Intra-Arterial Urokinase for Acute Superior Mesenteric Artery Occlusion: A Retrospective 12-Year Report of 13 Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Indication of Thrombolysis
2.3. Exclusion Criteria
2.4. Procedure
2.5. Definition
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Björck, M.; Acosta, S.; Lindberg, F.; Troëng, T.; Bergqvist, D. Revascularization of the superior mesenteric artery after acute thromboembolic occlusion. Br. J. Surg. 2002, 89, 923–927. [Google Scholar] [CrossRef]
- Gupta, P.K.; Natarajan, B.; Gupta, H.; Fang, X.; Fitzgibbons, R.J., Jr. Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery 2011, 150, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Koffeman, G.I.; Legemate, D.A.; Levi, M.; van Gulik, T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br. J. Surg. 2004, 91, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Tilsed, J.V.; Casamassima, A.; Kurihara, H.; Mariani, D.; Martinez, I.; Pereira, J.; Ponchietti, L.; Shamiyeh, A.; al-Ayoubi, F.; Barco, L.A.B.; et al. ESTES guidelines: Acute mesenteric ischaemia. Eur. J. Trauma Emerg. Surg. 2016, 42, 253–270. [Google Scholar] [CrossRef] [Green Version]
- Bala, M.; Kashuk, J.; Moore, E.E.; Kluger, Y.; Biffl, W.; Gomes, C.A.; Ben-Ishay, O.; Rubinstein, C.; Balogh, Z.J.; Civilet, I.; et al. Acute Mesenteric Ischemia: Guidelines of the World Society of Emergency Surgery. World J. Emerg. Surg. 2017, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Schoenbaum, S.W.; Pena, C.; Koenigsberg, P.; Katzen, B.T. Superior mesenteric artery embolism: Treatment with intra arterial urokinase. J. Vasc. Interv. Radiol. 1992, 3, 485–490. [Google Scholar] [CrossRef]
- Simo, G.; Echenagusia, A.J.; Camunez, F.; Turegano, F.; Cabrera, A.; Urbano, J. Superior mesenteric arterial embolism, local fibrinolytic treatment with urokinase. Radiology 1997, 204, 775–779. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Saeki, M.; Iwasaki, Y.; Ishikawa, M.; Hayakawa, M.; Sakuyama, K.; Ishikawa, T.; Ashidaet, H. Local thrombolytic therapy for superior mesenteric artery embolism: Complications and long-term clinical follow-up. Radiat. Med. 1999, 17, 27–33. [Google Scholar]
- Schoots, I.G.; Levi, M.M.; Reekers, J.A.; Lameris, J.S.; van Gulik, T.M. Thrombolytic therapy for acute superior mesenteric artery occlusion. J. Vasc. Interv. Radiol. 2005, 16, 317–329. [Google Scholar] [CrossRef]
- Björnsson, S.; Björck, M.; Block, T.; Resch, T.; Acosta, S. Thrombolysis for acute occlusion of the superior mesenteric artery. J. Vasc. Surg. 2011, 54, 1734–1742. [Google Scholar] [CrossRef] [Green Version]
- Agha, R.A.; Borrelli, M.R.; Farwana, R.; Koshy, K.; Fowler, A.J.; Orgill, D.P. The PROCESS 2018 statement: Updating consensus preferred reporting of case series in surgery (PROCESS) guidelines. Int. J. Surg. 2018, 60, 279–282. [Google Scholar] [CrossRef]
- Endean, E.D.; Barnes, S.L.; Kwolek, C.J.; Minion, D.J.; Schwarcz, T.H.; Mentzer, R.M., Jr. Surgical management of thrombotic acute intestinal ischemia. Ann. Surg. 2001, 233, 801–808. [Google Scholar] [CrossRef]
- Park, W.M.; Gloviczki, P.; Cherry, K.J., Jr.; Hallett, J.W., Jr.; Bower, T.C.; Panneton, J.M.; Schleck, C.; Ilstrup, D.; Harmsen, W.S.; Noel, A.A. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J. Vasc. Surg. 2002, 35, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Acosta, S.; Nilsson, T. Current status on plasma biomarkers for acute mesenteric ischemia. J. Thromb. Thrombolysis 2012, 33, 355–361. [Google Scholar] [CrossRef]
- Montagnana, M.; Danese, E.; Lippi, G. Biochemical markers of acute intestinal ischemia: Possibilities and limitations. Ann. Transl. Med. 2018, 6, 341. [Google Scholar] [CrossRef]
- Horton, K.M.; Fishman, E.K. Multi-detector row CT of mesenteric ischemia: Can it be done? Radiographics 2001, 21, 1463–1473. [Google Scholar] [CrossRef]
- Wadman, M.; Block, T.; Ekberg, O.; Syk, I.; Elmståhl, S.; Acosta, S. Impact of MDCT with intravenous contrast on the survival in patients with acute superior mesenteric artery occlusion. Emerg. Radiol. 2010, 17, 171–178. [Google Scholar] [CrossRef]
- Menke, J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: Systematic review and meta-analysis. Radiology 2010, 256, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Acosta, S.; Sonesson, B.; Resch, T. Endovascular therapeutic approaches for acute superior mesenteric artery occlusion. Cardiovasc. Intervent. Radiol. 2009, 32, 896–905. [Google Scholar] [CrossRef]
- Heiss, P.; Loewenhardt, B.; Manke, C.; Hellinger, A.; Dietl, K.H.; Schlitt, H.J.; Scheibl, K.; Feuerbach, S.; Paetzelet, C. Primary percutaneous aspiration and thrombolysis for the treatment of acute embolic superior mesenteric artery occlusion. Eur. Radiol. 2010, 20, 2948–2958. [Google Scholar] [CrossRef]
- Jamieson, A.C.; Thomas, R.J.; Cade, J.F. Lysis of a superior mesenteric artery embolus following local infusion of streptokinase and heparin. Aust. N. Z. J. Surg. 1979, 49, 355–356. [Google Scholar] [CrossRef]
- Morano, J.U.; Harrison, R.B. Mesenteric ischemia: Angiographic diagnosis and intervention. Clin. Imaging 1991, 15, 91–98. [Google Scholar] [CrossRef]
- Rodde, A.; Peiffert, B.; Bazin, C.; Amrein, D.; Regent, D.; Mathieu, P. Intra-arterial fibrinolysis of superior mesenteric artery embolism. J. Radiol. 1991, 72, 239–242. [Google Scholar]
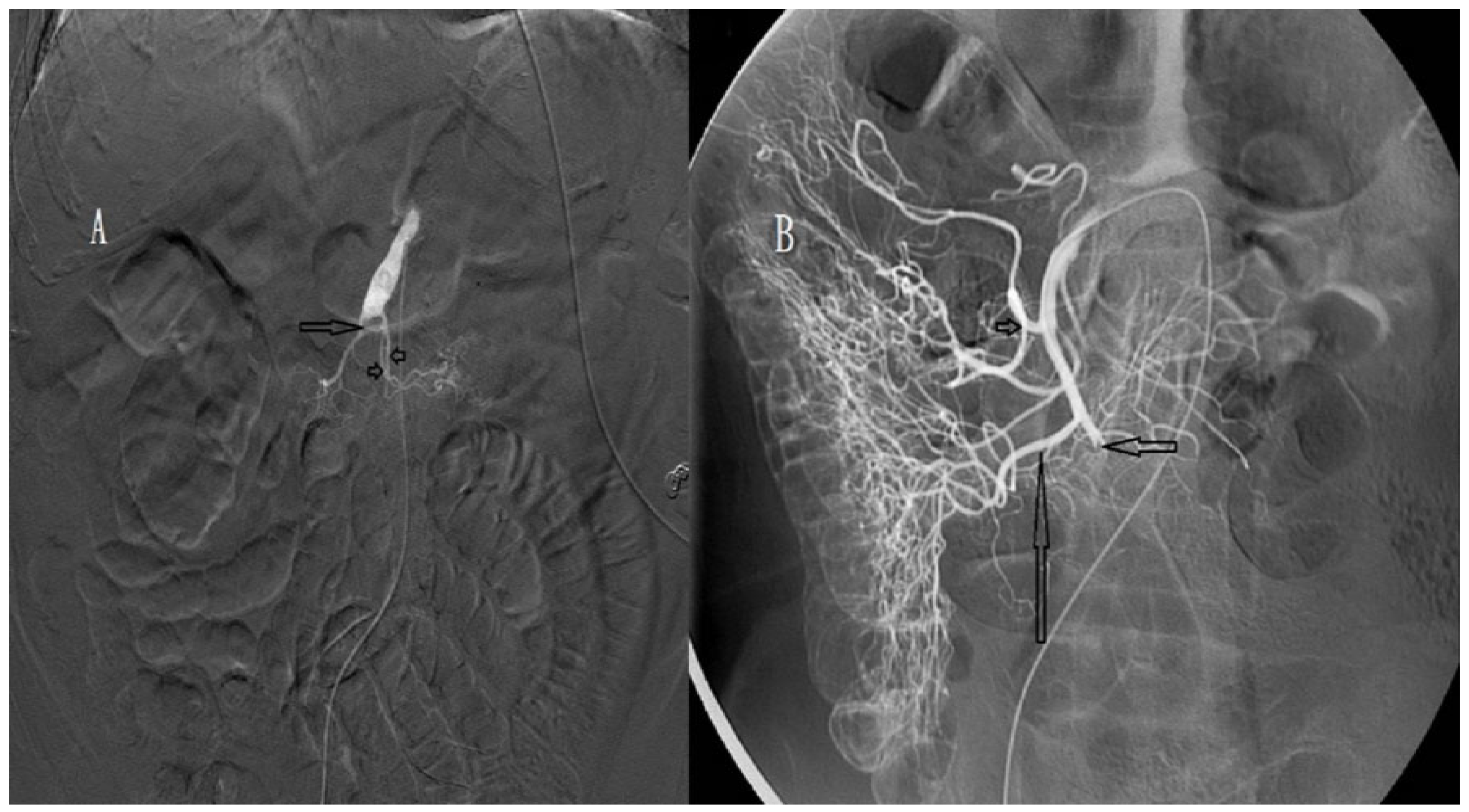
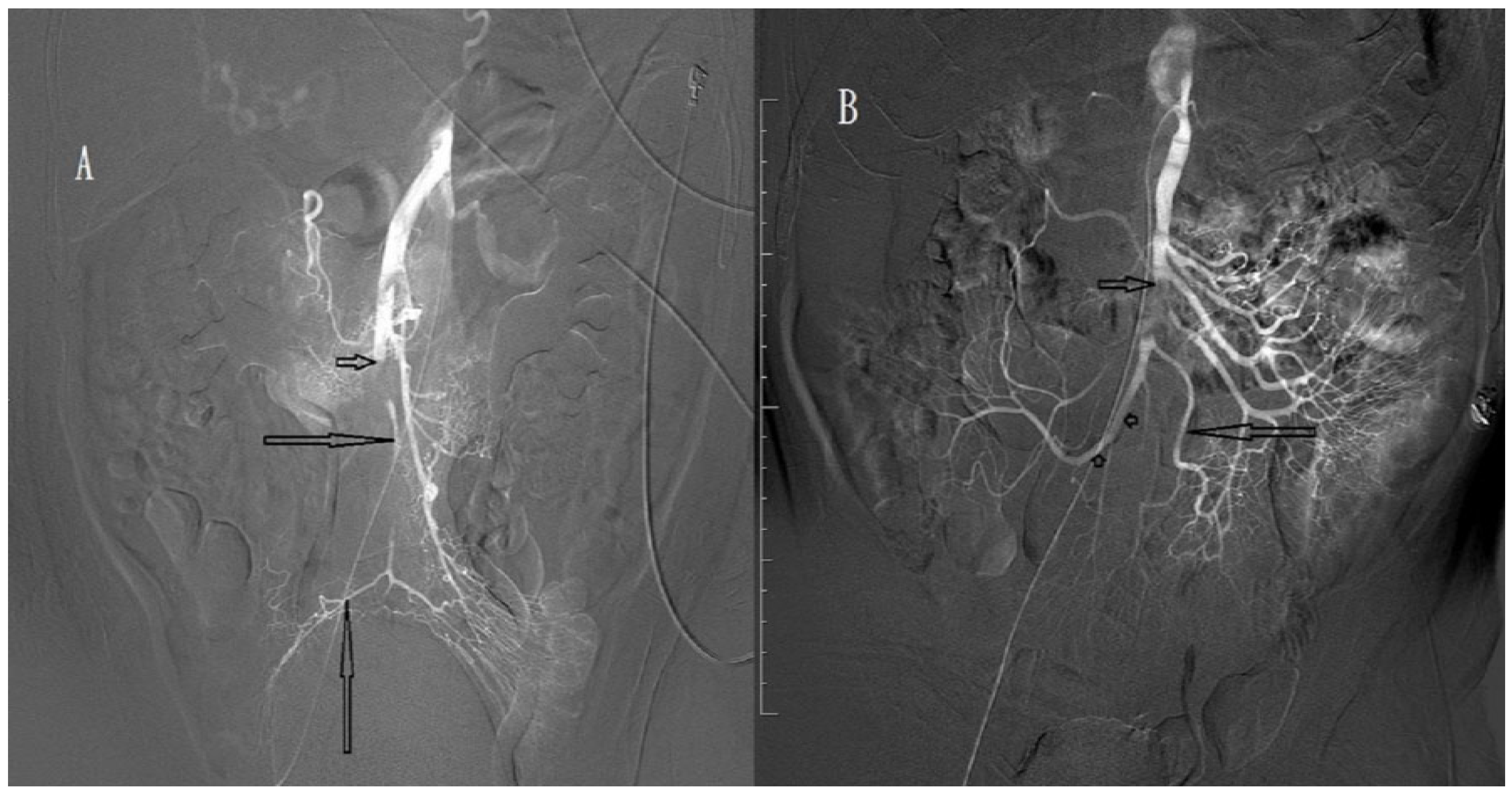


| Characteristics | Patients (n = 13) |
|---|---|
| Gender | |
| Male, n (%) | 9 (69.2) |
| Female, n (%) | 4 (30.8) |
| Age (years) Median (IQR) | 73.0 (24.0) |
| History | |
| Hypertension, n (%) | 11 (84.6) |
| Atrial fibrillation, n (%) | 9 (69.2) |
| Heart disease (CAD, CHF etc.), n (%) | 6 (46.2) |
| Diabetes mellitus, n (%) | 4 (30.8) |
| CVA, n (%) | 3 (23.1) |
| Time from abdominal pain to emergency department (hours) Median (IQR) | 9.0 (7.5) |
| Shock at triage, n (%) | 0 (0) |
| Bloody stool, n (%) | 5 (38.5) |
| Initial serum WBC (U/L) Median (IQR) | 13,200 (8350.0) |
| Initial serum hemoglobin (g/dL) Median (IQR) | 14.0 (4.5) |
| Initial serum amylase (U/L) Median (IQR) | 121.0 (79.0) |
| Synchronous intra-abdominal organ ischemia on CT, | 2 (15.4%) |
| Time from abdominal pain to urokinase (hours) Median (IQR) | 15.0 (6.0) |
| Duration of urokinase (hours) Median (IQR) | 42.0 (45.0) |
| Site of occlusion from SMA origin (cm) Median (IQR) | 5.0 (2.0) |
| Location of occlusion a | |
| Proximal, n (%) | 4 (30.8) |
| Distal, n (%) | 9 (69.2) |
| Degree of SMA occlusion b | |
| Complete, n (%) | 7 (53.8) |
| Incomplete, n (%) | 6 (46.2) |
| Degree of SMA recanalization after urokinase | |
| Total, n (%) | 0 |
| Near-total, n (%) | 5 |
| Partial, n (%) | 8 |
| Surgery, n (%) | 7 (53.8) |
| Bowel resection, n (%) | 6 (46.2) |
| Partial omentectomy, n (%) | 1 (7.7) |
| Laparoscopy c, n (%) | 4 (30.8) |
| Repeated surgery, n (%) | 5 (38.5) |
| Overall in-hospital mortality rate, n (%) | 4 (30.8) |
| Length of stay (days) Median (IQR) | 17.0 (34.0) |
| No. | Age | Sex | History | Bloody Stool | Synchronous Ischemia on CT | SMA Occlusion | Intra-Arterial Urokinase | Laparotomy | Outcome | Hospital Stay (Days) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To Orifice (cm) | Degree | Time a (Hours) | Recanalization | Time b (Hours) | Procedure | ||||||||
| 1 | 50 | M | H/T | No | No | D, 5.0 | Incomplete | 9 | Partial | 32 | Omenectomy | Alive | 9 |
| 2 | 80 | M | H/T, AF, CVA | Yes | No | D, 8.0 | Complete | 15 | Partial | 24 | Bowel resection c | Alive | 23 |
| 3 | 88 | M | H/T, CVA | No | No | D, 5.5 | Complete | 16 | Partial | 55 | Bowel resection c | Alive | 45 |
| 4 d | 83 | F | H/T, AF, CHF | No | kidney | P, 3.5 | Complete | 19 | Partial | No | Dead | 1 | |
| 5 e | 77 | F | H/T, AF, CAD | Yes | No | D, 4.0 | Incomplete | 28 | Near-total | No | Alive | 17 | |
| 6 | 68 | M | H/T | Yes | No | D, 5.0 | Complete | 26 | Partial | 126 | Bowel resection c | Dead | 65 |
| 7 e | 67 | M | AF, AAA | No | Spleen, kidney | D, 4.5 | Incomplete | 15 | Near-total | No | Alive | 7 | |
| 8 | 73 | F | H/T, CVA, DM | Yes | No | P, 3.5 | Complete | 21 | Partial | 47 | Bowel resection c | Dead | 36 |
| 9 | 78 | M | H/T, AF, CAD | No | No | D, 6.0 | Incomplete | 14 | Near-total | No | Alive | 6 | |
| 10 | 78 | M | H/T, AF, DM | No | No | P, 1.5 | Complete | 15 | Partial | 60 | Bowel resection | Alive | 28 |
| 11 | 77 | M | H/T, AF, CAD | Yes | No | D, 5.0 | Incomplete | 12 | Near-total | No | Alive | 11 | |
| 12 | 61 | M | H/T, AF | No | No | P, 3.5 | Complete | 14 | Partial | 51 | Bowel resection c | Dead | 49 |
| 13 | 73 | F | AF, CAD, DM | No | No | D, 5.5 | Incomplete | 18.5 | Near-total | No | Alive | 7 | |
| Characteristics | Restored Bowel Perfusion | Unrestored Bowel Perfusion | p-Value |
|---|---|---|---|
| No. of patients | 6 a | 7 b | |
| Gender | >0.999 | ||
| Male, n (%) | 4 (66.7) | 5 (71.4) | |
| Female, n (%) | 2 (33.3) | 2 (28.6) | |
| Age (years) median, IQR | 75.0 (15.0) | 78.0 (15.0) | 0.251 |
| Bloody stool | 2/6 (33.3) | 3/7 (42.9) | >0.999 |
| Initial serum WBC (U/L) median, IQR | 12,850 (7825.0) | 14,500 (9900.0) | 0.252 |
| Initial serum hemoglobin (g/dL) median, IQR | 14.1 (4.5) | 13.2 (4.7) | 0.830 |
| Synchronous intra-abdominal organ ischemia on CT, | 1 (16.7) | 1 (14.3) | >0.999 |
| Time from abdominal pain to urokinase (hours) Median (IQR) | 14.5 (9.6) | 16.0 (6.0) | 0.281 |
| Location of SMA occlusion | 0.105 | ||
| Proximal, n (%) Distal n (%) | 0 (0) 6 (100) | 4 (57.1) 3 (42.9) | |
| Degree of SMA occlusion | 0.002 | ||
| Complete, n (%) | 0 (0) | 7 (100) | |
| Incomplete, n (%) | 6 (100) | 0 (0) | |
| Degree of SMA recanalization after urokinase | 0.012 | ||
| Near-total Partial | 5 (83.3) 1 (16.7) | 0 (0) 7( 100) | |
| Length of stay (days) median, IQR | 8.0 (6.0) | 36.0 (26.0) | 0.032 |
| Mortality, n (%) | 0 (0) | 4 (57.1) | 0.0105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.-C.; Wu, C.-H.; Wong, Y.-C.; Hung, S.-C.; Hsin, M.-C. Intra-Arterial Urokinase for Acute Superior Mesenteric Artery Occlusion: A Retrospective 12-Year Report of 13 Cases. Biomedicines 2023, 11, 267. https://doi.org/10.3390/biomedicines11020267
Lin B-C, Wu C-H, Wong Y-C, Hung S-C, Hsin M-C. Intra-Arterial Urokinase for Acute Superior Mesenteric Artery Occlusion: A Retrospective 12-Year Report of 13 Cases. Biomedicines. 2023; 11(2):267. https://doi.org/10.3390/biomedicines11020267
Chicago/Turabian StyleLin, Being-Chuan, Cheng-Hsien Wu, Yon-Cheong Wong, Sheng-Che Hung, and Ming-Che Hsin. 2023. "Intra-Arterial Urokinase for Acute Superior Mesenteric Artery Occlusion: A Retrospective 12-Year Report of 13 Cases" Biomedicines 11, no. 2: 267. https://doi.org/10.3390/biomedicines11020267






