Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines
Abstract
:1. Introduction
2. Search Methods
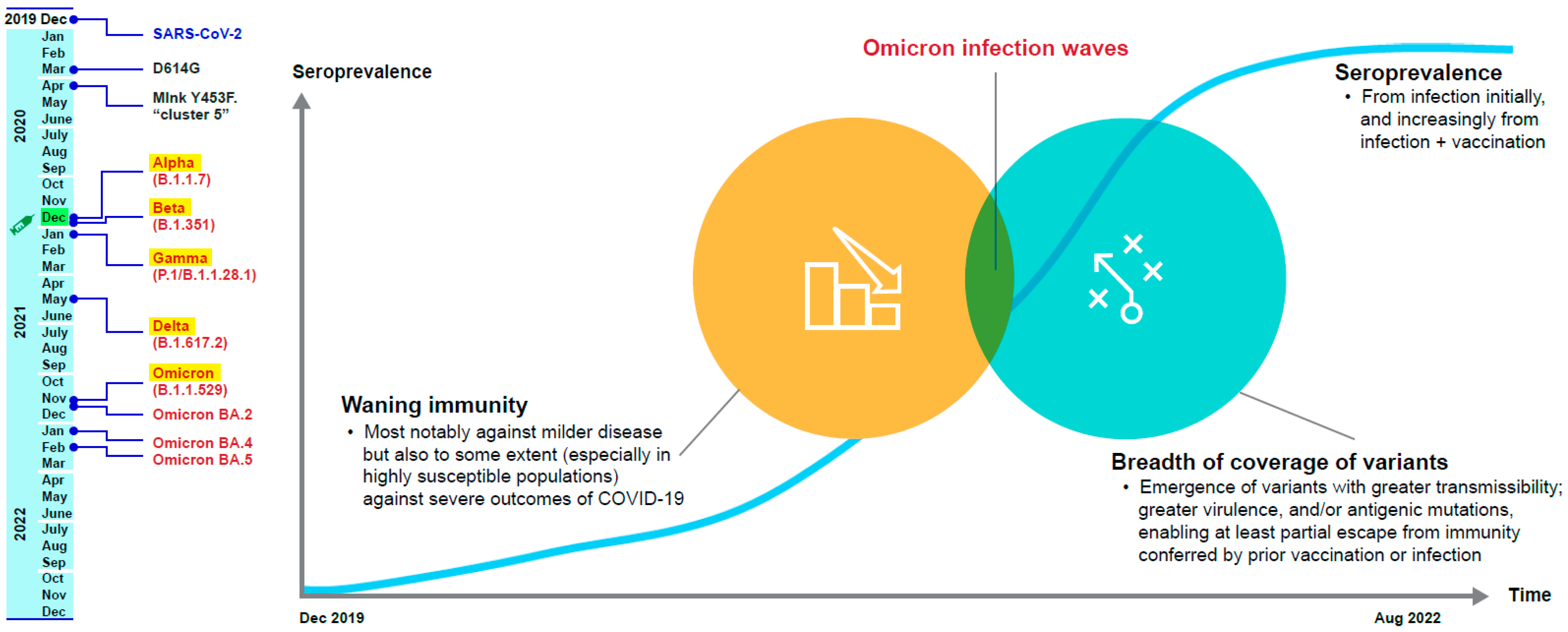
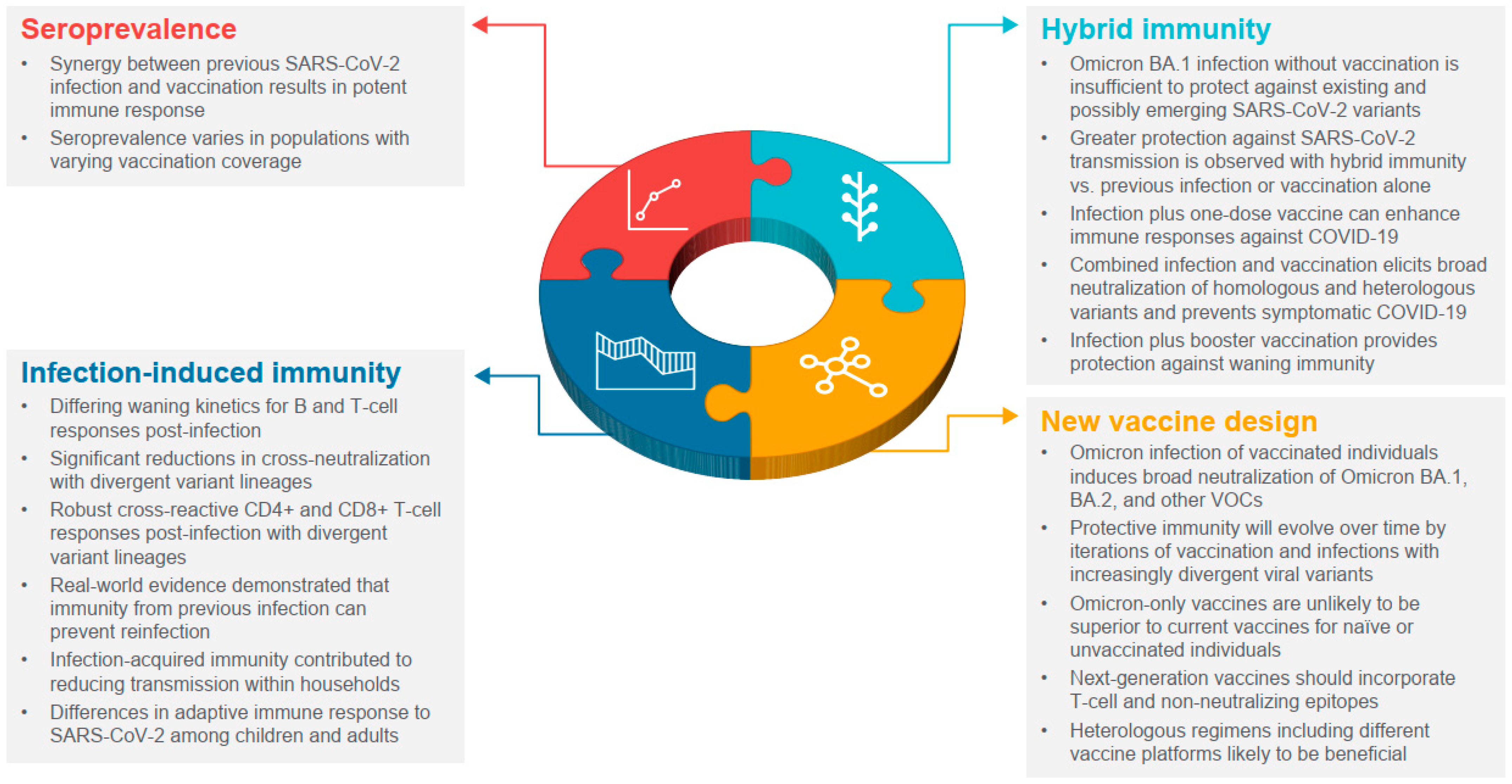
2.1. Seroprevalence after the First Two Years of the COVID-19 Pandemic
2.2. Infection-Induced Immunity
2.2.1. Immune Persistence and Duration of Protection
2.2.2. Breadth of Coverage against SARS-CoV-2 Variants
2.2.3. Protection from Reinfection
2.3. Hybrid Immunity
2.3.1. Immune Persistence and Quality
2.3.2. Breadth of Coverage of Variants
2.3.3. Effect on Reinfection
2.3.4. Effect on Transmission
2.3.5. Omicron and Hybrid Immunity
2.4. New COVID-19 Vaccines
2.4.1. Design Considerations for New COVID-19 Vaccines
2.4.2. Regulatory Considerations for New COVID-19 Vaccines
2.4.3. Implementation Considerations for New COVID-19 Vaccines
2.4.4. Evolution of Hybrid Immunity with New COVID-19 Vaccines
3. Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 19 January 2023).
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Goldblatt, D.; Alter, G.; Crotty, S.; Plotkin, S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol. Rev. 2022, 310, 6–26. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Levy, M.E.; Gaglani, M.; Irving, S.A.; Stockwell, M.; Dascomb, K.; DeSilva, M.B.; Reese, S.E.; Liao, I.-C.; Ong, T.C. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA. 1 and BA. 2/BA. 2.12. 1 sublineages predominated—VISION network, 10 states, December 2021–June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 931. [Google Scholar]
- Rubin, R. COVID-19 vaccines vs. variants-determining how much immunity is enough. JAMA 2021, 325, 1241–1243. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and their emerging variants with escape mutations. Front. Immunol. 2022, 13, 801522. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Nordstrom, P.; Ballin, M.; Nordstrom, A. Association between risk of COVID-19 infection in nonimmune individuals and COVID-19 immunity in their family members. JAMA Intern. Med. 2021, 181, 1589–1595. [Google Scholar]
- Tenforde, M.W.; Patel, M.M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; Gaglani, M.; McNeal, T.; et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin. Infect. Dis. 2022, 74, 1515–1524. [Google Scholar] [CrossRef]
- World Health Organization. Origin of SARS-CoV-2: March 26, 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332197/WHO-2019-nCoV-FAQ-Virus_origin-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 22 July 2022).
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 19 July 2022).
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827.e819. [Google Scholar]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar]
- O’Toole, A.; Hill, V. P.1. 2022-12-22. Available online: https://cov-lineages.org/global_report_P.1.html (accessed on 4 August 2022).
- European Center for Disease Prevention and Control. SARS-CoV-2 Variants of Concern as of 15 July 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 22 July 2022).
- Larsen, C.S.; Paludan, S.R. Corona’s new coat: SARS-CoV-2 in Danish minks and implications for travel medicine. Travel Med. Infect. Dis. 2020, 38, 101922. [Google Scholar] [CrossRef]
- Jones, J.M.; Opsomer, J.D.; Stone, M.; Benoit, T.; Ferg, R.A.; Stramer, S.L.; Busch, M.P. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020-December 2021. JAMA 2022, 328, 298–301. [Google Scholar]
- US Food and Drug Administration. FDA Takes Key Action in Fight against COVID-19 by Issuing Emergency Use Authorization for First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed on 28 November 2022).
- Hatcher, S.M.; Endres-Dighe, S.M.; Angulo, F.J.; Srivastava, A.; Nguyen, J.L.; Khan, F.; Martin, C.; Swerdlow, D.L.; McLaughlin, J.M.; Ubaka-Blackmore, N.; et al. COVID-19 vaccine effectiveness: A review of the first 6 months of COVID-19 vaccine availability (1 January-30 June 2021). Vaccines 2022, 10, 393. [Google Scholar] [CrossRef]
- Higdon, M.M.; Wahl, B.; Jones, C.B.; Rosen, J.G.; Truelove, S.A.; Baidya, A.; Nande, A.A.; ShamaeiZadeh, P.A.; Walter, K.K.; Feikin, D.R.; et al. A systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease. Open Forum Infect. Dis. 2022, 9, ofac138. [Google Scholar]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espie, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the Omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef]
- Bergeri, I.; Whelan, M.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global epidemiology of SARS-CoV-2 infection: A systematic review and meta-analysis of standardized population-based seroprevalence studies, Jan 2020–Dec 2021. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population immunity and COVID-19 severity with Omicron variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef]
- Bingham, J.; Cable, R.; Coleman, C.; Glatt, T.N.; Grebe, E.; Mhlanga, L.; Nyano, C.; Pieterson, N.; Swanevelder, R.; Swarts, A.; et al. Estimates of prevalence of anti-SARS-CoV-2 antibodies among blood donors in South Africa in March 2022. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Aguilera, X.; Gonzalez, C.; Apablaza, M.; Rubilar, P.; Icaza, G.; Ramirez-Santana, M.; Perez, C.; Cortes, L.J.; Núñez-Franz, L.; Quezada-Gaete, R.; et al. Immunization and SARS-CoV-2 antibody seroprevalence in a country with high vaccination coverage: Lessons from Chile. Vaccines 2022, 10, 1002. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schaffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2021, 10, 64. [Google Scholar]
- Jung, J.H.; Rha, M.S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Nagelkerke, N.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J. Travel Med. 2022, 29, taac109. [Google Scholar] [CrossRef]
- Nordstrom, P.; Ballin, M.; Nordstrom, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar]
- Dupont, L.; Snell, L.B.; Graham, C.; Seow, J.; Merrick, B.; Lechmere, T.; Maguire, T.J.A.; Hallett, S.R.; Pickering, S.; Charalampous, T.; et al. Neutralizing antibody activity in convalescent sera from infection in humans with SARS-CoV-2 and variants of concern. Nat. Microbiol. 2021, 6, 1433–1442. [Google Scholar] [CrossRef]
- Laurie, M.T.; Liu, J.; Sunshine, S.; Peng, J.; Black, D.; Mitchell, A.M.; Mann, S.A.; Pilarowski, G.; Zorn, K.C.; Rubio, L.; et al. SARS-CoV-2 variant exposures elicit antibody responses with differential cross-neutralization of established and emerging strains including delta and 0micron. J. Infect. Dis. 2022, 225, 1909–1914. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; de Oliveira, T.; Vermeulen, M.; van der Berg, K.; et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar]
- Rossler, A.; Knabl, L.; von Laer, D.; Kimpel, J. Neutralization profile after recovery from SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022, 386, 1764–1766. [Google Scholar] [CrossRef]
- Zou, J.; Kurhade, C.; Xia, H.; Liu, M.; Xie, X.; Ren, P.; Shi, P.Y. Cross-neutralization of Omicron BA.1 against BA.2 and BA.3 SARS-CoV-2. Nat. Commun. 2022, 13, 2956. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar]
- Maier, H.E.; Balmaseda, A.; Saborio, S.; Ojeda, S.; Barilla, C.; Sanchez, N.; Lopez, R.; Plazaola, M.; Cerpas, C.; van Bakel, H.; et al. Protection associated with previous SARS-CoV-2 infection in Nicaragua. N. Engl. J. Med. 2022, 387, 568–570. [Google Scholar]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protective effect of previous SARS-CoV-2 infection against Omicron BA.4 and BA.5 subvariants. N. Engl. J. Med. 2022, 387, 1620–1622. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar]
- Moss, P.; Ladhani, S. Cross-reactive adaptive immunity against coronaviruses in young children. Nat. Immunol. 2022, 23, 11–12. [Google Scholar]
- Tang, J.; Novak, T.; Hecker, J.; Grubbs, G.; Zahra, F.T.; Bellusci, L.; Pourhashemi, S.; Chou, J.; Moffitt, K.; Halasa, N.B.; et al. Cross-reactive immunity against the SARS-CoV-2 Omicron variant is low in pediatric patients with prior COVID-19 or MIS-C. Nat. Commun. 2022, 13, 2979. [Google Scholar] [CrossRef]
- Lavinder, J.J.; Ippolito, G.C. Boosted immunity to the common cold might protect children from COVID-19. Nat. Immunol. 2022, 23, 8–10. [Google Scholar]
- Zar, H.J.; Nicol, M.P.; MacGinty, R.; Workman, L.; Petersen, W.; Johnson, M.; Goldblatt, D. Antibodies to seasonal coronaviruses rarely cross-react with SARS-CoV-2: Findings from an African birth cohort. Pediatr. Infect. Dis. J. 2021, 40, e516–e519. [Google Scholar]
- Miyara, M.; Sterlin, D.; Anna, F.; Marot, S.; Mathian, A.; Atif, M.; Quentric, P.; Mohr, A.; Claër, L.; Parizot, C.; et al. Pre-COVID-19 humoral immunity to common coronaviruses does not confer cross-protection against SARS-CoV-2. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Mantus, G.; Nyhoff, L.E.; Edara, V.V.; Zarnitsyna, V.I.; Ciric, C.R.; Flowers, M.W.; Norwood, C.; Ellis, M.; Hussaini, L.; Manning, K.E.; et al. Pre-existing SARS-CoV-2 immunity influences potency, breadth, and durability of the humoral response to SARS-CoV-2 vaccination. Cell Rep. Med. 2022, 3, 100603. [Google Scholar]
- Patalon, T.; Saciuk, Y.; Hadad, H.O.; Perez, G.; Peretz, A.; Ben-Tov, A.; Gazit, S. Naturally-acquired immunity dynamics against SARS-CoV-2 in children and adolescents. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, P.; Whiteman, N.B.; Moghaddam, A.S.; Zuiani, A.; Habibi, S.; Gautam, A.; Xiao, T.; Cai, Y.; Chen, B. Differential antibody dynamics to SARS-CoV-2 infection and vaccination. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Bowman, K.A.; Stein, D.; Shin, S.; Ferbas, K.G.; Tobin, N.H.; Mann, C.; Fischinger, S.; Ollmann Saphire, E.; Lauffenburger, D.; Rimoin, A.W.; et al. Hybrid immunity shifts the Fc-effector quality of SARS-CoV-2 mRNA vaccine-induced immunity. mBio 2022, 13, e0164722. [Google Scholar]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef]
- Bekliz, M.; Adea, K.; Vetter, P.; Eberhardt, C.S.; Hosszu-Fellous, K.; Vu, D.-L.; Puhach, O.; Essaidi-Laziosi, M.; Waldvogel-Abramowski, S.; Stephan, C.; et al. Neutralization of ancestral SARS-CoV-2 and variants Alpha, Beta, Gamma, Delta, Zeta and Omicron by mRNA vaccination and infection-derived immunity through homologous and heterologous variants. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Cele, S.; Bernstein, M.; Karim, F.; Khan, K.; Ganga, Y.; Jule, Z.; Reedoy, K.; Lustig, G.; Samsunder, N.; Mazibuko, M.; et al. Beta infection combined with Pfizer BNT162b2 vaccination leads to broadened neutralizing immunity against Omicron. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Jung, M.K.; Jeong, S.D.; Noh, J.Y.; Kim, D.U.; Jung, S.; Song, J.Y.; Jeong, H.W.; Park, S.H.; Shin, E.C. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat. Microbiol. 2022, 7, 909–917. [Google Scholar] [CrossRef]
- Mazzoni, A.; Di Lauria, N.; Maggi, L.; Salvati, L.; Vanni, A.; Capone, M.; Lamacchia, G.; Mantengoli, E.; Spinicci, M.; Zammarchi, L.; et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J. Clin. Investig. 2021, 131, e149150. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar]
- Ekstrom, N.; Haveri, A.; Solastie, A.; Virta, C.; Osterlund, P.; Nohynek, H.; Nieminen, T.; Ivaska, L.; Tahtinen, P.A.; Lempainen, J. Strong neutralizing antibody responses to SARS-CoV-2 variants following a single vaccine dose in subjects with previous SARS-CoV-2 infection. medRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Young, B.E.; Zhu, F.; Lim, B.L.; Sia, W.R.; Thein, T.L.; Chen, M.I.; Leo, Y.S.; Lye, D.C.; et al. Pan-sarbecovirus neutralizing antibodies in BNT162b2-immunized SARS-CoV-1 survivors. N. Engl. J. Med. 2021, 385, 1401–1406. [Google Scholar] [CrossRef]
- Zar, H.J.; MacGinty, R.; Workman, L.; Botha, M.; Johnson, M.; Hunt, A.; Burd, T.; Nicol, M.P.; Flasche, S.; Quilty, B.J.; et al. Natural and hybrid immunity following four COVID-19 waves: A prospective cohort study of mothers in South Africa. EClinicalMedicine 2022, 53, 101655. [Google Scholar] [CrossRef]
- Collier, A.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of immune responses in fully vaccinated individuals after breakthrough infection with the SARS-CoV-2 delta variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Smolenov, I.; Han, H.H.; Li, P.; Baccarini, C.; Verhoeven, C.; Rockhold, F.; Clemens, S.A.C.; Ambrosino, D.; Richmond, P.; Siber, G.; et al. Impact of previous exposure to SARS-CoV-2 and of S-Trimer (SCB-2019) COVID-19 vaccination on the risk of reinfection: A randomised, double-blinded, placebo-controlled, phase 2 and 3 trial. Lancet Infect. Dis. 2022, 22, 990–1001. [Google Scholar] [CrossRef]
- Almadhi, M.; Alsayyad, A.S.; Conroy, R.; Atkin, S.; Awadhi, A.A.; Al-Tawfiq, J.A.; AlQahtani, M. Epidemiological assessment of SARS-CoV-2 reinfection. Int. J. Infect. Dis. 2022, 123, 9–16. [Google Scholar]
- Lewis, N.; Chambers, L.C.; Chu, H.T.; Fortnam, T.; De Vito, R.; Gargano, L.M.; Chan, P.A.; McDonald, J.; Hogan, J.W. Effectiveness associated with vaccination after COVID-19 recovery in preventing reinfection. JAMA Netw. Open 2022, 5, e2223917. [Google Scholar] [CrossRef]
- Braeye, T.; Catteau, L.; Brondeel, R.; van Loenhout, J.A.F.; Proesmans, K.; Cornelissen, L.; Van Oyen, H.; Stouten, V.; Hubin, P.; Billuart, M.; et al. Vaccine effectiveness against onward transmission of SARS-CoV2-infection by variant of concern and time since vaccination, Belgian contact tracing, 2021. Vaccine 2022, 40, 3027–3037. [Google Scholar] [CrossRef]
- Gazit, S.; Mizrahi, B.; Kalkstein, N.; Neuberger, A.; Peretz, A.; Mizrahi-Reuveni, M.; Ben-Tov, A.; Patalon, T. BNT162b2 mRNA vaccine effectiveness given confirmed exposure: Analysis of household members of COVID-19 patients. Clin. Infect. Dis. 2022, 75, e734–e740. [Google Scholar] [CrossRef]
- Park, Y.J.; Pinto, D.; Walls, A.C.; Liu, Z.; De Marco, A.; Benigni, F.; Zatta, F.; Silacci-Fregni, C.; Bassi, J.; Sprouse, K.R.; et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar]
- Muik, A.; Lui, B.G.; Bacher, M.; Wallisch, A.-K.; Toker, A.; Finlayson, A.; Krüger, K.; Ozhelvaci, O.; Grikscheit, K.; Hoehl, S.; et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Ganga, Y.; Bernstein, M.; Jule, Z.; Reedoy, K.; Cele, S.; Lustig, G.; Amoako, D.; Wolter, N.; et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 2022, 13, 4686. [Google Scholar]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022, 185, 2422–2433.e2413. [Google Scholar] [CrossRef]
- Quandt, J.; Muik, A.; Salisch, N.; Lui, B.G.; Lutz, S.; Kruger, K.; Wallisch, A.K.; Adams-Quack, P.; Bacher, M.; Finlayson, A.; et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci. Immunol. 2022, 7, eabq2427. [Google Scholar]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Sahin, U.; Jansen, K.U.; et al. Neutralization of Omicron sublineages and Deltacron SARS-CoV-2 by three doses of BNT162b2 vaccine or BA.1 infection. Emerg. Microbes Infect. 2022, 11, 1828–1832. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar]
- Townsend, J.P.; Hassler, H.B.; Wang, Z.; Miura, S.; Singh, J.; Kumar, S.; Ruddle, N.H.; Galvani, A.P.; Dornburg, A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe 2021, 2, e666–e675. [Google Scholar] [CrossRef]
- Stegger, M.; Edslev, S.M.; Sieber, R.N.; Cäcilia Ingham, A.; Ng, K.L.; Tang, M.-H.E.; Alexandersen, S.; Fonager, J.; Legarth, R.; Utko, M.; et al. Occurrence and significance of Omicron BA.1 infection followed by BA.2 reinfection. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- van Zelm, M.C. Immune memory to SARS-CoV-2 Omicron BA.1 breakthrough infections: To change the vaccine or not? Sci Immunol 2022, 7, eabq5901. [Google Scholar] [CrossRef]
- Kaku, C.I.; Bergeron, A.J.; Ahlm, C.; Normark, J.; Sakharkar, M.; Forsell, M.N.E.; Walker, L.M. Recall of pre-existing cross-reactive B cell memory following Omicron BA.1 breakthrough infection. Sci. Immunol. 2022, 7, eabq3511. [Google Scholar]
- Rössler, A.; Netzl, A.; Knabl, L.; Schäfer, H.; Wilks, S.H.; Bante, D.; Falkensammer, B.; Borena, W.; von Laer, D.; Smith, D.; et al. BA.2 Omicron differs immunologically from both BA.1 Omicron and pre-Omicron variants. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Wang, W.; Lusvarghi, S.; Subramanian, R.; Epsi, N.J.; Wang, R.; Goguet, E.; Fries, A.C.; Echegaray, F.; Vassell, R.; Coggins, S.A.; et al. Post-vaccination Omicron infections induce broader immunity across antigenic space than prototype mRNA COVID-19 booster vaccination or primary infection. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Richardson, S.I.; Madzorera, V.S.; Spencer, H.; Manamela, N.P.; van der Mescht, M.A.; Lambson, B.E.; Oosthuysen, B.; Ayres, F.; Makhado, Z.; Moyo-Gwete, T.; et al. SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe 2022, 30, 880–886.e884. [Google Scholar]
- Suryawanshi, R.K.; Chen, I.P.; Ma, T.; Syed, A.M.; Brazer, N.; Saldhi, P.; Simoneau, C.R.; Ciling, A.; Khalid, M.M.; Sreekumar, B.; et al. Limited cross-variant immunity from SARS-CoV-2 Omicron without vaccination. Nature 2022, 607, 351–355. [Google Scholar]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Franchi, F.; Cusi, M.G. Omicron infection evokes cross-protection against SARS-CoV-2 variants in vaccinees. Vaccines 2022, 10, 808. [Google Scholar] [CrossRef]
- Swanson, K. Pfizer/BioNTech COVID-19 Omicron-modified vaccine options: Vaccines and Related Biological Products Advisory Committee Meeting. Available online: https://www.fda.gov/media/159496/download (accessed on 13 September 2022).
- Muik, A.; Lui, B.G.; Bacher, M.; Wallisch, A.K.; Toker, A.; Couto, C.I.C.; Güler, A.; Mampilli, V.; Schmitt, G.J.; Mottl, J.; et al. Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice. Sci. Immunol. 2022, 7, eade9888. [Google Scholar]
- Hoge, S. mRNA-1273.214 Moderna COVID-19 Investigational Bivalent Vaccine (original + Omicron): Vaccines and Related Biological Products Advisory Committee Meeting. Available online: https://www.fda.gov/media/159492/download (accessed on 13 September 2022).
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A bivalent Omicron-containing booster vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Zou, J.; Kurhade, C.; Patel, S.; Kitchin, N.; Tompkins, K.; Cutler, M.; Cooper, D.; Yang, Q.; Cai, H.; Muik, A.; et al. Improved neutralization of Omicron BA.4/5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with bivalent BA.4/5 vaccine. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Davis-Gardner, M.E.; Lai, L.; Wali, B.; Samaha, H.; Solis, D.; Lee, M.; Porter-Morrison, A.; Hentenaar, I.T.; Yamamoto, F.; Godbole, S.; et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA bivalent booster. N. Engl. J. Med. 2023, 388, 183–185. [Google Scholar]
- Surie, D.; DeCuir, J.; Zhu, Y.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated hospitalization among immunocompetent adults aged ≥65 years—IVY Network, 18 states, September 8-November 30, 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1625–1630. [Google Scholar]
- Tenforde, M.W.; Weber, Z.A.; Natarajan, K.; Klein, N.P.; Kharbanda, A.B.; Stenehjem, E.; Embi, P.J.; Reese, S.E.; Naleway, A.L.; Grannis, S.J.; et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19-associated emergency department or urgent care encounters and hospitalizations among immunocompetent adults—VISION Network, nine states, September-November 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1616–1624. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes Moderna and Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose in Younger Age Groups. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-bivalent-covid-19-vaccines (accessed on 3 November 2022).
- European Medicines Agency. COVID-19 Vaccines: Authorised. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#adapted-covid-19-vaccines-section (accessed on 9 January 2023).
- World Health Organization. Good Practice Statement on the Use of Variant-Containing COVID-19 Vaccines. 17 October 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Variants-2022.1 (accessed on 9 January 2023).
- Marks, P.; Cavaleri, M. International Coalition of Medicines Regulatory Authorities SARS-CoV-2 Variant Workshop. Available online: https://icmra.info/drupal/covid-19/30june2022 (accessed on 13 September 2022).
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#:~:text=People%20ages%205%20years%20and,monovalent%20booster%20dose(s) (accessed on 3 November 2022).
- Centers for Disease Control and Prevention. Summary Document for Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized or Approved in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf (accessed on 3 November 2022).
- European Medicines Agency. ETF Concludes that Bivalent Original/Omicron BA.4-5 mRNA Vaccines May be Used for Primary Vaccination. Available online: https://www.ema.europa.eu/en/news/etf-concludes-bivalent-original-omicron-ba4-5-mrna-vaccines-may-be-used-primary-vaccination#:~:text=4%2D5%20mRNA%20vaccines%20may%20be%20used%20for%20primary%20vaccination,-Share&text=EMA's%20Emergency%20Task%20Force%20(ETF,for%20primary%20(initial)%20vaccination (accessed on 9 January 2023).
- Walls, A.C.; Sprouse, K.R.; Bowen, J.E.; Joshi, A.; Franko, N.; Navarro, M.J.; Stewart, C.; Cameroni, E.; McCallum, M.; Goecker, E.A.; et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell 2022, 185, 872–880.e873. [Google Scholar]
- Chin, E.T.; Leidner, D.; Lamson, L.; Lucas, K.; Studdert, D.M.; Goldhaber-Fiebert, J.D.; Andrews, J.R.; Salomon, J.A. Protection against Omicron from vaccination and previous infection in a prison system. N. Engl. J. Med. 2022, 387, 1770–1782. [Google Scholar] [CrossRef]
- Tan, S.T.; Kwan, A.T.; Rodríguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat. Med. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar]
- Sidik, S.M. COVID vaccine plus infection can lead to months of immunity. Nature 2022. online ahead of print. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; de Araujo Oliveira, V.; Paixao, E.S.; Florentino, P.T.V.; Penna, G.O.; Pearce, N.; Werneck, G.L.; Barreto, M.L.; Boaventura, V.S.; Barral-Netto, M. Vaccination plus previous infection: Protection during the omicron wave in Brazil. Lancet Infect. Dis. 2022, 22, 945–946. [Google Scholar]
- Garg, P.K.; Thiruvengadam, R. Effectiveness of SARS-CoV-2 vaccines in the post-natural infection world. Lancet Infect. Dis. 2022, 22, 745–747. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar]
- Goldblatt, D. SARS-CoV-2: From herd immunity to hybrid immunity. Nat. Rev. Immunol. 2022, 22, 333–334. [Google Scholar]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Madhavan, M.; Ritchie, A.J.; Aboagye, J.; Jenkin, D.; Provstgaad-Morys, S.; Tarbet, I.; Woods, D.; Davies, S.; Baker, M.; Platt, A.; et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. eBioMedicine 2022, 85, 104298. [Google Scholar]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinardi, J.R.; Srivastava, A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines 2023, 11, 370. https://doi.org/10.3390/biomedicines11020370
Spinardi JR, Srivastava A. Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines. 2023; 11(2):370. https://doi.org/10.3390/biomedicines11020370
Chicago/Turabian StyleSpinardi, Julia R., and Amit Srivastava. 2023. "Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines" Biomedicines 11, no. 2: 370. https://doi.org/10.3390/biomedicines11020370
APA StyleSpinardi, J. R., & Srivastava, A. (2023). Hybrid Immunity to SARS-CoV-2 from Infection and Vaccination—Evidence Synthesis and Implications for New COVID-19 Vaccines. Biomedicines, 11(2), 370. https://doi.org/10.3390/biomedicines11020370