Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review
Abstract
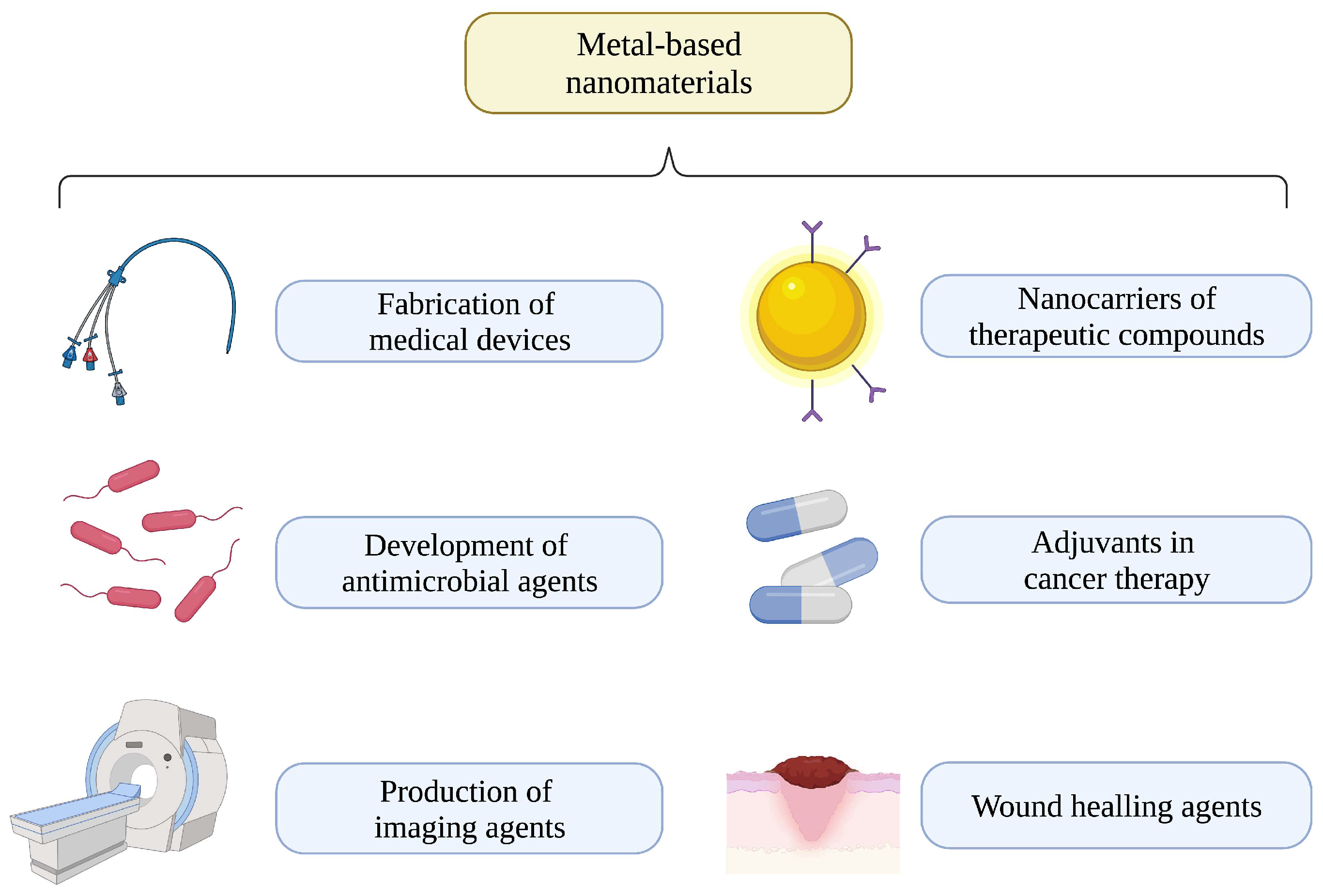
:1. Introduction
2. An Overview of Lung Cancer and Its Subtypes
2.1. Small Cell Lung Cancer (SCLC)
2.2. Non-Small Cell Lung Cancer (NSCLC)
3. A Brief History and General Features of Noble Metals with Biological Activity
4. Biological Sources for AgNPs Synthesis
4.1. Microbial Cell Cultures
4.2. Plant Extracts and Phytochemicals
4.3. Amino Acids
5. Capping Agents
6. Possible Anti-Lung Cancer Mechanisms of Biosynthesized AgNPs
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in Developmental Organic and Inorganic Nanomaterial: A Review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Virlan, M.J.R.; Miricescu, D.; Radulescu, R.; Sabliov, C.M.; Totan, A.; Calenic, B.; Greabu, M. Organic Nanomaterials and Their Applications in the Treatment of Oral Diseases. Molecules 2016, 21, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, A.; Zhang, Z.; Zhao, Q.; Li, J.; Mei, Y.; Yin, Y.; Wang, W. Multifunctional Inorganic Nanomaterials for Cancer Photoimmunotherapy. Cancer Commun. 2022, 42, 141–163. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V.; Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; et al. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. IntechOpen: London, UK, 2018; ISBN 978-1-78985-284-4. [Google Scholar]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1–23. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener Synthesis of Chemical Compounds and Materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Xin, X.; Qi, C.; Xu, L.; Gao, Q.; Liu, X. Green Synthesis of Silver Nanoparticles and Their Antibacterial Effects. Front. Chem. Eng. 2022, 4, 69. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent Advances in Metal Nanoparticles in Cancer Therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Cesario, T.C.; Wang, X.; Yuan, J.S.; Rentzepis, P.M. Synergistic Reaction of Silver Nitrate, Silver Nanoparticles, and Methylene Blue against Bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 13612–13617. [Google Scholar] [CrossRef] [Green Version]
- Arem, H.; Loftfield, E. Cancer Epidemiology: A Survey of Modifiable Risk Factors for Prevention and Survivorship. Am. J. Lifestyle Med. 2017, 12, 200–210. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating Intrinsic and Non-Intrinsic Cancer Risk Factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gospodarowicz, M.; Brierley, J.; O’Sullivan, B. Principles of Cancer Staging for Clinical Obstetrics and Gynecology. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 767–775. [Google Scholar] [CrossRef]
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 21 December 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA: A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Cancer of the Lung and Bronchus-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 9 November 2022).
- Bradley, S.H.; Kennedy, M.P.T.; Neal, R.D. Recognising Lung Cancer in Primary Care. Adv. Ther. 2019, 36, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeros, M.; Laversanne, M.; Barrios, E.; Cancela, M.d.C.; Vries, E.d.; Pardo, C.; Bray, F. An Updated Profile of the Cancer Burden, Patterns and Trends in Latin America and the Caribbean. Lancet Reg. Health–Am. 2022, 13, 100294. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The Epidemiology of Lung Cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, K.; Graulus, G.-J.; Mesotten, L.; Thomeer, M.; Derveaux, E.; Noben, J.-P.; Guedens, W.; Adriaensens, P. The Metabolic Landscape of Lung Cancer: New Insights in a Disturbed Glucose Metabolism. Front. Oncol. 2019, 9, 1215. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, L.; Chan, D.W.; Li, Q.K.; Zhang, H. Protein Signatures of Molecular Pathways in Non-Small Cell Lung Carcinoma (NSCLC): Comparison of Glycoproteomics and Global Proteomics. Clin. Proteom. 2017, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Eymin, B.; Gazzeri, S. Role of Cell Cycle Regulators in Lung Carcinogenesis. Cell Adhes. Migr. 2010, 4, 114–123. [Google Scholar] [CrossRef]
- Xie, X.; Li, X.; Tang, W.; Xie, P.; Tan, X. Primary Tumor Location in Lung Cancer: The Evaluation and Administration. Chin. Med. J. (Engl.) 2022, 135, 127–136. [Google Scholar] [CrossRef]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-Cell Lung Cancer. Nat. Rev. Dis. Primers. 2021, 7, 3. [Google Scholar] [CrossRef]
- Denninghoff, V.; Russo, A.; de Miguel-Pérez, D.; Malapelle, U.; Benyounes, A.; Gittens, A.; Cardona, A.F.; Rolfo, C. Small Cell Lung Cancer: State of the Art of the Molecular and Genetic Landscape and Novel Perspective. Cancers 2021, 13, 1723. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Salgia, R. Managing Patients With Relapsed Small-Cell Lung Cancer. JOP 2018, 14, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J. The Evolving TNM Cancer Staging System: An Essential Component of Cancer Care. CMAJ 2006, 174, 155–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telloni, S.M. Tumor Staging and Grading: A Primer. In Molecular Profiling: Methods and Protocols; Espina, V., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 1–17. ISBN 978-1-4939-6990-6. [Google Scholar]
- Wu, A.J.; Gillis, A.; Foster, A.; Woo, K.; Zhang, Z.; Gelblum, D.Y.; Downey, R.J.; Rosenzweig, K.E.; Ong, L.; Perez, C.A.; et al. Patterns of Failure in Limited-Stage Small Cell Lung Cancer: Implications of TNM Stage for Prophylactic Cranial Irradiation. Radiother. Oncol. 2017, 125, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P. Staging and Imaging of Small Cell Lung Cancer. Cancer Imaging 2012, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.-M.C.; Früh, M.; Ardizzoni, A.; Besse, B.; Faivre-Finn, C.; Hendriks, L.E.; Lantuejoul, S.; Peters, S.; Reguart, N.; Rudin, C.M.; et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2021, 32, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.P.; Govindan, R.; Grecula, J.C.; et al. Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Li, Y.; Wang, H.; Jia, T.; Wang, E.; Luo, Y.; Wei, Y.; Qin, Z.; Ma, X. Small Cell Lung Cancer Transformation: From Pathogenesis to Treatment. Semin. Cancer Biol. 2022, 86, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Raso, M.G.; Bota-Rabassedas, N.; Wistuba, I.I. Pathology and Classification of SCLC. Cancers 2021, 13, 820. [Google Scholar] [CrossRef]
- Augert, A.; Zhang, Q.; Bates, B.; Cui, M.; Wang, X.; Wildey, G.; Dowlati, A.; MacPherson, D. Small Cell Lung Cancer Exhibits Frequent Inactivating Mutations in the Histone Methyltransferase KMT2D/MLL2: CALGB 151111 (Alliance). J. Thorac. Oncol. 2017, 12, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Mollaoglu, G.; Guthrie, M.R.; Böhm, S.; Brägelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Zhou, N.; Zhu, W.; Zhang, J.; Wei, T.; Guo, L.; Luo, P.; Zhang, J. Genomic and Immunological Profiles of Small-Cell Lung Cancer between East Asians and Caucasian. Cancer Cell Int. 2022, 22, 173. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, T.; Xiong, Y.; Yang, Y. Roles of NOTCH1 as a Therapeutic Target and a Biomarker for Lung Cancer: Controversies and Perspectives. Dis. Mrk. 2015, 2015, e520590. [Google Scholar] [CrossRef] [Green Version]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; West, W.W.; Zhen, W. Current Concepts in the Management of Small Cell Lung Cancer. Indian J. Med. Res. 2013, 137, 1043–1051. [Google Scholar]
- Tariq, S.; Kim, S.Y.; Monteiro de Oliveira Novaes, J.; Cheng, H. Update 2021: Management of Small Cell Lung Cancer. Lung 2021, 199, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Gensheimer, M.F.; Loo, B.W. Optimal Radiation Therapy for Small Cell Lung Cancer. Curr. Treat. Options Oncol. 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zimmermann, S.; Parikh, K.; Mansfield, A.S.; Adjei, A.A. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin. Proc. 2019, 94, 1599–1622. [Google Scholar] [CrossRef]
- Byers, L.A.; Rudin, C.M. Small Cell Lung Cancer: Where Do We Go from Here? Cancer 2015, 121, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zou, S.; Zhao, Z.; Liu, P.; Ke, C.; Xu, S. New Insights into Small-Cell Lung Cancer Development and Therapy. Cell. Biol. Int. 2020, 44, 1564–1576. [Google Scholar] [CrossRef] [Green Version]
- Hiddinga, B.I.; Raskin, J.; Janssens, A.; Pauwels, P.; Meerbeeck, J.P.V. Recent Developments in the Treatment of Small Cell Lung Cancer. Eur. Respir. Rev. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging Therapies for Small Cell Lung Cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Lee, C.; Park, Y.S. Location of Stage I–III Non-small Cell Lung Cancer and Survival Rate: Systematic Review and Meta-analysis. Thorac. Cancer 2018, 9, 1614–1622. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-Small-Cell Lung Cancers: A Heterogeneous Set of Diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Bareschino, M.A.; Schettino, C.; Rossi, A.; Maione, P.; Sacco, P.C.; Zeppa, R.; Gridelli, C. Treatment of Advanced Non Small Cell Lung Cancer. J. Thorac. Dis. 2011, 3, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic Alterations Defining NSCLC Subtypes and Their Therapeutic Implications. Lung Cancer 2013, 82, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Socinski, M.A.; Obasaju, C.; Gandara, D.; Hirsch, F.R.; Bonomi, P.; Bunn, P.; Kim, E.S.; Langer, C.J.; Natale, R.B.; Novello, S.; et al. Clinicopathologic Features of Advanced Squamous NSCLC. J. Thorac. Oncol. 2016, 11, 1411–1422. [Google Scholar] [CrossRef] [Green Version]
- Zappa, C.; Mousa, S.A. Non-Small Cell Lung Cancer: Current Treatment and Future Advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Rodig, S.J.; Mino-Kenudson, M.; Dacic, S.; Yeap, B.Y.; Shaw, A.; Barletta, J.A.; Stubbs, H.; Law, K.; Lindeman, N.; Mark, E.; et al. Unique Clinicopathologic Features Characterize ALK-Rearranged Lung Adenocarcinoma in the Western Population. Clin. Cancer Res. 2009, 15, 5216–5223. [Google Scholar] [CrossRef] [Green Version]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef]
- Xie, M.; Xu, X.; Fan, Y. KRAS-Mutant Non-Small Cell Lung Cancer: An Emerging Promisingly Treatable Subgroup. Front. Oncol. 2021, 11, 672612. [Google Scholar] [CrossRef] [PubMed]
- Friedlaender, A.; Banna, G.; Malapelle, U.; Pisapia, P.; Addeo, A. Next Generation Sequencing and Genetic Alterations in Squamous Cell Lung Carcinoma: Where Are We Today? Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, L.G. Lung Cancer: Diagnosis and Management. Lung Cancer 2007, 75, 8. [Google Scholar]
- Goebel, C.; Louden, C.L.; Mckenna, R.J.; Onugha, O.; Wachtel, A.; Long, T. Diagnosis of Non-Small Cell Lung Cancer for Early Stage Asymptomatic Patients. Cancer Genom. Proteom. 2019, 16, 229–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heineman, D.J.; Daniels, J.M.; Schreurs, W.H. Clinical Staging of NSCLC: Current Evidence and Implications for Adjuvant Chemotherapy. Ther. Adv. Med. Oncol. 2017, 9, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Majem, M.; Juan, O.; Insa, A.; Reguart, N.; Trigo, J.M.; Carcereny, E.; García-Campelo, R.; García, Y.; Guirado, M.; Provencio, M. SEOM Clinical Guidelines for the Treatment of Non-Small Cell Lung Cancer (2018). Clin. Transl. Oncol. 2019, 21, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Maguire, F.B.; Morris, C.R.; Parikh-Patel, A.; Cress, R.D.; Keegan, T.H.M.; Li, C.-S.; Lin, P.S.; Kizer, K.W. First-Line Systemic Treatments for Stage IV Non-Small Cell Lung Cancer in California: Patterns of Care and Outcomes in a Real-World Setting. JNCI Cancer Spectr. 2019, 3, pkz020. [Google Scholar] [CrossRef]
- König, D.; Savic Prince, S.; Rothschild, S.I. Targeted Therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An Update on Treatment of the Most Important Actionable Oncogenic Driver Alterations. Cancers 2021, 13, 804. [Google Scholar] [CrossRef]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-Based Drug Delivery for Therapy of Lung Cancer: Progress and Challenges. J. Nanomater. 2013, 2013, e863951. [Google Scholar] [CrossRef] [Green Version]
- Kichloo, A.; Albosta, M.; Dahiya, D.; Guidi, J.C.; Aljadah, M.; Singh, J.; Shaka, H.; Wani, F.; Kumar, A.; Lekkala, M. Systemic Adverse Effects and Toxicities Associated with Immunotherapy: A Review. World J. Clin. Oncol. 2021, 12, 150–163. [Google Scholar] [CrossRef]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Current Advances and Future Trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble Metals in Medicine: Latest Advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.F.; Zoroddu, M.A. Noble Metals in Pharmaceuticals: Applications and Limitations. In Biomedical Applications of Metals; Rai, M., Ingle, A.P., Medici, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–48. ISBN 978-3-319-74814-6. [Google Scholar]
- Gibier, P. Koch’s Discovery. N. Am. Rev. 1890, 151, 726–731. [Google Scholar]
- Benedek, T.G. The History of Gold Therapy for Tuberculosis. J. Hist. Med. Allied Sci. 2004, 59, 50–89. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-Based Therapy: From Past to Present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef]
- Markowska, A.; Kasprzak, B.; Jaszczyńska-Nowinka, K.; Lubin, J.; Markowska, J. Noble Metals in Oncology. Contemp. Oncol 2015, 19, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A Repertoire of Biomedical Applications of Noble Metal Nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Schlamp, G. Noble Metals and Noble Metal Alloys. In Springer Handbook of Materials Data; Warlimont, H., Martienssen, W., Eds.; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2018; pp. 339–412. ISBN 978-3-319-69743-7. [Google Scholar]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Mussa Farkhani, S.; Asoudeh Fard, A.; Zakeri-Milani, P.; Shahbazi Mojarrad, J.; Valizadeh, H. Enhancing Antitumor Activity of Silver Nanoparticles by Modification with Cell-Penetrating Peptides. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Pietro, P.D.; Zaccaro, L.; Comegna, D.; Gatto, A.D.; Saviano, M.; Snyders, R.; Cossement, D.; Satriano, C.; Rizzarelli, E. Silver Nanoparticles Functionalized with a Fluorescent Cyclic RGD Peptide: A Versatile Integrin Targeting Platform for Cells and Bacteria. RSC Adv. 2016, 6, 112381–112392. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver Nanoparticles Outperform Gold Nanoparticles in Radiosensitizing U251 Cells in Vitro and in an Intracranial Mouse Model of Glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2020, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Al-Khedhairy, A.A.; Wahab, R. Silver Nanoparticles: An Instantaneous Solution for Anticancer Activity against Human Liver (HepG2) and Breast (MCF-7) Cancer Cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical Potential of Silver Nanoparticles Synthesized from Calli Cells of Citrullus Colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Silver Nanoparticles Enhance the Apoptotic Potential of Gemcitabine in Human Ovarian Cancer Cells: Combination Therapy for Effective Cancer Treatment. Int. J. Nanomed. 2017, 12, 6487–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K.; et al. Biogenic Silver Nanoparticles for Cancer Treatment: An Experimental Report. Colloids Surf. B: Biointerfaces 2013, 106, 86–92. [Google Scholar] [CrossRef]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells. Pharmaceutics 2018, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Patil, M.P.; Ngabire, D.; Thi, H.H.P.; Kim, M.-D.; Kim, G.-D. Eco-Friendly Synthesis of Gold Nanoparticles and Evaluation of Their Cytotoxic Activity on Cancer Cells. J. Clust Sci. 2017, 28, 119–132. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green Chemistry Approach for the Synthesis and Stabilization of Biocompatible Gold Nanoparticles and Their Potential Applications in Cancer Therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Kang, Y.; Zhao, L.; Guo, S. Design and Synthesis of Copper Nanoparticles for the Treatment of Human Esophageal Cancer: Introducing a Novel Chemotherapeutic Supplement. J. Exp. Nanosci. 2022, 17, 274–284. [Google Scholar] [CrossRef]
- Kiriyanthan, R.M.; Sharmili, S.A.; Balaji, R.; Jayashree, S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Govindarajan, M.; Vaseeharan, B. Photocatalytic, Antiproliferative and Antimicrobial Properties of Copper Nanoparticles Synthesized Using Manilkara Zapota Leaf Extract: A Photodynamic Approach. Photodiagnosis Photodyn. Ther. 2020, 32, 102058. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of Copper Nanoparticles Using Aqueous Tilia Extract: Antimicrobial and Anticancer Activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Dashtestani, F.; AlizadehZeinabad, H. Cytotoxic Effect of Albumin Coated Copper Nanoparticle on Human Breast Cancer Cells of MDA-MB 231. PLoS ONE 2017, 12, e0188639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Kim, E.; Han, J.W.; Park, J.H.; Kim, J.-H. Green Chemistry Approach for Synthesis of Effective Anticancer Palladium Nanoparticles. Molecules 2015, 20, 22476–22498. [Google Scholar] [CrossRef] [PubMed]
- Gulbagca, F.; Aygün, A.; Gülcan, M.; Ozdemir, S.; Gonca, S.; Şen, F. Green Synthesis of Palladium Nanoparticles: Preparation, Characterization, and Investigation of Antioxidant, Antimicrobial, Anticancer, and DNA Cleavage Activities. Appl. Organomet. Chem. 2021, 35, e6272. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Combination of Palladium Nanoparticles and Tubastatin-A Potentiates Apoptosis in Human Breast Cancer Cells: A Novel Therapeutic Approach for Cancer. Int. J. Nanomed. 2017, 12, 6503–6520. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Melatonin Enhances Palladium-Nanoparticle-Induced Cytotoxicity and Apoptosis in Human Lung Epithelial Adenocarcinoma Cells A549 and H1229. Antioxidants 2020, 9, 357. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Anticancer Properties of Platinum Nanoparticles and Retinoic Acid: Combination Therapy for the Treatment of Human Neuroblastoma Cancer. Int. J. Mol. Sci. 2020, 21, 6792. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Rasu Manimuthu, T. Cytotoxic Potentials of Biologically Fabricated Platinum Nanoparticles from Streptomyces Sp. on MCF-7 Breast Cancer Cells. IET Nanobiotechnol. 2017, 11, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Abedi, A.; Akbarzadeh, A.; Mokhtari, M.J.; Shahmabadi, H.E.; Mehrabi, M.R.; Javadian, S.; Chiani, M. Evaluation of Synthesized Platinum Nanoparticles on the MCF-7 and HepG-2 Cancer Cell Lines. Int. Nano Lett. 2013, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, S.; Junaid Bashir, D.; Imtiyaz, K.; Rizvi, M.M.A.; Ahamad, I.; Fatma, T.; Bharal Agarwal, N.; Arora, I.; Samim, M. Biofabricated Platinum Nanoparticles: Therapeutic Evaluation as a Potential Nanodrug against Breast Cancer Cells and Drug-Resistant Bacteria. RSC Adv. 2021, 11, 24900–24916. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Park, H.; Leary, J.F.; Key, J. Top-down Fabrication-Based Nano/Microparticles for Molecular Imaging and Drug Delivery. Int. J. Nanomed. 2019, 14, 6631–6644. [Google Scholar] [CrossRef] [Green Version]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marbella, L.E.; Millstone, J.E. NMR Techniques for Noble Metal Nanoparticles. Chem. Mater. 2015, 27, 2721–2739. [Google Scholar] [CrossRef] [Green Version]
- Walsh, A.G.; Chen, Z.; Zhang, P. X-Ray Spectroscopy of Silver Nanostructures toward Antibacterial Applications. J. Phys. Chem. C 2020, 124, 4339–4351. [Google Scholar] [CrossRef]
- Daphedar, A.; Taranath, T.C. Characterization and Cytotoxic Effect of Biogenic Silver Nanoparticles on Mitotic Chromosomes of Drimia Polyantha (Blatt. & McCann) Stearn. Toxicol. Rep. 2018, 5, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Mejía-Rosales, S.; José-Yacamán, M. Scanning Transmission Electron Microscopy Methods for the Analysis of Nanoparticles. Methods Mol. Biol. 2012, 906, 453–471. [Google Scholar] [CrossRef]
- Sikes, J.C.; Wonner, K.; Nicholson, A.; Cignoni, P.; Fritsch, I.; Tschulik, K. Characterization of Nanoparticles in Diverse Mixtures Using Localized Surface Plasmon Resonance and Nanoparticle Tracking by Dark-Field Microscopy with Redox Magnetohydrodynamics Microfluidics. ACS Phys. Chem. Au 2022, 2, 289–298. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Arredondo-Ochoa, T.; Silva-Martínez, G.A. Microemulsion Based Nanostructures for Drug Delivery. Front. Nanotechnol. 2022, 3, 753947. [Google Scholar] [CrossRef]
- Mishra, R.; Mishra, S.; Barot, Y.B. Chapter 12-Greener Synthesis and Stabilization of Metallic Nanoparticles in Ionic Liquids. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Kharisov, B., Kharissova, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–276. ISBN 978-0-12-822446-5. [Google Scholar]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Krishna, G.; Srileka, V.; Singara Charya, M.A.; Abu Serea, E.S.; Shalan, A.E. Biogenic Synthesis and Cytotoxic Effects of Silver Nanoparticles Mediated by White Rot Fungi. Heliyon 2021, 7, e06470. [Google Scholar] [CrossRef]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria Ternatea and Solanum Nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, e928204. [Google Scholar] [CrossRef] [Green Version]
- Matur, M.; Madhyastha, H.; Shruthi, T.S.; Madhyastha, R.; Srinivas, S.P.; Navya, P.N.; Daima, H.K. Engineering Bioactive Surfaces on Nanoparticles and Their Biological Interactions. Sci. Rep. 2020, 10, 19713. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 575415. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green Synthesis of Silver Nanoparticles Using Teucrium Polium Leaf Extract and Assessment of Their Antitumor Effects against MNK45 Human Gastric Cancer Cell Line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green Biosynthesis of Silver Nanoparticles Using Leaves Extract of Artemisia Vulgaris and Their Potential Biomedical Applications. Colloids Surf. B: Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of Silver Nanoparticles Using the Extract of Alternanthera Sessilis—Antiproliferative Effect against Prostate Cancer Cells. Cancer Nano 2013, 4, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis Prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, Antibacterial Activity and Anticancer Effects Against Prostate Cancer (PC-3) Cells of Silver Nanoparticles Using Dimocarpus Longan Lour. Peel Extract. Nanoscale Res. Lett. 2016, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of Silver Nanoparticles Using Taxus Yunnanensis Callus and Their Antibacterial Activity and Cytotoxicity in Human Cancer Cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nuairi, A.G.; Mosa, K.A.; Mohammad, M.G.; El-Keblawy, A.; Soliman, S.; Alawadhi, H. Biosynthesis, Characterization, and Evaluation of the Cytotoxic Effects of Biologically Synthesized Silver Nanoparticles from Cyperus Conglomeratus Root Extracts on Breast Cancer Cell Line MCF-7. Biol. Trace Elem. Res. 2020, 194, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Gopal, P.K.; Paul, S.; Pal, R. Cyanobacteria Assisted Biosynthesis of Silver Nanoparticles—A Potential Antileukemic Agent. J. Appl. Phycol. 2016, 28, 3387–3394. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective Cytotoxicity of Green Synthesized Silver Nanoparticles against the MCF-7 Tumor Cell Line and Their Enhanced Antioxidant and Antimicrobial Properties. IJN 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of Silver Nanoparticles Mediated by Extracellular Pigment from Talaromyces Purpurogenus and Their Biomedical Applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Wypij, M.; Jędrzejewski, T.; Ostrowski, M.; Trzcińska, J.; Rai, M.; Golińska, P. Biogenic Silver Nanoparticles: Assessment of Their Cytotoxicity, Genotoxicity and Study of Capping Proteins. Molecules 2020, 25, 3022. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.-K. Biosynthesis, Antimicrobial and Cytotoxic Effect of Silver Nanoparticles Using a Novel Nocardiopsis Sp. MBRC-1. BioMed Res. Int. 2013, 2013, e287638. [Google Scholar] [CrossRef] [Green Version]
- Fageria, L.; Pareek, V.; Dilip, R.V.; Bhargava, A.; Pasha, S.S.; Laskar, I.R.; Saini, H.; Dash, S.; Chowdhury, R.; Panwar, J. Biosynthesized Protein-Capped Silver Nanoparticles Induce ROS-Dependent Proapoptotic Signals and Prosurvival Autophagy in Cancer Cells. ACS Omega 2017, 2, 1489–1504. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and Biological Characterization of Silver Nanoparticles Derived from the Cyanobacterium Oscillatoria Limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Jan Iftikhar, F.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal Nanoparticles Fabricated by Green Chemistry Using Natural Extracts: Biosynthesis, Mechanisms, and Applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal Nanoparticles Synthesis through Natural Phenolic Acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kim, B.; Belfield, K.D.; Norman, D.; Brennan, M.; Ali, G.S. Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia Absinthium Aqueous Extract—A Comprehensive Study. Mater. Sci. Eng. C 2016, 58, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zahrani, S.A.; Bhat, R.S.; Al-Onazi, M.A.; Alwhibi, M.S.; Soliman, D.A.; Aljebrin, N.A.; Al-Suhaibani, L.S.; Daihan, S.A. Anticancer Potential of Biogenic Silver Nanoparticles Using the Stem Extract of Commiphora Gileadensis against Human Colon Cancer Cells. Green Process. Synth. 2022, 11, 435–444. [Google Scholar] [CrossRef]
- Sparkman, O.D.; Penton, Z.E.; Kitson, F.G. Chapter 12-Amino Acids. In Gas Chromatography and Mass Spectrometry, 2nd ed.; Sparkman, O.D., Penton, Z.E., Kitson, F.G., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 265–271. ISBN 978-0-12-373628-4. [Google Scholar]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- de Matos, R.A.; Courrol, L.C. Biocompatible Silver Nanoparticles Prepared with Amino Acids and a Green Method. Amino Acids 2017, 49, 379–388. [Google Scholar] [CrossRef]
- Sethi, M.; Knecht, M.R. Understanding the Mechanism of Amino Acid-Based Au Nanoparticle Chain Formation. Langmuir 2010, 26, 9860–9874. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Bajaj, G.; Mukherji, S.; Mukherji, S. Arginine-Assisted Immobilization of Silver Nanoparticles on ZnO Nanorods: An Enhanced and Reusable Antibacterial Substrate without Human Cell Cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef] [Green Version]
- Laban, B.; Ralević, U.; Petrović, S.; Leskovac, A.; Vasić-Anićijević, D.; Marković, M.; Vasić, V. Green Synthesis and Characterization of Nontoxic L-Methionine Capped Silver and Gold Nanoparticles. J. Inorg. Biochem. 2020, 204, 110958. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Asik, R.; Manikkaraja, C.; Tamil Surya, K.; Suganthy, N.; Priya Aarthy, A.; Mathe, D.; Sivakumar, M.; Archunan, G.; Padmanabhan, P.; Gulyas, B. Anticancer Potential of L-Histidine-Capped Silver Nanoparticles against Human Cervical Cancer Cells (SiHA). Nanomaterials 2021, 11, 3154. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Munyayi, T.A.; Vorster, B.C.; Mulder, D.W. The Effect of Capping Agents on Gold Nanostar Stability, Functionalization, and Colorimetric Biosensing Capability. Nanomaterials 2022, 12, 2470. [Google Scholar] [CrossRef]
- Toh, H.S.; Jurkschat, K.; Compton, R.G. The Influence of the Capping Agent on the Oxidation of Silver Nanoparticles: Nano-Impacts versus Stripping Voltammetry. Chem.–Eur. J. 2015, 21, 2998–3004. [Google Scholar] [CrossRef]
- Javed, R.; Sajjad, A.; Naz, S.; Sajjad, H.; Ao, Q. Significance of Capping Agents of Colloidal Nanoparticles from the Perspective of Drug and Gene Delivery, Bioimaging, and Biosensing: An Insight. Int. J. Mol. Sci. 2022, 23, 10521. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An Easy Sonochemical Route for Synthesis, Characterization and Photocatalytic Performance of Nanosized FeVO4 in the Presence of Aminoacids as Green Capping Agents. J. Mater. Sci.: Mater. Electron. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Kumar, A.; Das, N.; Satija, N.K.; Mandrah, K.; Roy, S.K.; Rayavarapu, R.G. A Novel Approach towards Synthesis and Characterization of Non-Cytotoxic Gold Nanoparticles Using Taurine as Capping Agent. Nanomaterials 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Nyamu, S.N.; Ombaka, L.; Masika, E.; Ng’ang’a, M. One-Pot Microwave-Assisted Synthesis of Size-Dependent l-Glutathione-Capped Spherical Silver Nanoparticles Suitable for Materials with Antibacterial Properties. J. Interdiscip. Nanomed. 2019, 4, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological Agents for Synthesis of Nanoparticles and Their Applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, M.; He, F.; Li, Z.; Zhu, X.; Ma, Y.; Zhou, Z.; Yang, Z.; Gao, F.; Zeng, M. Biosynthesis and Antibacterial Activity of Silver Nanoparticles Using Yeast Extract as Reducing and Capping Agents. Nanoscale Res Lett 2020, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Unveiling the Potentials of Biocompatible Silver Nanoparticles on Human Lung Carcinoma A549 Cells and Helicobacter Pylori. Sci. Rep. 2019, 9, 5787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagtap, R.R.; Garud, A.; Puranik, S.S.; Rudrapal, M.; Ansari, M.A.; Alomary, M.N.; Alshamrani, M.; Salawi, A.; Almoshari, Y.; Khan, J.; et al. Biofabrication of Silver Nanoparticles (AgNPs) Using Embelin for Effective Therapeutic Management of Lung Cancer. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity Of Shikonin-Silver Nanoparticles As An Opportunity For Lung Cancer. J. Label. Compd. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef]
- Noorbazargan, H.; Amintehrani, S.; Dolatabadi, A.; Mashayekhi, A.; Khayam, N.; Moulavi, P.; Naghizadeh, M.; Mirzaie, A.; Mirzaei Rad, F.; Kavousi, M. Anti-Cancer & Anti-Metastasis Properties of Bioorganic-Capped Silver Nanoparticles Fabricated from Juniperus Chinensis Extract against Lung Cancer Cells. AMB Express. 2021, 11, 61. [Google Scholar] [CrossRef]
- Kis, B.; Moacă, E.-A.; Tudoran, L.B.; Muntean, D.; Magyari-Pavel, I.Z.; Minda, D.I.; Lombrea, A.; Diaconeasa, Z.; Dehelean, C.A.; Dinu, Ș.; et al. Green Synthesis of Silver Nanoparticles Using Populi Gemmae Extract: Preparation, Physicochemical Characterization, Antimicrobial Potential and In Vitro Antiproliferative Assessment. Materials 2022, 15, 5006. [Google Scholar] [CrossRef]
- Farshori, N.N.; Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Saquib, Q.; Siddiqui, M.A.; Wahab, R.; Al-Khedhairy, A.A. Green Synthesis of Silver Nanoparticles Using Phoenix Dactylifera Seed Extract and Its Anticancer Effect against Human Lung Adenocarcinoma Cells. J. Drug Deliv. Sci. Technol. 2022, 70, 103260. [Google Scholar] [CrossRef]
- Janakiraman, V.; Govindarajan, K.; Magesh, C.R. Biosynthesis of Silver Nanoparticles from Endophytic Fungi, and Its Cytotoxic Activity. BioNanoScience 2019, 9, 573–579. [Google Scholar] [CrossRef]
- Kummara, S.; Patil, M.B.; Uriah, T. Synthesis, Characterization, Biocompatible and Anticancer Activity of Green and Chemically Synthesized Silver Nanoparticles–A Comparative Study. Biomed. Pharmacother. 2016, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kanipandian, N.; Kannan, S.; Ramesh, R.; Subramanian, P.; Thirumurugan, R. Characterization, Antioxidant and Cytotoxicity Evaluation of Green Synthesized Silver Nanoparticles Using Cleistanthus Collinus Extract as Surface Modifier. Mater. Res. Bull. 2014, 49, 494–502. [Google Scholar] [CrossRef]
- Vera-Nuñez, L.D.C.; Cornejo-Ruiz, J.O.; Arenas-Chávez, C.A.; de Hollanda, L.M.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Davies, N.M.; Yáñez, J.A.; Vera-Gonzales, C. Green Synthesis of a Novel Silver Nanoparticle Conjugated with Thelypteris Glandulosolanosa (Raqui-Raqui): Preliminary Characterization and Anticancer Activity. Processes 2022, 10, 1308. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum Vulgare Mediated Biosynthesis of Silver Nanoparticles for Its Antibacterial and Anticancer Activity. Colloids Surf. B: Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Rajendran, R.; Pullani, S.; Thavamurugan, S.; Radhika, R.; Lakshmi Prabha, A. Green Fabrication of Silver Nanoparticles from Salvia Species Extracts: Characterization and Anticancer Activities against A549 Human Lung Cancer Cell Line. Appl. Nanosci. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Ahn, E.-Y.; Jin, H.; Park, Y. Assessing the Antioxidant, Cytotoxic, Apoptotic and Wound Healing Properties of Silver Nanoparticles Green-Synthesized by Plant Extracts. Mater. Sci. Eng. C 2019, 101, 204–216. [Google Scholar] [CrossRef]
- Murugesan, A.K.; Pannerselvam, B.; Javee, A.; Rajenderan, M.; Thiyagarajan, D. Facile Green Synthesis and Characterization of Gloriosa Superba L. Tuber Extract-Capped Silver Nanoparticles (GST-AgNPs) and Its Potential Antibacterial and Anticancer Activities against A549 Human Cancer Cells. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100460. [Google Scholar] [CrossRef]
- Zhang, D.; Ramachandran, G.; Mothana, R.A.; Siddiqui, N.A.; Ullah, R.; Almarfadi, O.M.; Rajivgandhi, G.; Manoharan, N. Biosynthesized Silver Nanoparticles Using Caulerpa Taxifolia against A549 Lung Cancer Cell Line through Cytotoxicity Effect/Morphological Damage. Saudi J. Biol. Sci. 2020, 27, 3421–3427. [Google Scholar] [CrossRef]
- Castro Aceituno, V.; Ahn, S.; Simu, S.Y.; Wang, C.; Mathiyalagan, R.; Yang, D.C. Silver Nanoparticles from Dendropanax Morbifera Léveille Inhibit Cell Migration, Induce Apoptosis, and Increase Generation of Reactive Oxygen Species in A549 Lung Cancer Cells. Vitr. Cell. Dev. Biol.-Anim. 2016, 52, 1012–1019. [Google Scholar] [CrossRef]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-Cancer Activity of Biosynthesized Silver Nanoparticles Using Avicennia Marina against A549 Lung Cancer Cells through ROS/Mitochondrial Damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef]
- Suseela, V.; Nirmaladevi, R.; Pallikondaperumal, M.; Priya, R.S.; Shaik, M.R.; Shaik, A.H.; Khan, M.; Shaik, B. Eco-Friendly Preparation of Silver Nanoparticles and Their Antiproliferative and Apoptosis-Inducing Ability against Lung Cancer. Life 2022, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, R.; Kaur, J. Alkylating Agents and Cancer Therapy. Expert Opin. Ther. Pat. 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Finch, G.L.; Burns-Naas, L.A. Cancer Chemotherapeutic Agents. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 630–641. ISBN 978-0-12-386455-0. [Google Scholar]
- Beretta, G.L.; Zunino, F. Molecular Mechanisms of Anthracycline Activity. In Anthracycline Chemistry and Biology II: Mode of Action, Clinical Aspects and New Drugs; Krohn, K., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–19. ISBN 978-3-540-75813-6. [Google Scholar]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Jabeen, S.; Qureshi, R.; Munazir, M.; Maqsood, M.; Munir, M.; Shah, S.S.H.; Rahim, B.Z. Application of Green Synthesized Silver Nanoparticles in Cancer Treatment—A Critical Review. Mater. Res. Express 2021, 8, 092001. [Google Scholar] [CrossRef]
- Hembram, K.C.; Kumar, R.; Kandha, L.; Parhi, P.K.; Kundu, C.N.; Bindhani, B.K. Therapeutic Prospective of Plant-Induced Silver Nanoparticles: Application as Antimicrobial and Anticancer Agent. Artif. Cells Nanomed. Biotechnol. 2018, 46, S38–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Wang, X.; Li, J.; Shang, M.; Niu, S.; Zhang, W.; Li, Y.; Sun, Z.; Gan, J.; Li, W.; et al. Silver Nanoparticles Induced Cytotoxicity in HT22 Cells through Autophagy and Apoptosis via PI3K/AKT/MTOR Signaling Pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111696. [Google Scholar] [CrossRef]
- Narasimha, V.R.; Latha, T.S.; Pallu, R.; Panati, K.; Narala, V.R. Anticancer Activities of Biogenic Silver Nanoparticles Targeting Apoptosis and Inflammatory Pathways in Colon Cancer Cells. J. Clust Sci. 2022, 33, 2215–2231. [Google Scholar] [CrossRef]
- Abass Sofi, M.; Sunitha, S.; Ashaq Sofi, M.; Khadheer Pasha, S.K.; Choi, D. An Overview of Antimicrobial and Anticancer Potential of Silver Nanoparticles. J. King Saud Univ.-Sci. 2022, 34, 101791. [Google Scholar] [CrossRef]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of Intrinsic Apoptotic Signaling Pathway in A549 Lung Cancer Cells Using Silver Nanoparticles from Gossypium Hirsutum and Evaluation of in Vivo Toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Que, Y.M.; Fan, X.Q.; Lin, X.J.; Jiang, X.L.; Hu, P.P.; Tong, X.Y.; Tan, Q.Y. Size Dependent Anti-Invasiveness of Silver Nanoparticles in Lung Cancer Cells. RSC Adv. 2019, 9, 21134–21138. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K.; et al. Effects of Green-Synthesized Silver Nanoparticles on Lung Cancer Cells in Vitro and Grown as Xenograft Tumors in Vivo. IJN 2016, 11, 1879–1887. [Google Scholar] [CrossRef] [Green Version]
- Padmini, R.; Nallal, V.U.M.; Razia, M.; Sivaramakrishnan, S.; Alodaini, H.A.; Hatamleh, A.A.; Al-Dosary, M.A.; Ranganathan, V.; Chung, W.J. Cytotoxic Effect of Silver Nanoparticles Synthesized from Ethanolic Extract of Allium Sativum on A549 Lung Cancer Cell Line. J. King Saud Univ.-Sci. 2022, 34, 102001. [Google Scholar] [CrossRef]
- Pallavi, S.S.; Rudayni, H.A.; Bepari, A.; Niazi, S.K.; Nayaka, S. Green Synthesis of Silver Nanoparticles Using Streptomyces Hirsutus Strain SNPGA-8 and Their Characterization, Antimicrobial Activity, and Anticancer Activity against Human Lung Carcinoma Cell Line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. [Google Scholar] [CrossRef]
- Larsen, J.E.; Govindan, R.; Minna, J.D. 32-Molecular Basis of Lung Cancer. In The Molecular Basis of Cancer, 4th ed.; Mendelsohn, J., Gray, J.W., Howley, P.M., Israel, M.A., Thompson, C.B., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2015; pp. 475–490.e1. ISBN 978-1-4557-4066-6. [Google Scholar]
- Montanino, A.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Sforza, V.; Costanzo, R.; Sandomenico, C.; Botti, G.; Piccirillo, M.C.; et al. Angiogenesis Inhibitors in Small Cell Lung Cancer. Front. Oncol. 2021, 11, 655316. [Google Scholar] [CrossRef]
- Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, M.C.; Daniele, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. [Google Scholar] [CrossRef] [Green Version]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Grace Intasa-Ard, S.; Birault, A. Nanoparticles Characterization Using the CAM Assay. Enzymes 2019, 46, 129–160. [Google Scholar] [CrossRef] [PubMed]
- Buhr, C.R.; Wiesmann, N.; Tanner, R.C.; Brieger, J.; Eckrich, J. The Chorioallantoic Membrane Assay in Nanotoxicological Research—An Alternative for In Vivo Experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.C.; Nicosia, R.F. The Rat Aortic Ring Model of Angiogenesis. Methods Mol. Biol. 2015, 1214, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Ortega, D.A.; Martínez-Castañón, G.; Palestino, G.; Navarro-Tovar, G.; Gonzalez, C. Two Methods of AuNPs Synthesis Induce Differential Vascular Effects. The Role of the Endothelial Glycocalyx. Front. Med. 2022, 9. [Google Scholar] [CrossRef]
- Pavan, S.R.; Venkatesan, J.; Prabhu, A. Anticancer Activity of Silver Nanoparticles from the Aqueous Extract of Dictyota Ciliolata on Non-Small Cell Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-Angiogenesis Effect of Biogenic Silver Nanoparticles Synthesized Using Saliva Officinalis on Chick Chorioalantoic Membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef] [Green Version]
- Dela Cruz, C.S.; Tanoue, L.T.; Matthay, R.A. Lung Cancer: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [Green Version]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Ragavan, M.V.; Patel, M.I. Understanding Sex Disparities in Lung Cancer Incidence: Are Women More at Risk? Lung Cancer Manag. 2020, 9, LMT34. [Google Scholar] [CrossRef] [PubMed]
- El-Telbany, A.; Ma, P.C. Cancer Genes in Lung Cancer. Genes Cancer 2012, 3, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, X.; Zhang, Z.; Tang, C.; Ye, H.; Jones, L.; Lou, F.; Zhang, D.; Jiang, S.; Sun, H.; et al. Identification of Genetic Mutations in Human Lung Cancer by Targeted Sequencing. Cancer Inform. 2015, 14, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, S.P.; Soeroso, N.N.; Tumanggor, C.A.K.; Gani, S.; Pradana, A. Clinical Profile of Male Patients with Non-Small Cell Lung Cancer in Adam Malik General Hospital, Medan, Indonesia. Open Access. Maced. J. Med. Sci. 2019, 7, 2612–2614. [Google Scholar] [CrossRef] [Green Version]
- Rivera, M.P.; Detterbeck, F.; Mehta, A.C. Diagnosis of Lung Cancer*: The Guidelines. Chest 2003, 123, 129S–136S. [Google Scholar] [CrossRef]
- Long, K.; Suresh, K. Pulmonary Toxicity of Systemic Lung Cancer Therapy. Respirology 2020, 25 (Suppl. S2), 72–79. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.d.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of Adverse Effects of Cisplatin Administration in Patients Affected by Solid Tumours: A Retrospective Evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Faijanur-Rob-Siddiquee, M.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 2581. [Google Scholar]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Minhaj, M.A.; Nayak, A.K. 6-Degradation and Failure of Dental Composite Materials. In Applications of Nanocomposite Materials in Dentistry; Asiri, A.M., Inamuddin, Mohammad, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2019; pp. 107–121. ISBN 978-0-12-813742-0. [Google Scholar]
- Basavaraj, K.H. Nanotechnology in Medicine and Relevance to Dermatology: Present Concepts. Indian J. Dermatol. 2012, 57, 169. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Borkowski, M.; Barzowska, A.; Kobielusz, M.; Dąbrowski, J.M. Synthesis and Characterization of Size- and Charge-Tunable Silver Nanoparticles for Selective Anticancer and Antibacterial Treatment. ACS Appl. Mater. Interfaces 2022, 14, 14981–14996. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D.; et al. Metal Nanoparticles in Cancer: From Synthesis and Metabolism to Cellular Interactions. J. Nanostruct. Chem. 2022, 15, 1–28. [Google Scholar] [CrossRef]
- Ocran Mattila, P.; Ahmad, R.; Hasan, S.S.; Babar, Z.-U.-D. Availability, Affordability, Access, and Pricing of Anti-Cancer Medicines in Low- and Middle-Income Countries: A Systematic Review of Literature. Front. Public Health 2021, 9, 628744. [Google Scholar] [CrossRef]
- Saluja, R.; Arciero, V.S.; Cheng, S.; McDonald, E.; Wong, W.W.L.; Cheung, M.C.; Chan, K.K.W. Examining Trends in Cost and Clinical Benefit of Novel Anticancer Drugs Over Time. JOP 2018, 14, e280–e294. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; De Jesús, K.; Mailankody, S. The High Price of Anticancer Drugs: Origins, Implications, Barriers, Solutions. Nat. Rev. Clin. Oncol. 2017, 14, 381–390. [Google Scholar] [CrossRef]
- Shin, G.; Kwon, H.-Y.; Bae, S. For Whom the Price Escalates: High Price and Uncertain Value of Cancer Drugs. Int. J. Environ. Res. Public Health 2022, 19, 4204. [Google Scholar] [CrossRef]
- Siddiqui, M.; Rajkumar, S.V. The High Cost of Cancer Drugs and What We Can Do About It. Mayo Clin. Proc. 2012, 87, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Satoh, E.; Sasaki, Y.; Ohkuma, R.; Takahashi, T.; Kubota, Y.; Ishida, H.; Hamada, K.; Kiuchi, Y.; Tsunoda, T. Lack of Correlation between the Costs of Anticancer Drugs and Clinical Benefits in Japan. Cancer Sci. 2018, 109, 3896–3901. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A Review on Silver Nanoparticles: Classification, Various Methods of Synthesis, and Their Potential Roles in Biomedical Applications and Water Treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Arshad, H.; Sadaf, S.; Hassan, U. De-Novo Fabrication of Sunlight Irradiated Silver Nanoparticles and Their Efficacy against E. Coli and S. Epidermidis. Sci. Rep. 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Zaki, A.A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Colby, A.H.; Liu, R.; Doyle, R.P.; Merting, A.; Zhang, H.; Savage, N.; Chu, N.-Q.; Hollister, B.A.; McCulloch, W.; Burdette, J.E.; et al. Pilot-Scale Production of Expansile Nanoparticles: Practical Methods for Clinical Scale-Up. J. Control. Release 2021, 337, 144–154. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically Synthesized Metal Nanoparticles: Recent Advancement and Future Perspectives in Cancer Theranostics. Future Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef] [Green Version]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Shin, K.; Soh, M.; Chang, H.; Kim, J.; Lee, J.; Ko, G.; Kim, B.H.; Kim, D.; Hyeon, T. Large-Scale Synthesis and Medical Applications of Uniform-Sized Metal Oxide Nanoparticles. Adv. Mater. 2018, 30, 1704290. [Google Scholar] [CrossRef]
- Skoglund, S.; Hedberg, J.; Yunda, E.; Godymchuk, A.; Blomberg, E.; Wallinder, I.O. Difficulties and Flaws in Performing Accurate Determinations of Zeta Potentials of Metal Nanoparticles in Complex Solutions—Four Case Studies. PLoS ONE 2017, 12, e0181735. [Google Scholar] [CrossRef]
- Ferreyra Maillard, A.P.V.; Espeche, J.C.; Maturana, P.; Cutro, A.C.; Hollmann, A. Zeta Potential beyond Materials Science: Applications to Bacterial Systems and to the Development of Novel Antimicrobials. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183597. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra Maillard, A.P.V.; Gonçalves, S.; Santos, N.C.; López de Mishima, B.A.; Dalmasso, P.R.; Hollmann, A. Studies on Interaction of Green Silver Nanoparticles with Whole Bacteria by Surface Characterization Techniques. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, J.-P.; Pluquet, E.; Lescure, F.X.; Bentayeb, H.; Lecuyer, E.; Boutemy, M.; Dumont, P.; Jounieaux, V.; Schmit, J.L.; Dayen, C.; et al. Bacterial Infection Profiles in Lung Cancer Patients with Febrile Neutropenia. BMC Infect. Dis. 2011, 11, 183. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-Extract-Mediated Synthesis of Metal Nanoparticles. J. Chem. 2021, 2021, e6562687. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niknejad, F.; Nabili, M.; Daie Ghazvini, R.; Moazeni, M. Green Synthesis of Silver Nanoparticles: Advantages of the Yeast Saccharomyces Cerevisiae Model. Curr. Med. Mycol. 2015, 1, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, e270974. [Google Scholar] [CrossRef] [Green Version]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]




| Noble Metal | Precursor Salt | Size (nm) | Shape | Results | Reference |
|---|---|---|---|---|---|
| Silver | AgNO3 | 13 | Spherical |
| [91] |
| Silver | AgNO3 | 31 | Spherical |
| [92] |
| Silver | AgNO3 | 10 | Spherical |
| [93] |
| Silver | AgNO3 | 22 | Spherical |
| [94] |
| Gold | HAuCl4 | 27 | Spherical |
| [95] |
| Gold | HAuCl4·3H2O | 40 | Oval and spherical |
| [96] |
| Gold | HAuCl4 | 200 | Hexagonal and triangular |
| [97] |
| Copper | Cu(NO3)2·3H2O | 13 | Spherical |
| [98] |
| Copper | CuSO4·5H2O | 42 | Spherical |
| [99] |
| Copper | CuSO4·5H2O | 18 | Hemispherical |
| [100] |
| Copper | Cu(NO3)2·5H2O | 63 | Spherical |
| [101] |
| Palladium | PdCl2 | 5 | Spherical |
| [102] |
| Palladium | PdCl2 | 7 | Spherical |
| [103] |
| Palladium | PdCl2 | 25 | Spherical |
| [104] |
| Palladium | PdCl2 | 10 | Spherical |
| [105] |
| Platinum | PtCl6 | 25 | Spherical |
| [106] |
| Platinum | K2PtCl6 | 45 | Spherical |
| [107] |
| Platinum | K2PtCl6 | 34 | Spherical |
| [108] |
| Platinum | H2PtCl6·6H2O | 113 | Spherical |
| [109] |
| Reducing Agent | Size (nm) | Shape | ζ-Potential (mV) | Results | Reference |
|---|---|---|---|---|---|
| Ginkgo biloba leaves extract | 40 | Spherical or oval | −34.5 |
| [132] |
| Teucrium polium extract | 100 | Spherical | N.I. |
| [133] |
| Artemisia vulgaris extract | 25 | Spherical | N.I. |
| [134] |
| Alternanthera sessilis extract | 50 | Spherical | N.I. |
| [135] |
| Cucumis prophetarum aqueous leaf extract | 90 | Spherical | −36.7 |
| [136] |
| Dimocarpus Longan Lour., peel extract | 32 | Cubic | N.I. |
| [137] |
| Taxus yunnanensis extract | 27 | Crystal | −28 |
| [138] |
| Cyperus conglomeratus root extract | 468 | Spherical | N.I. |
| [139] |
| Lyngbya majuscula strain | 149 | Spherical | −35.2 |
| [140] |
| Walnut green husk aqueous extract | 31 | Spherical | −33.8 |
| [141] |
| Talaromyces purpurogenus extracellular pigment | 41 | −24.8 |
| [142] | |
| Actinobacterial strain SH11 | 16 | Spherical | −17.1 |
| [143] |
| Nocardiopsis sp. MBRC-1 | 45 | Spherical | N.I. |
| [144] |
| Penicillium shearii AJP05 | 8.0 | Spherical | N.I. |
| [145] |
| Noble Metal | Precursor Salt | Reducing Agent | Shape | Activities | Reference |
|---|---|---|---|---|---|
| Silver | AgNO3 | Populus nigra L. extract | Spherical, rhombohedral, and triangular |
| [178] |
| Silver | AgNO3 | Phoenix dactylifera extract | Spherical |
| [179] |
| Silver | AgNO3 | Botryodiplodia theobromae mycelium | N.I. |
| [180] |
| Silver | AgNO3 | Azadirachta indica extract | Spherical |
| [181] |
| Silver | AgNO3 | Cleistanthus collinus extract | Spherical |
| [182] |
| Silver | AgNO3 | Thelypteris glandulosolanosa extract | Spherical |
| [183] |
| Silver | AgNO3 | Origanum vulgare | Spherical |
| [184] |
| Silver | AgNO3 | Salvia coccinea, Salvia leucantha, Salvia splendens extracts | Spherical |
| [185] |
| Silver | AgNO3 | Cratoxylum formosum Mucuna birdwoodiana Lindera strychnifolia extracts | Spherical |
| [186] |
| Silver | AgNO3 | Juniperus chinensis extract | Spherical |
| [177] |
| Silver | AgNO3 | Gloriosa superba L. extract | Spherical |
| [187] |
| Silver | AgNO3 | Caulerpa taxifolia extract | Spherical |
| [188] |
| Silver | AgNO3 | Toxicodendron vernicifluum extract | Spherical and oval |
| [174] |
| Silver | AgNO3 | Dendropanax morbifera Léveille extract | N.I. |
| [189] |
| Silver | AgNO3 | Avicennia marina extract | Spherical |
| [190] |
| Silver | AgNO3 | Tabebuiaroseo-alba extract | Spherical |
| [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía-Méndez, J.L.; López-Mena, E.R.; Sánchez-Arreola, E. Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review. Biomedicines 2023, 11, 389. https://doi.org/10.3390/biomedicines11020389
Mejía-Méndez JL, López-Mena ER, Sánchez-Arreola E. Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review. Biomedicines. 2023; 11(2):389. https://doi.org/10.3390/biomedicines11020389
Chicago/Turabian StyleMejía-Méndez, Jorge L., Edgar R. López-Mena, and Eugenio Sánchez-Arreola. 2023. "Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review" Biomedicines 11, no. 2: 389. https://doi.org/10.3390/biomedicines11020389
APA StyleMejía-Méndez, J. L., López-Mena, E. R., & Sánchez-Arreola, E. (2023). Activities against Lung Cancer of Biosynthesized Silver Nanoparticles: A Review. Biomedicines, 11(2), 389. https://doi.org/10.3390/biomedicines11020389







