Type 2 Diabetes Mellitus Coincident with Clinical and Subclinical Thyroid Dysfunctions Results in Dysregulation of Circulating Chemerin, Resistin and Visfatin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Subjects and Study Design
2.3. Blood Sample Collection
2.4. Biochemical Analysis
2.5. Adipocytokines and Proinflammatory Cytokine Measurements
2.6. Assessment of Insulin Resistance
2.7. Statistical Analysis
3. Results
3.1. Demographic Information of Studied Population
3.2. Insulin Resistance-Markers of All Participants
3.3. Thyroid-Hormone Profile in All Participants
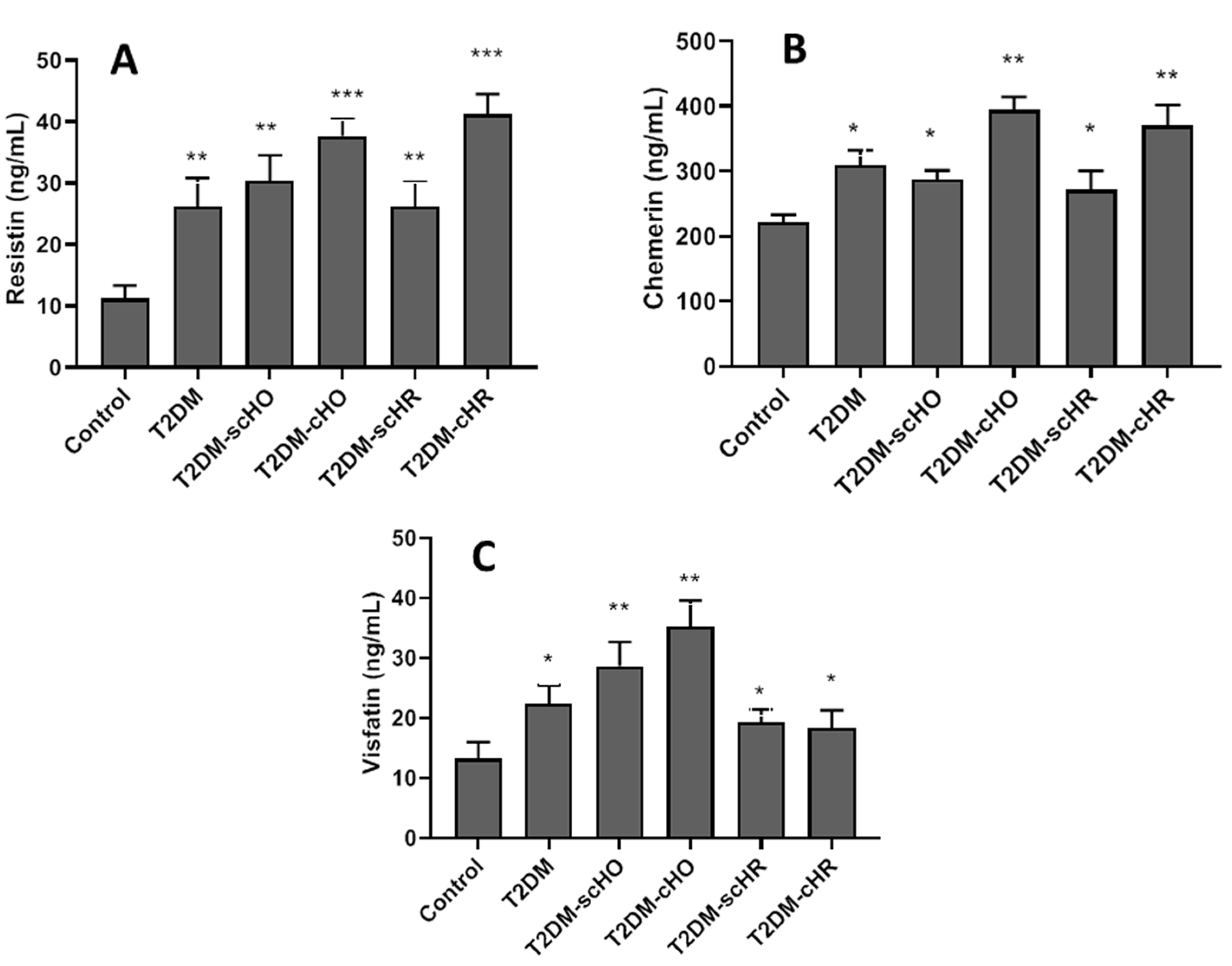
3.4. Serum Adipocytokines in Different Studied Populations
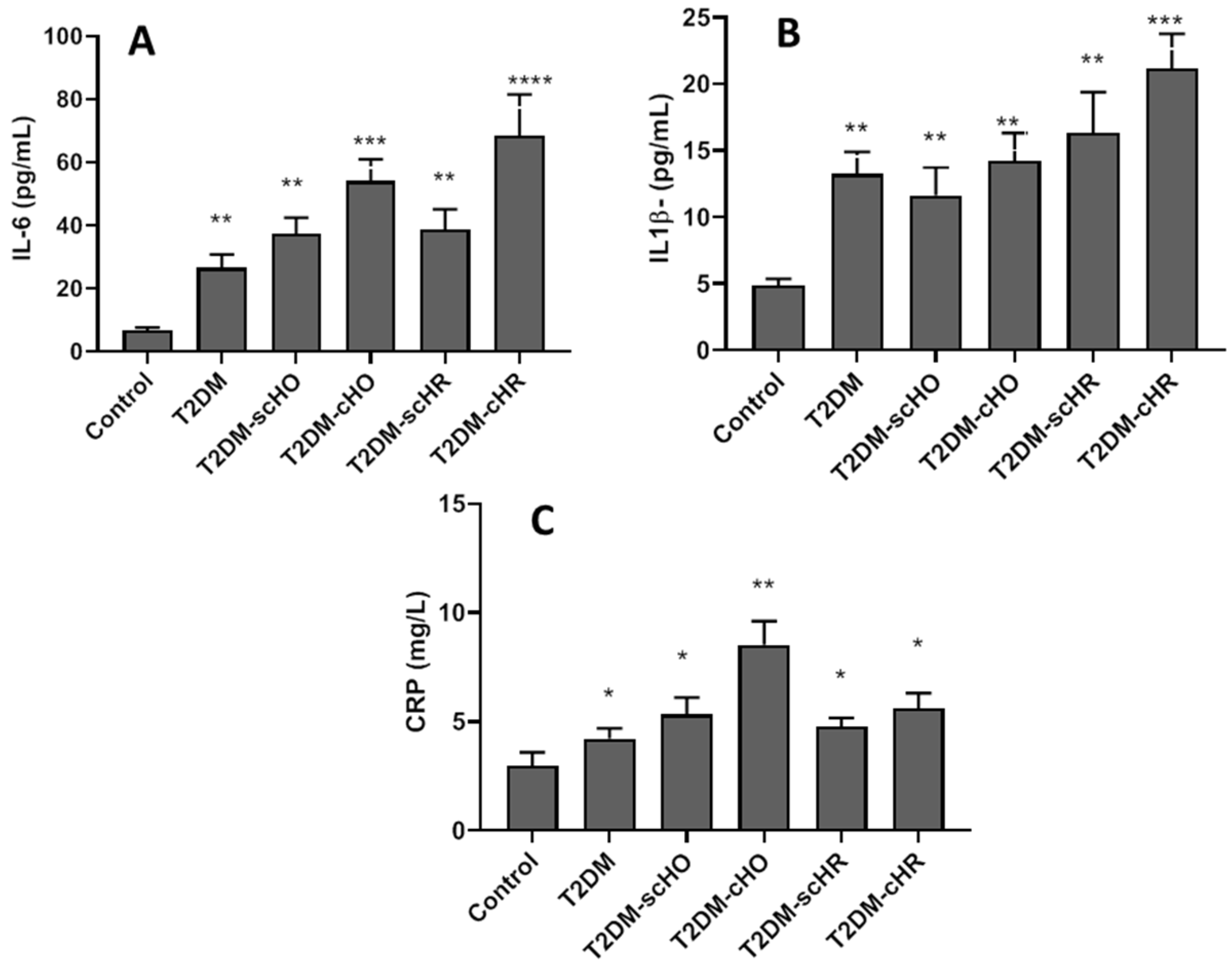
3.5. Proinflammatory Cytokines in All Participants
3.6. Association between Serum Adipocytokines and Insulin-Resistant Markers, Thyroid-Hormone Profile and Inflammatory Factors in T2DM Patients with Hypothyroidism
3.7. Association between Adipocytokines and Metabolic and Inflammatory Factors in Hyperthyroid Patients with T2DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hage, M.; Zantout, M.S.; Azar, S.T. Thyroid disorders and diabetes mellitus. J. Thyroid Res. 2011, 2011, 439463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid dysfunction and diabetes mellitus: Two closely associated disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteghamati, A.; Gouya, M.M.; Abbasi, M.; Delavari, A.; Alikhani, S.; Alaedini, F.; Safaie, A.; Forouzanfar, M.; Gregg, E.W. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care 2008, 31, 96–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansournia, N.; Riyahi, S.; Tofangchiha, S.; Riahi, M.; Heidari, Z.; Hazrati, E.; Mansournia, M.A. Subclinical hypothyroidism and diabetic nephropathy in Iranian patients with type 2 diabetes. J. Endocrinol. Investig. 2017, 40, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kumar, J.A.; Prajna, K.; Shetty, S.K.; Rai, T.; Shrinidhi; Begum, M.; Shashikala. Thyroid function in type 2 diabetes mellitus and in diabetic nephropathy. J. Clin. Diagn. Res. 2013, 7, 1583–1585. [Google Scholar]
- Kapadia, K.B.; Bhatt, P.A.; Shah, J.S. Association between altered thyroid state and insulin resistance. J. Pharmacol. Pharmacother. 2012, 3, 156–160. [Google Scholar] [CrossRef]
- Aydogan, B.I.; Sahin, M. Adipocytokines in thyroid dysfunction. Int. Scholar. Res. Not. 2013, 2013, 646271. [Google Scholar] [CrossRef]
- Santini, F.; Marzullo, P.; Rotondi, M.; Ceccarini, G.; Pagano, L.; Ippolito, S.; Chiovato, L.; Biondi, B. The crosstalk between thyroid gland and adipose tissue: Signal integration in health and disease. Eur. J. Endocrinol. 2014, 171, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Seifi, S.; Tabandeh, M.R.; Nazifi, S.; Saeb, M.; Shirian, S.; Sarkoohi, P. Regulation of adiponectin gene expression in adipose tissue by thyroid hormones. J. Physiol. Biochem. 2012, 68, 193–203. [Google Scholar] [CrossRef]
- Alshaikh, E.M.; Omar, U.M.; Alsufiani, H.M.; Mansouri, R.A.; Tarbiah, N.I.; Alshaikh, A.A.; Rahimulddin, S.A.; Al Doghaither, H.A. The potential influence of hyperthyroidism on circulating adipokines chemerin, visfatin, and omentin. Int. J. Health Sci. 2019, 13, 44. [Google Scholar]
- El-Mesallamy, H.O.; El-Derany, M.O.; Hamdy, N.M. A study on serum level of chemerin in experimentaly induced thyroid dysfunctions. Bas. Sci. Med. 2018, 7, 21–26. [Google Scholar]
- Cinar, N.; Gurlek, A. Association between novel adipocytokines adiponectin, vaspin, visfatin, and thyroid: An experimental and clinical update. Endocr. Connect. 2013, 2, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-J.; Zhao, W.; Zhu, M.-Y.; Tang, S.-S.; Zhang, H. Thyroid-Stimulating Hormone Induces the Secretion of Tumor Necrosis Factor-α from 3T3-L1 Adipocytes via a Protein Kinase A-Dependent Pathway. Exp. Clin. Endocrinol. Diabetes 2013, 121, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Tabandeh, M.R.; Hosseini, S.A. Novel cardioprotective effect of L-carnitine on obese diabetic mice: Regulation of chemerin and CMKLRI expression in heart and adipose tissues. Arquivos. Brasileiros. Cardiol. 2021, 25, 715–725. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Kumari, B.; Yadav, U.C. Adipokine Visfatin’s role in pathogenesis of Diabesity and related metabolic derangements. Curr. Mol. Med. 2018, 18, 16–125. [Google Scholar] [CrossRef]
- Chu, C.-H.; Lee, J.-K.; Wang, M.-C.; Lu, C.-C.; Sun, C.-C.; Chuang, M.-J.; Lam, H.-C. Change of visfatin, C-reactive protein concentrations, and insulin sensitivity in patients with hyperthyroidism. Metabolism 2008, 57, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Caixàs, A.; Tirado, R.; Vendrell, J.; Gallart, L.; Megía, A.; Simón, I.; Llauradó, G.; González-Clemente, J.M.; Giménez-Palop, O. Plasma visfatin concentrations increase in both hyper and hypothyroid subjects after normalization of thyroid function and are not related to insulin resistance, anthropometric or inflammatory parameters. Clin. Endocrin. 2009, 71, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, M.; Sahin, M.; Cakal, E.; Yuzbasioglu, F.; Sezer, K.; Kilinc, M.; Imrek, S.S. Visfatin plasma concentrations in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function. J. Endocrinol. Investig. 2009, 32, 435–439. [Google Scholar] [CrossRef]
- Su, K.-Z.; Li, Y.-R.; Zhang, D.; Yuan, J.-H.; Zhang, C.-S.; Liu, Y.; Song, L.-M.; Lin, Q.; Li, M.-W.; Dong, J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 1399. [Google Scholar] [CrossRef] [Green Version]
- Eke Koyuncu, C.; Turkmen Yildirmak, S.; Temizel, M.; Ozpacaci, T.; Gunel, P.; Cakmak, M.; Ozbanazi, Y.G. Serum resistin and insu-lin-like growth factor-1 levels in patients with hypothyroidism and hyperthyroidism. J. Thyroid. Res. 2013, 2013, 306750. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Díez, J.J. Influence of thyroid dysfunction on serum concentrations of adipocytokines. Cytokine 2007, 40, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yaturu, S.; Prado, S.; Grimes, S.R. Changes in adipocyte hormones leptin, resistin, and adiponectin in thyroid dysfunction. J. Cell. Biochem. 2004, 93, 491–496. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 2008, 29, 76–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkovic, M.; Žarković, M. Subclinical thyroid dysfunction and the risk of cardiovascular disease. Curr. Pharmaceut. Des. 2020, 26, 5617–5627. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S43–S48. [Google Scholar]
- Hu, Y.; Zheng, J.; Ye, X.; Song, Y.; Wu, X. Association Between Elevated Thyroid Peroxidase Antibody and Abdominal Fat Distribution in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 863–871. [Google Scholar] [CrossRef]
- Vladu, I.M.; Forțofoiu, M.; Clenciu, D.; Forțofoiu, M.C.; Pădureanu, R.; Radu, L.; Cojan, Ș.T.; Rădulescu, P.M.; Pădureanu, V. Insulin resistance quantified by the value of HOMA IR and cardiovascular risk in patients with type 2 diabetes. Exp. Therap. Med. 2022, 23, 73. [Google Scholar] [CrossRef]
- Ogbonna, S.; Ezeani, I.; Okafor, C.; Chinenye, S. Association between glycemic status and thyroid dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Maratou, E.; Hadjidakis, D.J.; Peppa, M.; Alevizaki, M.; Tsegka, K.; Lambadiari, V.; Mitrou, P.; Boutati, E.; Kollias, A.; Economopoulos, T.; et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur. J. Endocrinol. 2010, 163, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.M.; Goswami, B.; Mallika, V. Association between insulin resistance and hypothyroidism in females attending a tertiary care hospital. Indian J. Clin. Biochem. 2010, 25, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Handisurya, A.; Pacini, G.; Tura, A.; Gessl, A.; Kautzky-Willer, A. Effects of T4 replacement therapy on glucose metabolism in subjects with subclinical (SH) and overt hypothyroidism (OH). Clin. Endocrin. 2008, 69, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Dessein, P.H.; Joffe, B.I.; Stanwix, A.E. Subclinical hypothyroidism is associated with insulin resistance in rheumatoid arthritis. Thyroid 2004, 14, 443–446. [Google Scholar] [CrossRef] [PubMed]
- AL Sayed, A.; AL Ali, N.; Abbas, Y.B.; Alfadhli, E. Subclinical Hypothyroidism Is Associated with Early Insulin Resistance in Kuwaiti Women. Endocr. J. 2006, 53, 653–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helfer, G.; Wu, Q.-F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Bermejo, A.; Chico-Julia, B.; Fernandez-Balsells, M.; Recasens, M.; Esteve, E.; Casamitjana, R.; Ricart, W.; Fernandez-Real, J.M. Serum visfatin increases with progressive beta-cell deterioration. Diabetes 2006, 55, 2871–2875. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Onyango, D.J.; Ramanjaneya, M.; Conner, A.C.; Patel, S.T.; Dunmore, S.J.; Randeva, H.S. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic β-cells. J. Mol. Endocrinol. 2010, 44, 171–178. [Google Scholar] [CrossRef]
- Sait, B.; Yesil, Y.; Akin, S.; Aksoy, D.Y.; Aydin, K.; Cinar, N.; Karakaya, J.; Grlek, A. Omentin, chemerin levels in lean patients with subclinical hypothyroidism and Hashimoto thyroiditis. Endocrin. Rev. 2013, 15, 1–6. [Google Scholar]
- Nogueiras, R.; Gualillo, O.; Caminos, J.E.; Casanueva, F.F.; Diéguez, C. Regulation of Resistin by Gonadal, Thyroid Hormone, and Nutritional Status. Obes. Res. 2003, 11, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.; Gagnon, A.; Dods, P.; Papineau, D.; Tiberi, M.; Sorisky, A. TSH signaling and cell survival in 3T3-L1 preadipocytes. Am. J. Physiol. 2002, 283, C1056–C1064. [Google Scholar] [CrossRef]
- Haider, D.G.; Schaller, G.; Kapiotis, S.; Maier, C.; Luger, A.; Wolzt, M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia 2006, 49, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Chen, J.; Farhatullah, S.; Adya, R.; Kaur, J.; Heutling, D.; Lewandowski, K.C.; O’Hare, J.P.; Lehnert, H.; Randeva, H.S. Insulin and Metformin Regulate Circulating and Adipose Tissue Chemerin. Diabetes 2009, 58, 1971–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, J.K.; Kim, H.I.; Lee, S.K.; Kim, S.J.; Park, S.H.; Kim, H.S. Visfatin, a novel adipokine, stimulates glucose uptake through the Ca2+-dependent AMPK-p38 MAPK pathway in C2C12 skeletal muscle cells. J. Mol. Endocrin. 2015, 54, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef] [Green Version]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an adipocytokine with proinflammatory and im-munomodulating properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, H.C.; Kwon, Y.W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.Y.; Lee, J.; Yang, H.M.; et al. Adenylyl Cyclase-Associated Protein 1(CAP1) is a Receptor for Human Resistin and Mediates Inflammatory Actions of Human Monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef] [Green Version]
- Song, R.-H.; Wang, B.; Yao, Q.-M.; Li, Q.; Jia, X.; Zhang, J.-A. The impact of obesity on thyroid autoimmunity and dysfunction: A systematic review and meta-analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Huang, F.; Zhang, S.; Lv, Y.; Liu, Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: A cohort study. BMC Endocr. Disord. 2019, 19, 58. [Google Scholar] [CrossRef] [Green Version]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; Feitosa, M.M.; Severo, J.S.; Freitas, T.E.C.; Morais, J.B.S.; Torres-Leal, F.L.; Henriques, G.S.; Marreiro, D.D.N. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm. Metab. Res. 2016, 48, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan, O.; De La Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Characterization, Regulation, and Medical Significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsigalou, C.; Vallianou, N.; Dalamaga, M. Autoantibody production in obesity: Is there evidence for a link between obesity and autoim-munity? Curr. Obes. Rep. 2020, 9, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Q.; Cai, D.; Guo, H.; Fang, J.; Cui, H.; Gou, L.; Deng, J.; Wang, Z.; Zuo, Z. Resistin, a Novel Host Defense Peptide of Innate Immunity. Front. Immunol. 2021, 12, 699807. [Google Scholar] [CrossRef] [PubMed]
| Variable (Mean ± SD) | Healthy | T2DM Euthyroidism | T2DM SC-Hypo | T2DM C-Hypo | T2DM SC-Hyper | T2DM C-Hyper |
|---|---|---|---|---|---|---|
| N | 55 | 64 | 41 | 43 | 36 | 33 |
| Gender (F/M) | 27/28 | 36/31 | 24/17 | 27/16 | 17/19 | 14/19 |
| Age (years) | 51.6 ± 10.4 | 57.6 ± 9.8 | 53.5 ± 11.2 | 53.5 ± 11.2 | 48.5 ± 8.6 | 50.3 ± 6.7 |
| BMI (kg/m2) | 21.2 ± 1.6 | 28.6 ± 2.2 * | 28.9 ± 1.7 * | 30.4 ± 2.8 * | 25.3 ± 3.1 | 23.7 ± 2.2 |
| FBG (mg/dL) | 87.2 ± 11.6 | 138.8 ± 23.2 * | 141.70 ± 16.5 * | 150.4 ± 19.3 * | 150.3 ± 16.1 * | 168.5 ± 13.8 ** |
| 2h PPG (mg/dL) | 95.4 ± 6.3 | 211.7 ± 20.3 ** | 218.9 ± 13.8 ** | 224.6 ± 19.6 ** | 253.0 ± 20.2 *** | 267.8 ± 11.8 *** |
| Insulin (uU/mL) | 5.3 ± 1.4 | 8.2 ± 3.1 * | 11.4 ± 2.2 ** | 12.2 ± 3.6 ** | 17.6 ± 4.2 *** | 20.2 ± 6.2 *** |
| HOMA-IR (mM*mU/L) | 1.1 ± 0.3 | 6.4 ± 0.6 ** | 5.8 ± 0.8 ** | 6.1 ± 0.6 ** | 5.6 ± 0.5 ** | 7 ± 0.7 *** |
| HbA1c (%) | 4.7 ± 1.1 | 6.7 ± 1.8 * | 6.5 ± 1.3 * | 7.8 ± 0.7 ** | 6.3 ± 0.9 * | 7.7 ± 1.2 ** |
| Variable (Mean ± SD) | Healthy | T2DM Euthyroidism | T2DM SC-HO | T2DM C-HO | T2DM SC-HR | T2DM C-HR |
|---|---|---|---|---|---|---|
| N | 55 | 64 | 41 | 43 | 36 | 33 |
| TSH (μIU/mL) | 2.24 ± 0.49 | 1.87 ± 0.36 | 11.31 ± 2.46 ** | 63.27 ± 8.19 **** | 0.16 ± 0.047 *** | 0.04 ± 0.01 **** |
| FT3 (pmol/L) | 4.21 ± 0.61 | 3.81 ± 0.52 | 3.81 ± 0.46 | 2.43 ± 0.72 * | 4.82 ± 0.71 ** | 11.49 ± 2.48 *** |
| FT4 (pmol/L) | 10.42 ± 1.8 | 9.84 ± 2.06 | 8.78 ± 1.21 | 4.22 ± 0.71 * | 12.32 ± 0.97 ** | 30.72 ± 4.67 *** |
| TPO-Ab positive; n (%) | 4 (7.27) | 8 (12.5) | 16 (39.02) *** | 23 (53.48) **** | 5 (13.88) | 7 (21.21) ** |
| T2DM with Clinical and Subclinical Hypothyroidism | ||||||
|---|---|---|---|---|---|---|
| Variables | Visfatin | Resistin | Chemerin | |||
| β(r) | p | β(r) | p < 0.0001 | β(r) | p | |
| TSH | 0.861 | p < 0.0001 | 0.829 | p < 0.0001 | 0.988 | p < 0.0001 |
| fT3 | −0.751 | p < 0.0001 | −0.767 | p < 0.0001 | −0.902 | p < 0.0001 |
| fT4 | −0.868 | p < 0.0001 | −0.838 | p < 0.0001 | −0.983 | p < 0.0001 |
| Anti-TPO | 0.362 | 0.039 | 0.466 | 0.002 | 0.226 | 0.078 |
| CRP | 0.810 | p < 0.0001 | 0.722 | p < 0.0001 | 0.820 | p < 0.0001 |
| IL-6 | 0.811 | 0.075 | 0.827 | p < 0.0001 | 0.954 | p < 0.0001 |
| IL1-β | 0.725 | 0.018 | 0.705 | 0.001 | 0.737 | p < 0.0001 |
| FBG | 0.726 | p < 0.0001 | 0.650 | p < 0.0001 | 0.795 | p < 0.0001 |
| HOMA-IR | 0.141 | 0.267 | 0.112 | 0.374 | 0.171 | 0.278 |
| Insulin | 0.275 | 0.098 | 0.124 | 0.302 | 0.174 | 0.269 |
| Visfatin | 1 | p < 0.0001 | 0.262 | 0.157 | 0.262 | 0.157 |
| Resistin | 0.262 | 0.157 | 1 | p < 0.0001 | 0.898 | p < 0.0001 |
| Chemerin | 0.378 | 0.096 | 0.898 | p < 0.0001 | 1 | p < 0.0001 |
| BMI | 0.378 | 0.019 | 0.334 | 0.031 | 0.447 | 0.003 |
| T2DM with Clinical and Subclinical Hyperthyroidism | ||||||
|---|---|---|---|---|---|---|
| Variables | Visfatin | Resistin | Chemerin | |||
| β(r) | p | β(r) | p < 0.0001 | β(r) | p | |
| TSH | −0.321 | 0.068 | −0.910 | p < 0.0001 | −0.975 | p < 0.0001 |
| fT3 | 0.317 | 0.072 | 0.906 | p < 0.0001 | 0.982 | p < 0.0001 |
| fT4 | 0.304 | 0.086 | 0.902 | p < 0.0001 | 0.864 | p < 0.0001 |
| Anti-TPO | 0.202 | 0.078 | 0.266 | 0.137 | 0.165 | 0.351 |
| CRP | 0.164 | 0.361 | 0.720 | p < 0.0001 | 0.722 | p < 0.0001 |
| IL-6 | 0.315 | 0.075 | 0.880 | p < 0.0001 | 0.958 | p < 0.0001 |
| IL1-β | 0.409 | 0.018 | 0.569 | 0.001 | 0.668 | p < 0.0001 |
| FBG | 0.451 | 0.008 | 0.686 | p < 0.0001 | 0.787 | p < 0.0001 |
| HOMA-IR | 0.365 | 0.037 | 0.793 | p < 0.0001 | 0.759 | p < 0.0001 |
| Insulin | 0.318 | 0.072 | 0.783 | p < 0.0001 | 0.849 | p < 0.0001 |
| Visfatin | 1 | p < 0.0001 | 0.267 | 0.122 | 0.319 | 0.069 |
| Resistin | 0.267 | 0.122 | 1 | p < 0.0001 | 0.898 | p < 0.0001 |
| Chemerin | 0.319 | 0.069 | 0.898 | p < 0.0001 | 1 | p < 0.0001 |
| BMI | −0.137 | 0.213 | −0.221 | 0.089 | −0.197 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabandeh, M.R.; Taha, A.S.; Addai Ali, H.; Razijalali, M.; Mohammadtaghvaei, N. Type 2 Diabetes Mellitus Coincident with Clinical and Subclinical Thyroid Dysfunctions Results in Dysregulation of Circulating Chemerin, Resistin and Visfatin. Biomedicines 2023, 11, 346. https://doi.org/10.3390/biomedicines11020346
Tabandeh MR, Taha AS, Addai Ali H, Razijalali M, Mohammadtaghvaei N. Type 2 Diabetes Mellitus Coincident with Clinical and Subclinical Thyroid Dysfunctions Results in Dysregulation of Circulating Chemerin, Resistin and Visfatin. Biomedicines. 2023; 11(2):346. https://doi.org/10.3390/biomedicines11020346
Chicago/Turabian StyleTabandeh, Mohammad Reza, Amal Sattar Taha, Hanaa Addai Ali, Mohammad Razijalali, and Narges Mohammadtaghvaei. 2023. "Type 2 Diabetes Mellitus Coincident with Clinical and Subclinical Thyroid Dysfunctions Results in Dysregulation of Circulating Chemerin, Resistin and Visfatin" Biomedicines 11, no. 2: 346. https://doi.org/10.3390/biomedicines11020346





