos, J.L.; Koop, Y.; van Dijk, A.P.J.; Duijnhouwer, A.L.; de Mast, Q.; van de Veerdonk, F.L.; Bosch, F.; Kok, B.; Netea, M.G.; et al. Cardiac function in relation to myocardial injury in hospitalised patients with COVID-19. Neth. Heart J. 2020, 28, 410–417. [Google Scholar] [CrossRef]
- Mahmoud-Elsayed, H.M.; Moody, W.E.; Bradlow, W.M.; Khan-Kheil, A.M.; Senior, J.; Hudsmith, L.E.; Steeds, R.P. Echocardiographic findings in patients with COVID-19 pneumonia. Can. J. Cardiol. 2020, 36, 1203–1207. [Google Scholar] [CrossRef]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Salvo, G.D.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart. J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef]
- Barman, H.A.; Atici, A.; Tekin, E.A.; Baycan, O.F.; Alici, G.; Meric, B.K.; Sit, O.; Genc, O.; Er, F.; Gungor, B.; et al. Echocardiographic features of patients with COVID-19 infection: A cross-sectional study. Int. J. Cardiovasc. Imaging 2021, 37, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Karagodin, I.; Carvalho Singulane, C.; Woodward, G.M.; Xie, M.; Tucay, E.S.; Tude Rodrigues, A.C.; Vasquez-Ortiz, Z.Y.; Alizadehasl, A.; Monaghan, M.J.; Ordonez Salazar, B.A.; et al. WASE-COVID Investigators. Echocardiographic Correlates of In-hospital death in patients with acute COVID-19 infection: The World Alliance Societies of Echocardiography (WASE-COVID) Study. J. Am. Soc. Echocardiogr. 2021, 34, 819–830. [Google Scholar] [CrossRef]
- McMahon, S.R.; De Francis, G.; Schwartz, S.; Duvall, W.L.; Arora, B.; Silverman, D.I. Tablet-based limited echocardiography to reduce sonographer scan and decontamination time during the COVID-19 pandemic. J. Am. Soc. Echocardiogr. 2020, 33, 895–899. [Google Scholar] [CrossRef]
- Dadon, Z.; Levi, N.; Alpert, E.A.; Orlev, A.; Belman, D.; Glikson, M.; Butnaru, A.; Gottlieb, S. The quality, safety, feasibility, and interpretive accuracy of echocardiographic and lung ultrasound assessment of COVID-19 patients using a hand-held ultrasound. Echocardiography 2022, 39, 886–894. [Google Scholar] [CrossRef]
- Maheshwarappa, H.M.; Mishra, S.; Kulkarni, A.V.; Gunaseelan, V.; Kanchi, M. Use of handheld ultrasound device with artificial intelligence for evaluation of cardiorespiratory system in COVID-19. Indian J. Crit. Care Med. 2021, 25, 524–527. [Google Scholar] [PubMed]
- Melamed, R.; Sprenkle, M.D.; Ulstad, V.K.; Herzog, C.A.; Leatherman, J.W. Assessment of left ventricular function by intensivists using hand-held echocardiography. Chest 2009, 135, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Voigt, J.U. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Chamsi-Pasha, M.A.; Sengupta, P.P.; Zoghbi, W.A. Handheld echocardiography: Current state and future perspectives. Circulation 2017, 136, 2178–2188. [Google Scholar] [CrossRef]
- Tsutsui, J.M.; Maciel, R.R.; Costa, J.M.; Andrade, J.L.; Ramires, J.F.; Mathias, W. Hand-carried ultrasound performed at bedside in cardiology inpatient setting–a comparative study with comprehensive echocardiography. Cardiovasc. Ultrasound 2004, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dadon, Z.; Levi, N.; Orlev, A.; Belman, D.; Alpert, E.A.; Glikson, M.; Gottlieb, S.; Butnaru, A. The utility of handheld cardiac and lung ultrasound in predicting outcomes of hospitalised patients with COVID-19. Can. J. Cardiol. 2022, 38, 338–346. [Google Scholar] [CrossRef]
- Sachpekidis, V.; Adamopoulos, C.; Datsios, A.; Mosialos, L.; Stamatiadis, N.; Gogos, C.; Poulianitis, V.; Galanos, O.; Stratilati, S.; Styliadis, I.; et al. A tricky case of cardiogenic shock: Diagnostic challenges in the COVID-19 era. Clin. Case Rep. 2021, 9, 420–424. [Google Scholar] [CrossRef]
- Sauza-Sosa, J.C.; Arratia-Carlin, K.; Fernandez-Tapia, J. Point-of-care ultrasound assessment with handheld ultrasound device attached to cell phone. J. Clin. Ultrasound 2022, 50, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Jeyashanmugaraja, G.P.; Shkolnik, E.; Akanya, D.T.; Stawiarski, K.; Winterbottom, C.; Zarich, S. One clot after another in COVID-19 patient: Diagnostic utility of handheld echocardiogram. Oxf. Med. Case Rep. 2021, 2021, omaa141. [Google Scholar] [CrossRef] [PubMed]
- Elikowski, W.A.; Malek-Elikowska, M.; Fertala, N.; Zawodna-Marszalek, M.; Wroblewski, D.; Zytkiewicz, M. Tablet-based limited echocardiography at COVID-19-dedicated hospital during the pandemic in the context of takotsubo syndrome. Pol. Merkur Lek. 2021, 49, 57–59. [Google Scholar]
- Mierzwa, A.P.; Huang, S.P.; Nguyen, K.T.; Culjat, M.O.; Singh, R.S. Wearable ultrasound array for point-of-care imaging and patient monitoring. Stud. Health Technol. Inf. 2016, 220, 241–244. [Google Scholar]
- Dadon, Z.; Butnaru, A.; Rosenmann, D.; Alper-Suissa, L.; Glikson, M.; Alpert, E.A. Use of artificial intelligence as a didactic tool to improve ejection fraction assessment in the emergency department: A randomized controlled pilot study. AEM Educ. Train. 2022, 6, e10738. [Google Scholar] [CrossRef]
- Dadon, Z.; Levi, N.; Orlev, A.; Belman, D.; Butnaru, A.; Glikson, M.; Gottlieb, S.; Alpert, E.A. Use of artificial intelligence for point-of-care echocardiographic assessment of left ventricular ejection fraction among COVID-19 patients. Eur. Heart J. 2022, 43 (Suppl. 2), ehac544-004. [Google Scholar] [CrossRef]



| Advantages | Challenges | Similarities † |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Type of Device * | HUD Photo † | Manufacturer/Headquarters | Cardiac Probe | Size (mm) ** | Weight (gr.) | Display Platform (Screen Size) | Wireless Probe |
|---|---|---|---|---|---|---|---|
| BenQ H1300 |  | BenQ/Taipei City, Taiwan | 2–4 MHz phased | S: 356 × 242 × 153 | S: 1200 | Designated console (8”) | - |
| Butterfly iQ+ |  | Butterfly Network/Burlington, Massachusetts | 2–10 MHz phased | P: 163 × 56 × 35 | P: 309 | Mobile device | - |
| Chison SonoEye |  | Chison/Wuxi, Jiangsu, China | 2.5 MHz phased | P: 173 × 64 × 24 | P: 100 | Mobile device | - |
| Clarius PA HD3 |  | Clarius/Vancouver, BC, CA | 1–5 MHz phased | P: 148 × 76 × 32 | P: 292 | Mobile device | √ |
| Kosmos Ultraportable Ultrasound |  | EchoNous/Redmond, WA | 2.0–5.0 MHz phased | S: 216 × 146 × 59 P: 150 × 56 × 35 | S: 653 P: 225 | Designated console (8”) or Android tablet | - |
| LeSONO LU710PA |  | LELTEK Inc./Taipei, Taiwan | 1.7–3.7 MHz phased | P: 194 × 74 × 40 | P: 350 | Mobile device | √ |
| Lumify S4-1 |  | Philips Medical Systems/Eindhoven, Holland | 1–4 MHz sector/phased | P: 10.2 × 5.1 | P: 96 | Mobile device | - |
| Vave health |  | Vave Health/Santa Clara, California | 1.5–3.5 MHz phased | P: 169 × 54 × 38 | P: 340 (w) | Mobile device | √ |
| Vscan Extend |  | GE Healthcare/Chicago, Illinois | 1.7–3.8 MHz Sector | S: 168 × 76 × 22 P: 129 × 39 × 28 | S: 321 P: 85 | Designated console (5.7”) | - |
| Assessed Entity/Chamber | Evaluated Parameters and Findings | Potential Diagnoses |
|---|---|---|
| LV ○ |
| Myocarditis Takotsubo cardiomyopathy Myocardial infarction |
| Pericardial space ○ |
| Pericarditis Tamponade |
| RV |
| Acute pulmonary hypertension Pulmonary embolism |
| IVC |
| Part of volume status/shock assessment † |
| Valvular function ○ |
| Regurgitation Stenosis |
| Others |
| Thrombus/tumor Pleural effusion Lung congestion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadon, Z.; Carasso, S.; Gottlieb, S. The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19. Biomedicines 2023, 11, 239. https://doi.org/10.3390/biomedicines11020239
Dadon Z, Carasso S, Gottlieb S. The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19. Biomedicines. 2023; 11(2):239. https://doi.org/10.3390/biomedicines11020239
Chicago/Turabian StyleDadon, Ziv, Shemy Carasso, and Shmuel Gottlieb. 2023. "The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19" Biomedicines 11, no. 2: 239. https://doi.org/10.3390/biomedicines11020239
APA StyleDadon, Z., Carasso, S., & Gottlieb, S. (2023). The Role of Hand-Held Cardiac Ultrasound in Patients with COVID-19. Biomedicines, 11(2), 239. https://doi.org/10.3390/biomedicines11020239







