Applications of Mass Spectrometry in Dentistry
Abstract
:1. Introduction
2. MS Applications in Dental Materials
2.1. Laboratory Analysis
2.2. Saliva as a Diagnostic Fluid
3. MS Proteomics
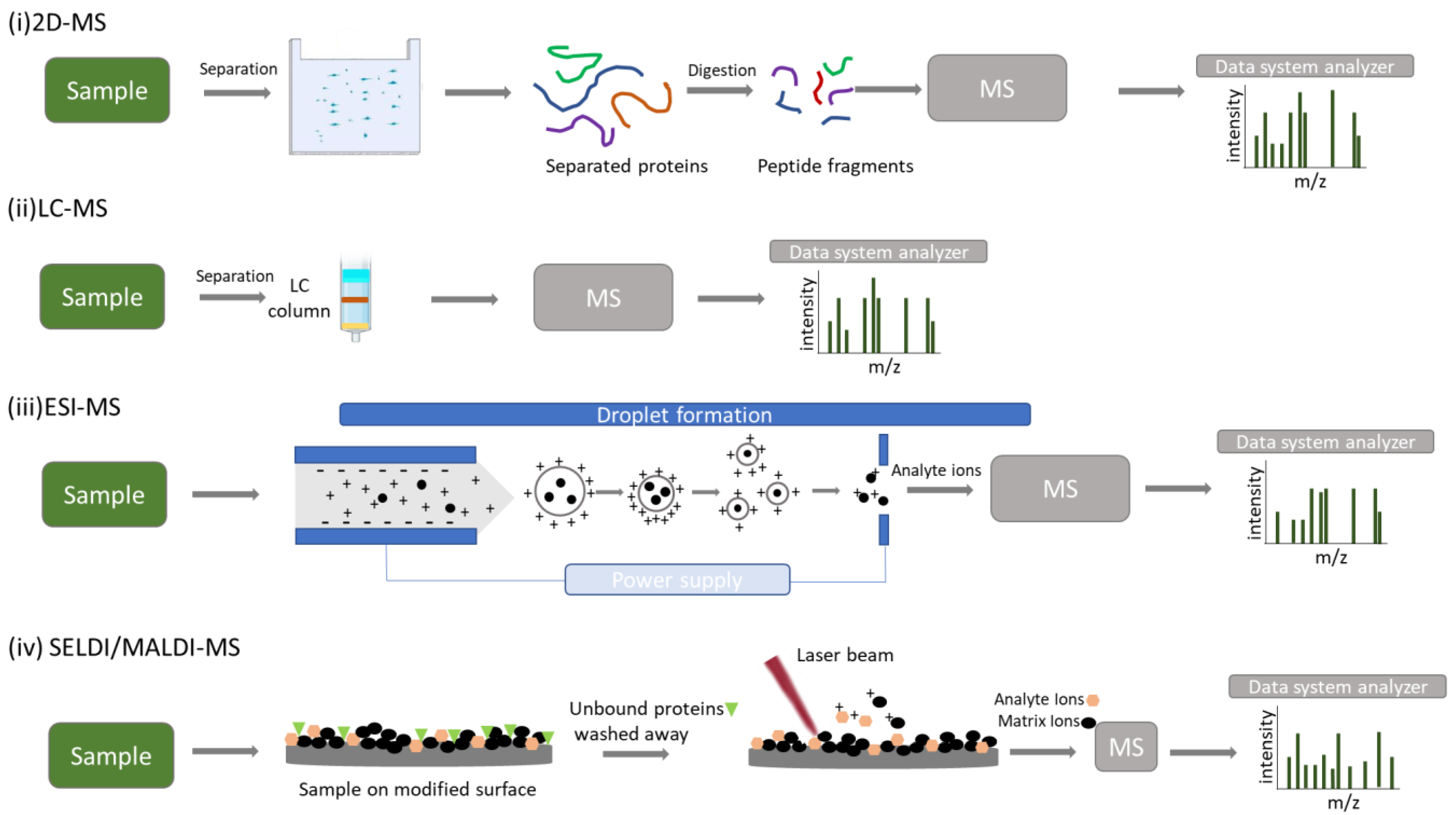
3.1. 2-DE/MS
3.2. LC–MS/MS
3.3. ESI–MS
3.4. MALDI-TOF/MS
3.5. SELDI-TOF/MS
4. MS Applications in Salivary Diagnostics
4.1. Oral Diseases
Caries
4.2. Periodontal Disease
4.3. Oral Cancer
4.4. Oral Lichen Planus
4.5. Oral Microbiome
4.6. Systematic Diseases
4.6.1. Sjogren’s Syndrome
4.6.2. Diabetes I and II
4.7. Cancer
4.8. Graft versus Host Disease
5. MS Applications in Hard and Soft Dental Tissues
5.1. Dental Hard Tissues
5.2. Acquired Enamel Pellicle
5.3. Other Applications
6. MS as a Real-Time Detector

7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paital, B. Mass Spectrophotometry: An Advanced Technique in Biomedical Sciences. Adv. Tech. Biol. Med. 2015, 4, 1000182. [Google Scholar] [CrossRef] [Green Version]
- Ciocan-Cartita, C.A.; Jurj, A.; Buse, M.; Gulei, D.; Braicu, C.; Raduly, L.; Cojocneanu, R.; Pruteanu, L.L.; Iuga, C.A.; Coza, O.; et al. The Relevance of Mass Spectrometry Analysis for Personalized Medicine through Its Successful Application in Cancer “Omics”. Int. J. Mol. Sci. 2019, 20, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.-H.; Kuo, C.-H. Gas Chromatography-Mass Spectrometry-Based Analytical Strategies for Fatty Acid Analysis in Biological Samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narukawa, T.; Iwai, T.; Chiba, K.; Feldmann, J. A Method for Methylmercury and Inorganic Mercury in Biological Samples Using High Performance Liquid Chromatography- Inductively Coupled Plasma Mass Spectrometry. Anal. Sci. 2018, 34, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Vaher, M.; Koel, M.; Kaljurand, M. Ionic Liquids as Electrolytes for Nonaqueous Capillary Electrophoresis. Electrophoresis 2002, 23, 426–430. [Google Scholar] [CrossRef]
- Vaiano, F.; Serpelloni, G.; Focardi, M.; Fioravanti, A.; Mari, F.; Bertol, E. LC–MS/MS and GC–MS Methods in Propofol Detection: Evaluation of the Two Analytical Procedures. Forensic Sci. Int. 2015, 256, 1–6. [Google Scholar] [CrossRef]
- March, R.E. Quadrupole Ion Trap Mass Spectrometry: A View at the Turn of the Century. Int. J. Mass Spectrom. 2000, 200, 285–312. [Google Scholar] [CrossRef]
- Hu, Q.; Noll, R.J.; Li, H.; Makarov, A.; Hardman, M.; Graham Cooks, R. The Orbitrap: A New Mass Spectrometer. J. Mass Spectrom. 2005, 40, 430–443. [Google Scholar] [CrossRef]
- Kaufmann, A. Analytical Performance of the Various Acquisition Modes in Orbitrap MS and MS/MS. J. Mass Spectrom. 2018, 53, 725–738. [Google Scholar] [CrossRef]
- Gerber, S.A.; Rush, J.; Stemman, O.; Kirschner, M.W.; Gygi, S.P. Absolute Quantification of Proteins and Phosphoproteins from Cell Lysates by Tandem MS. Proc. Natl. Acad. Sci. USA 2003, 100, 6940–6945. [Google Scholar] [CrossRef]
- Griffiths, J. A Brief History of Mass Spectrometry. Anal. Chem. 2008, 80, 5678–5683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spahl, W.; Budzikiewicz, H.; Geurtsen, W. Determination of Leachable Components from Four Commercial Dental Composites by Gas and Liquid Chromatography/Mass Spectrometry. J. Dent. 1998, 26, 137–145. [Google Scholar] [CrossRef]
- Manabe, A.; Kaneko, S.; Numazawa, S.; Itoh, K.; Inoue, M.; Hisamitsu, H.; Sasa, R.; Yoshida, T. Detection of Bisphenol-A in Dental Materials by Gas Chromatography-Mass Spectrometry. Dent. Mater. J. 2000, 19, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, B.W.; Jensen, E.; Örtengren, U.; Michelsen, V.B. Analysis of Organic Components in Resin-Modified Pulp Capping Materials: Critical Considerations. Eur. J. Oral Sci. 2017, 125, 183–194. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Sarbon, N.M.; Rana, S.S.; Chintagunta, A.D.; Prathibha, S.; Ingilala, S.K.; Jeevan Kumar, S.P.; Sai Anvesh, B.; Dirisala, V.R. Extraction of Bioactive Compounds from Psidium Guajava Leaves and Its Utilization in Preparation of Jellies. AMB Express 2021, 11, 36. [Google Scholar] [CrossRef]
- Zuanazzi, D.; Xiao, Y.; Siqueira, W.L. Evaluating Protein Binding Specificity of Titanium Surfaces through Mass Spectrometry–Based Proteomics. Clin. Oral Investig. 2021, 25, 2281–2296. [Google Scholar] [CrossRef]
- Chuang, S.-F.; Kang, L.-L.; Liu, Y.-C.; Lin, J.-C.; Wang, C.-C.; Chen, H.-M.; Tai, C.-K. Effects of Silane- and MDP-Based Primers Application Orders on Zirconia–Resin Adhesion—A ToF-SIMS Study. Dent. Mater. 2017, 33, 923–933. [Google Scholar] [CrossRef]
- Lima, R.B.W.; Barreto, S.C.; Alfrisany, N.M.; Porto, T.S.; De Souza, G.M.; De Goes, M.F. Effect of Silane and MDP-Based Primers on Physico-Chemical Properties of Zirconia and Its Bond Strength to Resin Cement. Dent. Mater. 2019, 35, 1557–1567. [Google Scholar] [CrossRef]
- Lapinska, B.; Rogowski, J.; Nowak, J.; Nissan, J.; Sokolowski, J.; Lukomska-Szymanska, M. Effect of Surface Cleaning Regimen on Glass Ceramic Bond Strength. Molecules 2019, 24, 389. [Google Scholar] [CrossRef] [Green Version]
- Papale, F.; Santonocito, S.; Polizzi, A.; Giudice, A.L.; Capodiferro, S.; Favia, G.; Isola, G. The New Era of Salivaomics in Dentistry: Frontiers and Facts in the Early Diagnosis and Prevention of Oral Diseases and Cancer. Metabolites 2022, 12, 638. [Google Scholar] [CrossRef]
- Kaufman, E.; Lamster, I.B. The Diagnostic Applications of Saliva—A Review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Pappa, E.; Kousvelari, E.; Vastardis, H. Saliva in the “Omics” Era: A Promising Tool in Paediatrics. Oral Dis. 2019, 25, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yu, Q.; Lin, Q.; Duan, Y. Emerging Salivary Biomarkers by Mass Spectrometry. Clin. Chim. Acta 2015, 438, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Koneru, S.; Tanikonda, R. Salivaomics—A Promising Future in Early Diagnosis of Dental Diseases. Dent. Res. J. 2014, 11, 11–15. [Google Scholar]
- Yamada, N.; Yuji, R.; Suzuki, E. The Current Status and Future Prospects of the Salivary Proteome. J. Health Sci. 2009, 55, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Paqué, P.N.; Herz, C.; Wiedemeier, D.B.; Mitsakakis, K.; Attin, T.; Bao, K.; Belibasakis, G.N.; Hays, J.P.; Jenzer, J.S.; Kaman, W.E.; et al. Salivary Biomarkers for Dental Caries Detection and Personalized Monitoring. JPM 2021, 11, 235. [Google Scholar] [CrossRef]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.-N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-Based Proteomics in Biomedical Research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef]
- Ali, A.; Abouleila, Y.; Shimizu, Y.; Hiyama, E.; Emara, S.; Mashaghi, A.; Hankemeier, T. Single-Cell Metabolomics by Mass Spectrometry: Advances, Challenges, and Future Applications. TrAC Trends Anal. Chem. 2019, 120, 115436. [Google Scholar] [CrossRef]
- Ardito, F.; Perrone, D.; Cocchi, R.; Lo Russo, L.; De Lillo, A.; Giannatempo, G.; Lo Muzio, L. Novel Possibilities in the Study of the Salivary Proteomic Profile Using SELDI-TOF/MS Technology. Oncol. Lett. 2016, 11, 1967–1972. [Google Scholar] [CrossRef] [Green Version]
- Chait, B.T. Mass Spectrometry: Bottom-Up or Top-Down? Science 2006, 314, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Timp, W.; Timp, G. Beyond Mass Spectrometry, the next Step in Proteomics. Sci. Adv. 2020, 6, eaax8978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angel, T.E.; Aryal, U.K.; Hengel, S.M.; Baker, E.S.; Kelly, R.T.; Robinson, E.W.; Smith, R.D. Mass Spectrometry-Based Proteomics: Existing Capabilities and Future Directions. Chem. Soc. Rev. 2012, 41, 3912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, C.V.; de Campos, W.G.; de Souza, M.M.; Lourenço, S.V.; Siqueira, W.L.; Lemos-Júnior, C.A. Diagnostic Potential of Saliva Proteome Analysis: A Review and Guide to Clinical Practice. Braz. Oral Res. 2019, 33, e043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domon, B.; Aebersold, R. Mass Spectrometry and Protein Analysis. Science 2006, 312, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Yang, X.; Huang, Y.; Tang, Z.; Chen, Y.; Liu, H.; Huang, M.; Qing, L.; Li, L.; Wang, Q.; et al. Saliva—A New Opportunity for Fluid Biopsy. Clin. Chem. Lab. Med. (CCLM) 2023, 61, 4–32. [Google Scholar] [CrossRef]
- Takahashi, N. Microbial Ecosystem in the Oral Cavity: Metabolic Diversity in an Ecological Niche and Its Relationship with Oral Diseases. Int. Congr. Ser. 2005, 1284, 103–112. [Google Scholar] [CrossRef]
- Ahmad, P.; Hussain, A.; Siqueira, W.L. Mass Spectrometry-based Proteomic Approaches for Salivary Protein Biomarkers Discovery and Dental Caries Diagnosis: A Critical Review. Mass Spectrom. Rev. 2022, 4, 1000182. [Google Scholar] [CrossRef]
- Vitorino, R.; de Morais Guedes, S.; Ferreira, R.; Lobo, M.J.C.; Duarte, J.; Ferrer-Correia, A.J.; Tomer, K.B.; Domingues, P.M.; Amado, F.M.L. Two-Dimensional Electrophoresis Study of in Vitro Pellicle Formation and Dental Caries Susceptibility. Eur. J. Oral Sci. 2006, 114, 147–153. [Google Scholar] [CrossRef]
- Preza, D.; Thiede, B.; Olsen, I.; Grinde, B. The Proteome of the Human Parotid Gland Secretion in Elderly with and without Root Caries. Acta Odontol. Scand. 2009, 67, 161–169. [Google Scholar] [CrossRef]
- Yan, G.; Huang, W.; Xue, H.; Jia, Y.; Yang, D. Relationship between dental caries and salivary proteome by electrospray ionization ion-trap tandem mass spectrometry in children aged 6 to 8 years. Hua Xi Kou Qiang Yi Xue Za Zhi 2014, 32, 297–302. [Google Scholar] [CrossRef]
- Pappa, E.; Vougas, K.; Zoidakis, J.; Papaioannou, W.; Rahiotis, C.; Vastardis, H. Downregulation of Salivary Proteins, Protective against Dental Caries, in Type 1 Diabetes. Proteomes 2021, 9, 33. [Google Scholar] [CrossRef]
- Petersen, P.E. The World Oral Health Report 2003: Continuous Improvement of Oral Health in the 21st Century—The Approach of the WHO Global Oral Health Programme: The World Oral Health Report 2003. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef]
- Antezack, A.; Chaudet, H.; Tissot-Dupont, H.; Brouqui, P.; Monnet-Corti, V. Rapid Diagnosis of Periodontitis, a Feasibility Study Using MALDI-TOF Mass Spectrometry. PLoS ONE 2020, 15, e0230334. [Google Scholar] [CrossRef]
- Ngo, L.H.; Veith, P.D.; Chen, Y.-Y.; Chen, D.; Darby, I.B.; Reynolds, E.C. Mass Spectrometric Analyses of Peptides and Proteins in Human Gingival Crevicular Fluid. J. Proteome Res. 2010, 9, 1683–1693. [Google Scholar] [CrossRef]
- Kojima, T.; Andersen, E.; Sanchez, J.C.; Wilkins, M.R.; Hochstrasser, D.F.; Pralong, W.F.; Cimasoni, G. Human Gingival Crevicular Fluid Contains MRP8 (S100A8) and MRP14 (S100A9), Two Calcium-Binding Proteins of the S100 Family. J. Dent. Res. 2000, 79, 740–747. [Google Scholar] [CrossRef]
- Pisano, E.; Cabras, T.; Montaldo, C.; Piras, V.; Inzitari, R.; Olmi, C.; Castagnola, M.; Messana, I. Peptides of Human Gingival Crevicular Fluid Determined by HPLC-ESI-MS. Eur. J. Oral Sci. 2005, 113, 462–468. [Google Scholar] [CrossRef]
- Ngo, L.H.; Darby, I.B.; Veith, P.D.; Locke, A.G.; Reynolds, E.C. Mass Spectrometric Analysis of Gingival Crevicular Fluid Biomarkers Can Predict Periodontal Disease Progression. J. Periodontal Res. 2013, 48, 331–341. [Google Scholar] [CrossRef]
- Wu, Y.; Shu, R.; Luo, L.-J.; Ge, L.-H.; Xie, Y.-F. Initial Comparison of Proteomic Profiles of Whole Unstimulated Saliva Obtained from Generalized Aggressive Periodontitis Patients and Healthy Control Subjects. J. Periodontal Res. 2009, 44, 636–644. [Google Scholar] [CrossRef]
- Gonçalves, L.D.R.; Soares, M.R.; Nogueira, F.C.S.; Garcia, C.; Camisasca, D.R.; Domont, G.; Feitosa, A.C.R.; de Pereira, D.A.; Zingali, R.B.; Alves, G. Comparative Proteomic Analysis of Whole Saliva from Chronic Periodontitis Patients. J. Proteom. 2010, 73, 1334–1341. [Google Scholar] [CrossRef]
- Rangé, H.; Léger, T.; Huchon, C.; Ciangura, C.; Diallo, D.; Poitou, C.; Meilhac, O.; Bouchard, P.; Chaussain, C. Salivary Proteome Modifications Associated with Periodontitis in Obese Patients. J. Clin. Periodontol. 2012, 39, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary Biomarkers for Oral Cancer and Pre-Cancer Screening: A Review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.-M.; Hsiao, Y.-C.; Chien, K.-Y.; Chen, S.-F.; Chuang, Y.-N.; Lin, S.-Y.; Wang, W.-S.; Chang, I.Y.-F.; Yang, C.; Chu, L.J.; et al. Assessment of Candidate Biomarkers in Paired Saliva and Plasma Samples from Oral Cancer Patients by Targeted Mass Spectrometry. J. Proteom. 2020, 211, 103571. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-L.; Liu, X.-Q.; Liu, W.; Cheng, B.; Li, M.-T. Comparative Analysis of Whole Saliva Proteomes for the Screening of Biomarkers for Oral Lichen Planus. Inflamm. Res. 2006, 55, 405–407. [Google Scholar] [CrossRef]
- Cruz, A.F.; Vitório, J.G.; Duarte-Andrade, F.F.; Diniz, M.G.; Canuto, G.A.B.; Toledo, J.S.; Fonseca, F.P.; Fernandes, A.P.; André, L.C.; Gomes, C.C.; et al. Reticular and Erosive Oral Lichen Planus Have a Distinct Metabolomic Profile: A Preliminary Study Using Gas Chromatography-mass Spectrometry. J. Oral Pathol. Med. 2019, 48, 400–405. [Google Scholar] [CrossRef]
- Buszewski, B.; Rogowska, A.; Pomastowski, P.; Złoch, M.; Railean-Plugaru, V. Identification of Microorganisms by Modern Analytical Techniques. J. AOAC Int. 2017, 100, 1607–1623. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 97, pp. 171–210. [Google Scholar] [CrossRef]
- Grenga, L.; Pible, O.; Armengaud, J. Pathogen Proteotyping: A Rapidly Developing Application of Mass Spectrometry to Address Clinical Concerns. Clin. Mass Spectrom. 2019, 14, 9–17. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Q.; Yan, G.; Yang, D. The Oral Microbiome and Salivary Proteins Influence Caries in Children Aged 6 to 8 Years. BMC Oral Health 2020, 20, 295. [Google Scholar] [CrossRef]
- Pei, J.; Li, F.; Xie, Y.; Liu, J.; Yu, T.; Feng, X. Microbial and Metabolomic Analysis of Gingival Crevicular Fluid in General Chronic Periodontitis Patients: Lessons for a Predictive, Preventive, and Personalized Medical Approach. EPMA J. 2020, 11, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Li, X.; Zheng, X.; Xia, Y.; Fu, Y.; Li, X.; Qian, Y.; Zou, J.; Zhao, A.; Guan, J.; et al. Pediatric Obstructive Sleep Apnea Is Associated with Changes in the Oral Microbiome and Urinary Metabolomics Profile: A Pilot Study. J. Clin. Sleep Med. 2018, 14, 1559–1567. [Google Scholar] [CrossRef]
- Bostanci, N.; Bao, K. Contribution of Proteomics to Our Understanding of Periodontal Inflammation. Proteomics 2017, 17, 1500518. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the Oral Microbiome in Personalized Dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Meijer, J.; Ieong, S.; Xie, Y.; Yu, T.; Zhou, H.; Henry, S.; Vissink, A.; Pijpe, J.; et al. Salivary Proteomic and Genomic Biomarkers for Primary Sjögren’s Syndrome. Arthritis Rheum. 2007, 56, 3588–3600. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Mu, Y.; Guo, C.; You, X.; Liu, X.; Li, Q.; Sun, W. Analysis of the Saliva Metabolic Signature in Patients with Primary Sjögren’s Syndrome. PLoS ONE 2022, 17, e0269275. [Google Scholar] [CrossRef]
- Pappa, E.; Vougas, K.; Zoidakis, J.; Vastardis, H. Proteomic Advances in Salivary Diagnostics. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140494. [Google Scholar] [CrossRef]
- Rao, P.V.; Reddy, A.P.; Lu, X.; Dasari, S.; Krishnaprasad, A.; Biggs, E.; Roberts, C.T.; Nagalla, S.R. Proteomic Identification of Salivary Biomarkers of Type-2 Diabetes. J. Proteome Res. 2009, 8, 239–245. [Google Scholar] [CrossRef]
- Hirtz, C.; Chevalier, F.; Sommerer, N.; Raingeard, I.; Bringer, J.; Rossignol, M.; de Périere, D.D. Salivary Protein Profiling in Type I Diabetes Using Two-Dimensional Electrophoresis and Mass Spectrometry. Clin. Proteom. 2006, 2, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Evers, S.; Lu, Z.H.; Shen, Y.; Chen, J. Two-Dimensional Protein Database of Human Pancreas. Electrophoresis 2004, 25, 512–518. [Google Scholar] [CrossRef]
- Sanchez, J.C.; Chiappe, D.; Converset, V.; Hoogland, C.; Binz, P.A.; Paesano, S.; Appel, R.D.; Wang, S.; Sennitt, M.; Nolan, A.; et al. The Mouse SWISS-2D PAGE Database: A Tool for Proteomics Study of Diabetes and Obesity. Proteomics 2001, 1, 136–163. [Google Scholar] [CrossRef]
- Ahmed, M.; Forsberg, J.; Bergsten, P. Protein Profiling of Human Pancreatic Islets by Two-Dimensional Gel Electrophoresis and Mass Spectrometry. J. Proteome Res. 2005, 4, 931–940. [Google Scholar] [CrossRef]
- Albrethsen, J.; Kaas, A.; Schönle, E.; Swift, P.; Kocova, M.; Gammeltoft, S.; Hansen, L.; Mortensen, H.B. Evaluation of a Type 1 Diabetes Serum Cohort by SELDI-TOF MS Protein Profiling. Prot. Clin. Appl. 2009, 3, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Bano, S.; Vavachan, V.M.; Taunk, K.; Seshadri, V.; Rapole, S. Application of Mass Spectrometry Based Proteomics to Understand Diabetes: A Special Focus on Interactomics. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140469. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Vastardis, H.; Mermelekas, G.; Gerasimidi-Vazeou, A.; Zoidakis, J.; Vougas, K. Saliva Proteomics Analysis Offers Insights on Type 1 Diabetes Pathology in a Pediatric Population. Front. Physiol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Streckfus, C.F.; Bigler, L.R.; Zwick, M. The Use of Surface-Enhanced Laser Desorption/Ionization Time-of-Flight Mass Spectrometry to Detect Putative Breast Cancer Markers in Saliva: A Feasibility Study. J. Oral Pathol. Med. 2006, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, O.; Haviv, Y.; Krief, G.; Keshet, N.; Westreich, R.; Stemmer, S.M.; Zaks, B.; Navat, S.P.; Yanko, R.; Lahav, O.; et al. Possible Proteomic Biomarkers for the Detection of Pancreatic Cancer in Oral Fluids. Sci. Rep. 2020, 10, 21995. [Google Scholar] [CrossRef]
- Asai, Y.; Itoi, T.; Sugimoto, M.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Mukai, S.; Fujita, M.; et al. Elevated Polyamines in Saliva of Pancreatic Cancer. Cancers 2018, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Z.; Wang, J.-G.; Zhang, X.-L. Diagnostic Model of Saliva Protein Finger Print Analysis of Patients with Gastric Cancer. WJG 2009, 15, 865. [Google Scholar] [CrossRef]
- Bassim, C.W.; Ambatipudi, K.S.; Mays, J.W.; Edwards, D.A.; Swatkoski, S.; Fassil, H.; Baird, K.; Gucek, M.; Melvin, J.E.; Pavletic, S.Z. Quantitative Salivary Proteomic Differences in Oral Chronic Graft-versus-Host Disease. J. Clin. Immunol. 2012, 32, 1390–1399. [Google Scholar] [CrossRef]
- Chiappelli, F.; Covani, U.; Giacomelli, L. Proteomics as It Pertains to Oral Pathologies and Dental Research. Bioinformation 2011, 5, 277. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, M.J.; Faught, M.J.; Carlisle, B.H.; Stockwell, P.A. ToothPrint, a Proteomic Database for Dental Tissues. Proteomics 2001, 1, 132–135. [Google Scholar] [CrossRef]
- Jágr, M.; Eckhardt, A.; Pataridis, S.; Broukal, Z.; Dušková, J.; Mikšík, I. Proteomics of Human Teeth and Saliva. Physiol. Res. 2014, S141–S154. [Google Scholar] [CrossRef]
- Park, E.-S.; Cho, H.-S.; Kwon, T.-G.; Jang, S.-N.; Lee, S.-H.; An, C.-H.; Shin, H.-I.; Kim, J.-Y.; Cho, J.-Y. Proteomics Analysis of Human Dentin Reveals Distinct Protein Expression Profiles. J. Proteome Res. 2009, 8, 1338–1346. [Google Scholar] [CrossRef]
- Jágr, M.; Eckhardt, A.; Pataridis, S.; Mikšík, I. Comprehensive Proteomic Analysis of Human Dentin. Eur. J. Oral Sci. 2012, 120, 259–268. [Google Scholar] [CrossRef]
- Hammarström, L.; Alatli, I.; Fong, C. Origins of Cementum. Oral Dis. 2008, 2, 63–69. [Google Scholar] [CrossRef]
- Salmon, C.R.; Tomazela, D.M.; Ruiz, K.G.S.; Foster, B.L.; Paes Leme, A.F.; Sallum, E.A.; Somerman, M.J.; Nociti, F.H. Proteomic Analysis of Human Dental Cementum and Alveolar Bone. J. Proteom. 2013, 91, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, W.L.; Zhang, W.; Helmerhorst, E.J.; Gygi, S.P.; Oppenheim, F.G. Identification of Protein Components in in Vivo Human Acquired Enamel Pellicle Using LC−ESI−MS/MS. J. Proteome Res. 2007, 6, 2152–2160. [Google Scholar] [CrossRef]
- Zimmerman, J.; Custodio, W.; Hatibovic-Kofman, S.; Lee, Y.; Xiao, Y.; Siqueira, W. Proteome and Peptidome of Human Acquired Enamel Pellicle on Deciduous Teeth. Int. J. Mol. Sci. 2013, 14, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Campanella, B.; Onor, M.; Lomonaco, T.; Benedetti, E.; Bramanti, E. HS-SPME-GC-MS Approach for the Analysis of Volatile Salivary Metabolites and Application in a Case Study for the Indirect Assessment of Gut Microbiota. Anal. Bioanal. Chem. 2019, 411, 7551–7562. [Google Scholar] [CrossRef]
- Yaprak, E.; Yolcubal, İ.; Sinanoğlu, A.; Doğrul-Demiray, A.; Guzeldemir-Akcakanat, E.; Marakoğlu, İ. High Levels of Heavy Metal Accumulation in Dental Calculus of Smokers: A Pilot Inductively Coupled Plasma Mass Spectrometry Study. J. Periodontal Res. 2017, 52, 83–88. [Google Scholar] [CrossRef]
- Warinner, C.; Hendy, J.; Speller, C.; Cappellini, E.; Fischer, R.; Trachsel, C.; Arneborg, J.; Lynnerup, N.; Craig, O.E.; Swallow, D.M.; et al. Direct Evidence of Milk Consumption from Ancient Human Dental Calculus. Sci. Rep. 2015, 4, 7104. [Google Scholar] [CrossRef]
- Buchholz, B.A.; Spalding, K.L. Year of Birth Determination Using Radiocarbon Dating of Dental Enamel: Forensic 14C Dating of Dental Enamel. Surf. Interface Anal. 2010, 42, 398–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orringer, D.A.; Pandian, B.; Niknafs, Y.S.; Hollon, T.C.; Boyle, J.; Lewis, S.; Garrard, M.; Hervey-Jumper, S.L.; Garton, H.J.L.; Maher, C.O.; et al. Rapid Intraoperative Histology of Unprocessed Surgical Specimens via Fibre-Laser-Based Stimulated Raman Scattering Microscopy. Nat. Biomed. Eng. 2017, 1, 0027. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-C.; Dorrestein, P.C. Visualizing Life with Ambient Mass Spectrometry. Curr. Opin. Biotechnol. 2015, 31, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.-H.; Dutkiewicz, E.P.; Pei, J.; Hsu, C.-C. Ambient Ionization Mass Spectrometry Today and Tomorrow: Embracing Challenges and Opportunities. Anal. Chem. 2020, 92, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Szaniszlo, T.; Schaefer, K.-C.; Denes, J.; Lopata, A.; Godorhazy, L.; Szalay, D.; Balogh, L.; Sasi-Szabo, L.; Toth, M.; et al. Identification of Biological Tissues by Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 7343–7350. [Google Scholar] [CrossRef]
- Fox, S.A.; Farah, C.S. Mass Spectrometry in the Palm of Your Hand: Future Applications of in Vivo Tissue Analysis. Oral Dis. 2019, 25, 639–642. [Google Scholar] [CrossRef]
- Zhang, J.; Rector, J.; Lin, J.Q.; Young, J.H.; Sans, M.; Katta, N.; Giese, N.; Yu, W.; Nagi, C.; Suliburk, J.; et al. Nondestructive Tissue Analysis for Ex Vivo and in Vivo Cancer Diagnosis Using a Handheld Mass Spectrometry System. Sci. Transl. Med. 2017, 9, eaan3968. [Google Scholar] [CrossRef] [Green Version]
- Fatou, B.; Saudemont, P.; Leblanc, E.; Vinatier, D.; Mesdag, V.; Wisztorski, M.; Focsa, C.; Salzet, M.; Ziskind, M.; Fournier, I. In Vivo Real-Time Mass Spectrometry for Guided Surgery Application. Sci. Rep. 2016, 6, 25919. [Google Scholar] [CrossRef]

| Study | Sample | MS Technique | Outcomes | Reference |
|---|---|---|---|---|
| Stahl et al. | Resin composite | GC/MS, LC/MS | (Co)monomers, different additives, and manufacturing-related impurities were found in all polymerized composite resin specimens. From these, the co-monomer TEGDMA was isolated in concentrations more significant than those found to be harmful in primary human oral fibroblast cells | [12] |
| Manabe et al. | Resin composite | GC–MS/MS | Bisphenol-A was being released from dental materials, but the leachable amount was less than the reported dose required for xenoestrogenisity in vivo | [13] |
| Nilsen et al. | Resin-based pulp-capping materials | GC–MS, UPLC–MS | Investigated resin-based pulp-capping materials contained and eluted several reactive, organic substances that were not declared in the safety data sheets of the respective materials | [14] |
| Sampath et al. | Psidium guajava | GC–MS | A polyherbal toothpaste was prepared with guava leaf powder as a significant ingredient and possessed antimicrobial and antioxidant properties | [15] |
| Zuanazzi et al. | Titanium surfaces and salivary pellicle | nLC–ESI–MS/MS | Despite reported differences in protein composition on titanium surfaces, most proteins were found on all different surfaces, showing a low surface specificity for protein binding in three modified titanium surfaces | [16] |
| Chuang et al. | zirconia–resin primers adhesion surface | ToF-SIMS | All MDP-treatment groups showed improved shear bond strength (SBS) before thermocycling, while MDP-base primer and MPS followed by MDP retained higher SBS after this | [17] |
| Lima et al. | zirconia–resin primers adhesion surface | ToF-SIMS | Chemical treatment influenced all surface parameters | [18] |
| Lapinska et al. | Contaminated with saliva ceramic surface | ToF-SIMS | Cleaning contaminated leucite with orthophosphoric acid or re-etching lithium disilicate with hydrofluoric acid were the most efficient methods for saliva-contaminated ceramic surfaces | [19] |
| Study | Disease Condition | Sample | Proteomic Tool | Outcomes | Reference |
|---|---|---|---|---|---|
| Vitorino et al. | Caries | Whole saliva | MALDI–TOF MS | Cystatins, acidic proline-rich proteins (PRPs) and lipocalin-1 are correlated with the absence of dental caries. Amylase, IgA, and lactoferrin levels were found to correlate with the caries-susceptible group. | [39] |
| Preza et al. | Caries on elderly people | Ductal parotid gland secretion | LC–MS/MS | Aging tends to modify parotid function, which could have an impact on caries activity. | [40] |
| Yan et al. | Caries in children aged from 6–8 | Unstimulated whole saliva | ESI–MS/MS | In contrast to the caries-free group, the total salivary protein was greater in the active-caries group. The initial discovery of distinct proteins (MMP9, MUC7, LTF, CA6, AZU, and cold agglutinin) may serve as a starting point for the study of biomarkers for dental caries susceptibility. | [41] |
| Pappa et al. | Caries in adolescents with regulated and unregulated type 1 diabetes | Unstimulated whole saliva | LC–MS | A substantial correlation exists between the frequency of caries and unregulated type 1 diabetes in adolescents. The increasing incidence of caries in this group may be explained by the downregulation of the majority of differentially expressed proteins with a protective effect against caries activity. | [42] |
| Ngo et al. | Periodontitis | Gingival crevicular fluid | MALDI–TOF/TOF MS, nanoLC–ESI–MS/MS | 33 peptides and 66 proteins were identified. All the peptides discovered in this study and 43 of the identified proteins had not been reported in GCF before. | [45] |
| Ngo et al. | Periodontitis | Gingival crevicular fluid | MALDI–TOF MS | The mass spectra of gingival crevicular fluid could be used to predict attachment loss sites. | [48] |
| Antezack et al. | Periodontitis | Unstimulated saliva, gingival crevicular fluid dental plaque | MALDI–TOF MS | Development of diagnostic tests based on protein profiles of the samples for periodontitis and healthy subjects. | [44] |
| Kaur et al. | Oral cancer | Whole saliva | MS/MS | Salivary biomarkers could be utilized as a screening technique to increase the accuracy of early detection and diagnosis of oral cancer and pre-cancer conditions. | [52] |
| Chi et al. | Oral Cancer | Saliva, plasma | LC–MRM–MS | Saliva, unlike blood samples, had more potential for the successful identification of protein biomarkers for the early detection of oral cancer. | [53] |
| Yang et al. | Oral lichen planus | Whole saliva | MALDI–TOF MS | Palate, lung, and nasal epithelium carcinoma associated protein (PLNEC), and urinary prokallikrein may be two novel biomarkers that are involved in OLP inflammation and immune response. | [54] |
| Cruz et al. | Oral lichen planus | Formalin-fixed and paraffin-embedded tissue | LC–MS/MS | There are differences between the metabolic profiles of the reticular and erosive forms of oral lichen planus. | [55] |
| Buszewski et al. | Oral microbiome | - | MALDI–TOF MS | The development of quick, inexpensive, accurate, and reproducible procedures for the screening and identification of microorganisms. | [56] |
| Chen et al. | Oral microbiome | Supragingival plaque and unstimulated saliva | LC–MS/MS | Some bacteria could be used as potential biomarkers for children with caries. | [59] |
| Pei et al. | Oral microbiome | Gingival crevicular fluid | LC–MS/MS | Differential microorganisms and metabolites between periodontal and healthy subjects may be used as potential biomarkers. | [60] |
| Hu et al. | Sjogren’s Syndrome | Whole saliva | MALDI–TOF MS | Whole saliva from patients with primary SS has molecular signals that reflect damaged glandular cells and a stimulated immune system response. | [64] |
| Li et al. | Sjogren’s Syndrome | Unstimulated whole saliva | UPLC–HRMS | Saliva metabolic profile of pSS is differentiated between the pSS group and the controls. Panels of metabolites may be useful for the diagnosis of pSS. | [65] |
| Rao et al. | Diabetes type II | Whole saliva | 2D-LC–MS/MS | Identification of Salivary Biomarkers of Type-2 Diabetes | [67] |
| Hirtz et al. | Diabetes type I | Whole saliva | MALDI–TOF MS | Saliva samples from healthy subjects and poorly managed type 1 diabetes patients showed modulation of 23 proteins. | [68] |
| Pappa et al. | Diabetes type I in children | Whole saliva | LC–MS | Different patterns of protein expression were found in the subjects who had poor glycemic regulation. These proteins participate in biological processes related to the pathophysiology of diabetes. | [74] |
| Streckfus et al. | Breast cancer | Stimulated whole saliva | SELDI–MS | SELDI mass spectrometry may be a valuable tool in the development of salivary biomarkers. | [75] |
| Deutsch et al. | Pancreatic cancer | Unstimulated oral fluid | ion-trap MS | A combination of five biomarkers for PC was found. The majority of these proteins have never been found in oral fluids before, despite being known to be relevant to PC or other gastric malignancies. | [76] |
| Wu et al. | gastric cancer | Whole saliva | MALDI–TOF MS | The differential expression of specific proteins can be used to create a diagnostic model for separating saliva samples from gastric cancer patients and healthy subjects. | [78] |
| Bassim et al. | Chronic graft-versus-host disease | Unstimulated whole saliva | LC–MS/MS | Mass spectrometry could be used for noninvasive tests for screening, early detection, and monitoring of cGVHD in large patient population. | [79] |
| Study | Sample | MS Technique | Outcomes | Reference |
|---|---|---|---|---|
| Jagr et al. | Dentin | LC–MS/MS | 289 proteins were identified with high confidence, 90 of which had not been previously detected in human dentin. | [84] |
| Salmon | Dental Cementum, alveolar bone | LC–MS/MS | The first analysis of the proteomic composition of human DC matrix and comparison to that of the alveolar bone. The discovery of potential biomarkers may result in periodontal regeneration treatments that are more effective and reliable. | [86] |
| Siquiera et al. | Acquired enamel pellicle | LC–ESI–MS/MS | Identification of proteins in in vivo acquired enamel pellicle. | [87] |
| Zimmerman et al. | Acquired enamel pellicle on deciduous teeth | LC–ESI–MS/MS | AEP proteome presents a unique composition. | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallianta, M.; Pappa, E.; Vastardis, H.; Rahiotis, C. Applications of Mass Spectrometry in Dentistry. Biomedicines 2023, 11, 286. https://doi.org/10.3390/biomedicines11020286
Kallianta M, Pappa E, Vastardis H, Rahiotis C. Applications of Mass Spectrometry in Dentistry. Biomedicines. 2023; 11(2):286. https://doi.org/10.3390/biomedicines11020286
Chicago/Turabian StyleKallianta, Meletia, Eftychia Pappa, Heleni Vastardis, and Christos Rahiotis. 2023. "Applications of Mass Spectrometry in Dentistry" Biomedicines 11, no. 2: 286. https://doi.org/10.3390/biomedicines11020286
APA StyleKallianta, M., Pappa, E., Vastardis, H., & Rahiotis, C. (2023). Applications of Mass Spectrometry in Dentistry. Biomedicines, 11(2), 286. https://doi.org/10.3390/biomedicines11020286







