Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity
Abstract
1. Introduction
2. Founding Studies to Connect PAKs with Human Cancer
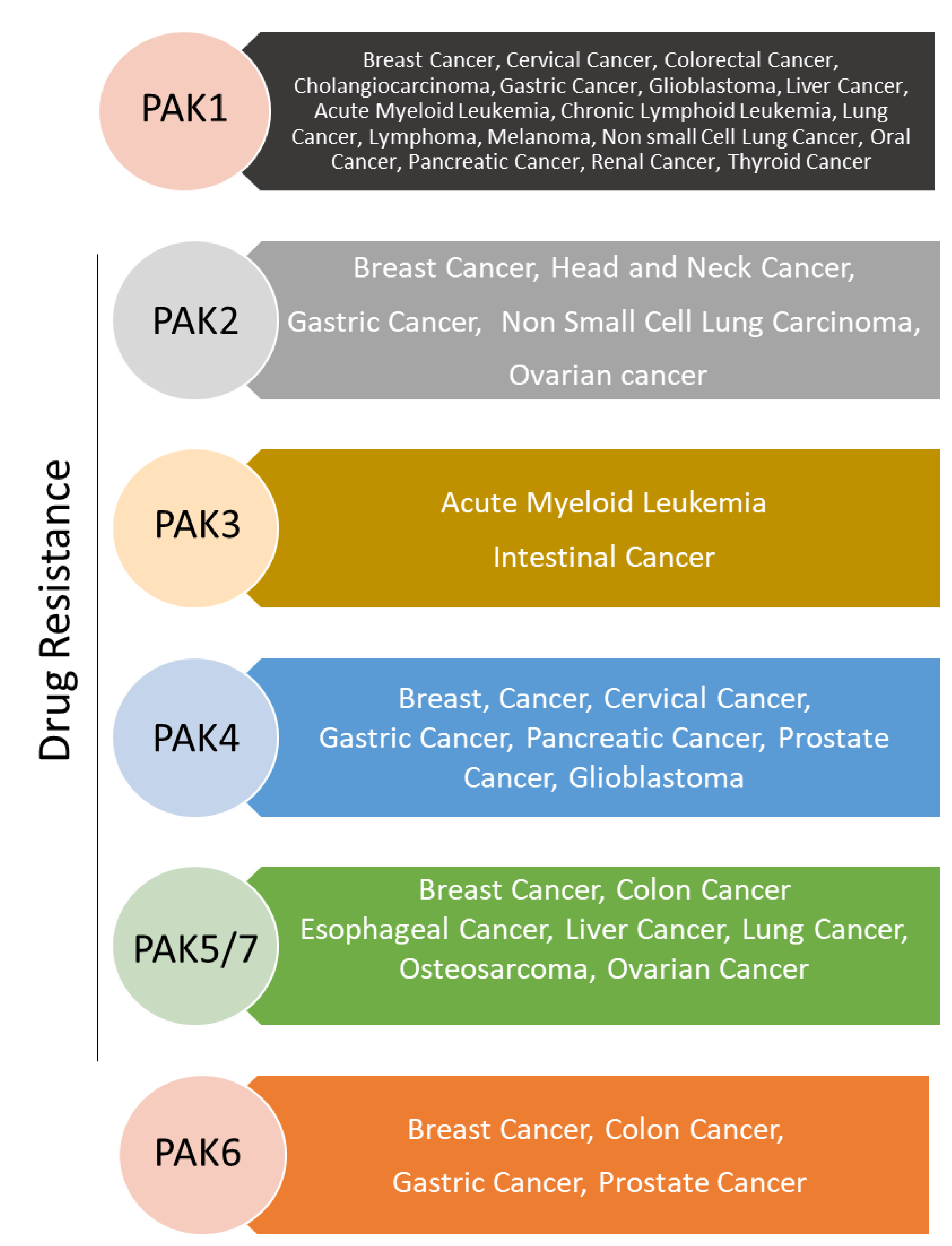
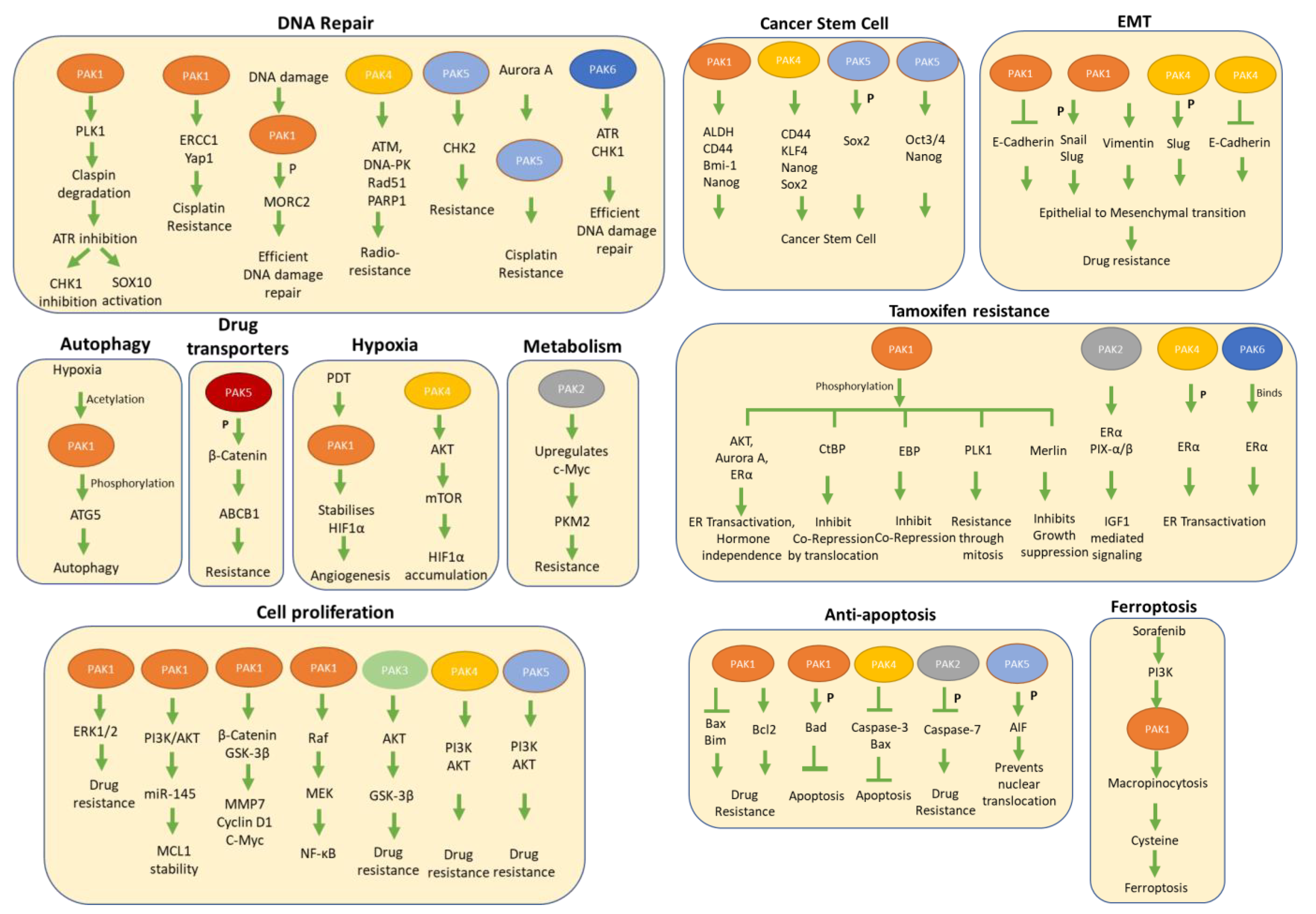
3. Activation Status of PAK Signaling and Therapeutic Sensitivity
3.1. PAK1 Signaling and Therapeutic Resistance
3.2. PAK2 Signaling in Therapeutic Resistance
3.3. PAK3 in Drug Resistance
3.4. PAK4 in Drug Resistance
3.5. PAK5/7 in Drug Resistance
3.6. PAK6 in Drug Resistance
4. Emerging PAK-Targeting Strategies
5. PAK-Directed Clinical Trials
6. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lonard, D.M.; Kumar, R.; O’Malley, B.W. Minireview: The SRC family of coactivators: An entree to understanding a subset of polygenic diseases? Mol. Endocrinol. 2010, 24, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, J.; Cyanam, D.; Mudvari, P.; Reddy, S.D.; Pakala, S.B.; Nair, S.S.; Florea, L.; Fuqua, S.A.; Godbole, S.; Kumar, R. Transcriptomic landscape of breast cancers through mRNA sequencing. Sci. Rep. 2012, 2, 264. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.; McLeod, H.L. Cancer pharmacogenetics. Br. J. Cancer 2004, 90, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Amjesh, R.; George, B.; Sankaran, D.; Sandiford, O.A.; Rameshwar, P.; Pillai, M.R.; Kumar, R. The Revelation of Continuously Organized, Co-Overexpressed Protein-Coding Genes with Roles in Cellular Communications in Breast Cancer. Cells 2022, 11, 3806. [Google Scholar] [CrossRef]
- Kumar, R.; Hung, M.C. Signaling intricacies take center stage in cancer cells. Cancer Res. 2005, 65, 2511–2515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; George, B.; Campbell, M.R.; Verma, N.; Paul, A.M.; Melo-Alvim, C.; Ribeiro, L.; Pillai, M.R.; da Costa, L.M.; Moasser, M.M. HER family in cancer progression: From discovery to 2020 and beyond. Adv. Cancer Res. 2020, 147, 109–160. [Google Scholar] [CrossRef]
- Ghosh, S.; Marrocco, I.; Yarden, Y. Roles for receptor tyrosine kinases in tumor progression and implications for cancer treatment. Adv. Cancer Res. 2020, 147, 1–57. [Google Scholar] [CrossRef]
- Kumar, R.; Mendelsohn, J. Polypeptide growth factors in the regulation of human tumor cell proliferation. Curr. Opin. Oncol. 1991, 3, 70–74. [Google Scholar] [CrossRef]
- Barnes, C.J.; Kumar, R. Epidermal growth factor receptor family tyrosine kinases as signal integrators and therapeutic targets. Cancer Metastasis Rev. 2003, 22, 301–307. [Google Scholar] [CrossRef]
- Mendelsohn, J. Jeremiah Metzger Lecture. Targeted cancer therapy. Trans. Am. Clin. Climatol. Assoc. 2000, 111, 95–110; discussion 110–111. [Google Scholar]
- Mendelsohn, J. Personalizing oncology: Perspectives and prospects. J. Clin. Oncol. 2013, 31, 1904–1911. [Google Scholar] [CrossRef]
- Kumar, R.; de Vijver, M.V.; Tortora, G.; Ciardiello, F.; Goldkorn, T.; Miller, W.H., Jr.; Norton, L. A Tribute to John Mendelsohn: A Pioneer in Targeted Cancer Therapy. Cancer Res. 2019, 79, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Murad, F.; Bogler, O.; O’Malley, B.W.; Hortobagyi, G.N. John Mendelsohn: A visionary scientist, oncologist and leader. Genes Cancer 2019, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. ErbB-dependent signaling as a determinant of trastuzumab resistance. Clin. Cancer Res. 2007, 13, 4657–4659. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gururaj, A.E.; Barnes, C.J. p21-activated kinases in cancer. Nat. Rev. Cancer 2006, 6, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Molli, P.R.; Li, D.Q.; Murray, B.W.; Rayala, S.K.; Kumar, R. PAK signaling in oncogenesis. Oncogene 2009, 28, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Li, D.Q. PAKs in Human Cancer Progression: From Inception to Cancer Therapeutic to Future Oncobiology. Adv. Cancer Res. 2016, 130, 137–209. [Google Scholar] [CrossRef]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, biochemistry, and biology of PAK kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef]
- Adam, L.; Vadlamudi, R.; Kondapaka, S.B.; Chernoff, J.; Mendelsohn, J.; Kumar, R. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 1998, 273, 28238–28246. [Google Scholar] [CrossRef]
- Bekri, S.; Adelaide, J.; Merscher, S.; Grosgeorge, J.; Caroli-Bosc, F.; Perucca-Lostanlen, D.; Kelley, P.M.; Pebusque, M.J.; Theillet, C.; Birnbaum, D.; et al. Detailed map of a region commonly amplified at 11q13-->q14 in human breast carcinoma. Cytogenet. Cell Genet. 1997, 79, 125–131. [Google Scholar] [CrossRef]
- Adam, L.; Vadlamudi, R.; Mandal, M.; Chernoff, J.; Kumar, R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem. 2000, 275, 12041–12050. [Google Scholar] [CrossRef]
- Vadlamudi, R.K.; Adam, L.; Wang, R.A.; Mandal, M.; Nguyen, D.; Sahin, A.; Chernoff, J.; Hung, M.C.; Kumar, R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J. Biol. Chem. 2000, 275, 36238–36244. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Adam, L.; Vadlamudi, R.K.; Zhou, H.; Sen, S.; Chernoff, J.; Mandal, M.; Kumar, R. p21-activated kinase 1 interacts with and phosphorylates histone H3 in breast cancer cells. EMBO Rep. 2002, 3, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.A.; Vadlamudi, R.K.; Bagheri-Yarmand, R.; Beuvink, I.; Hynes, N.E.; Kumar, R. Essential functions of p21-activated kinase 1 in morphogenesis and differentiation of mammary glands. J. Cell Biol. 2003, 161, 583–592. [Google Scholar] [CrossRef]
- Barnes, C.J.; Bagheri, R.; Mandal, M.; Yang, Z.; Clayman, G.L.; Hong, W.K.; Kumar, R. Suppression of Epidermal Growth Factor Receptor, Mitogen-activated Protein Kinase, and Pak1 Pathways and Invasiveness of Human Cutaneous Squamous Cancer Cells by the Tyrosine Kinase Inhibitor ZD1839 (Iressa). Mol. Cancer Thera 2003, 2, 345–351. [Google Scholar]
- George, B.; Pillai, P.M.; Paul, A.M.; Amjesh, R.; Leitzel, K.; Ali, S.M.; Sandiford, O.; Lipton, A.; Rameshwar, P.; Hortobagyi, G.N.; et al. Cellular Fitness Phenotypes of Cancer Target Genes from Oncobiology to Cancer Therapeutics. Cells 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Behan, F.M.; Iorio, F.; Picco, G.; Gonçalves, E.; Beaver, C.M.; Migliardi, G.; Santos, R.; Rao, Y.; Sassi, F.; Pinnelli, M.; et al. Prioritization of Cancer Therapeutic Targets Using CRISPR–Cas9 Screens. Nature 2019, 568, 511–516. [Google Scholar] [CrossRef]
- Kumar, R.; Paul, A.M.; Amjesh, R.; George, B.; Pillai, M.R. Coordinated dysregulation of cancer progression by the HER family and p21-activated kinases. Cancer Metastasis Rev. 2020, 39, 583–601. [Google Scholar] [CrossRef]
- Rayala, S.K.; Talukder, A.H.; Balasenthil, S.; Tharakan, R.; Barnes, C.J.; Wang, R.A.; Aldaz, C.M.; Khan, S.; Kumar, R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006, 66, 1694–1701. [Google Scholar] [CrossRef]
- Yao, D.; Li, C.; Rajoka, M.S.R.; He, Z.; Huang, J.; Wang, J.; Zhang, J. P21-Activated Kinase 1: Emerging biological functions and potential therapeutic targets in Cancer. Theranostics 2020, 10, 9741–9766. [Google Scholar] [CrossRef]
- Kastrati, I.; Semina, S.; Gordon, B.; Smart, E. Insights into how phosphorylation of estrogen receptor at serine 305 modulates tamoxifen activity in breast cancer. Mol. Cell Endocrinol. 2019, 483, 97–101. [Google Scholar] [CrossRef]
- Yao, J.; Deng, K.; Huang, J.; Zeng, R.; Zuo, J. Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer. Front. Pharmacol. 2020, 11, 592912. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.; Semenova, G.; Kosoff, R.; Chernoff, J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 2014, 14, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Akinmade, D.; Talukder, A.H.; Zhang, Y.; Luo, W.M.; Kumar, R.; Hamburger, A.W. Phosphorylation of the ErbB3 binding protein Ebp1 by p21-activated kinase 1 in breast cancer cells. Br. J. Cancer 2008, 98, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Ruff, E.F.; Muretta, J.M.; Thompson, A.R.; Lake, E.W.; Cyphers, S.; Albanese, S.K.; Hanson, S.M.; Behr, J.M.; Thomas, D.D.; Chodera, J.D.; et al. A dynamic mechanism for allosteric activation of Aurora kinase A by activation loop phosphorylation. Elife 2018, 7, e32776. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Guo, J.P.; Yang, H.; Kanai, M.; He, L.L.; Li, Y.Y.; Koomen, J.M.; Minton, S.; Gao, M.; Ren, X.B.; et al. Aurora-A is a determinant of tamoxifen sensitivity through phosphorylation of ERalpha in breast cancer. Oncogene 2014, 33, 4985–4996. [Google Scholar] [CrossRef]
- Le Romancer, M.; Poulard, C.; Cohen, P.; Sentis, S.; Renoir, J.M.; Corbo, L. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr. Rev. 2011, 32, 597–622. [Google Scholar] [CrossRef]
- Ghosh, A.; Awasthi, S.; Peterson, J.R.; Hamburger, A.W. Regulation of tamoxifen sensitivity by a PAK1-EBP1 signalling pathway in breast cancer. Br. J. Cancer 2013, 108, 557–563. [Google Scholar] [CrossRef]
- Rajendran, S.; Swaroop, S.S.; Roy, J.; Inemai, E.; Murugan, S.; Rayala, S.K.; Venkatraman, G. p21 activated kinase-1 and tamoxifen—A deadly nexus impacting breast cancer outcomes. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188668. [Google Scholar] [CrossRef]
- Jeong, S.B.; Im, J.H.; Yoon, J.H.; Bui, Q.T.; Lim, S.C.; Song, J.M.; Shim, Y.; Yun, J.; Hong, J.; Kang, K.W. Essential Role of Polo-like Kinase 1 (Plk1) Oncogene in Tumor Growth and Metastasis of Tamoxifen-Resistant Breast Cancer. Mol. Cancer Ther. 2018, 17, 825–837. [Google Scholar] [CrossRef]
- Nair, S.S.; Mishra, S.K.; Yang, Z.; Balasenthil, B.; Kumar, R.; Vadlamudi, R.K. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004, 64, 6416–6423. [Google Scholar] [CrossRef]
- Li, D.Q.; Nair, S.S.; Ohshiro, K.; Kumar, A.; Nair, V.S.; Pakala, S.B.; Reddy, S.D.; Gajula, R.P.; Eswaran, J.; Aravind, L.; et al. MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response. Cell Rep. 2012, 2, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Korobeynikov, V.; Borakove, M.; Feng, Y.; Wuest, W.M.; Koval, A.B.; Nikonova, A.S.; Serebriiskii, I.; Chernoff, J.; Borges, V.F.; Golemis, E.A.; et al. Combined inhibition of Aurora A and p21-activated kinase 1 as a new treatment strategy in breast cancer. Breast Cancer Res. Treat 2019, 177, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, H.S.; Kim, S.L.; Lee, D.S. The PAK1-Stat3 Signaling Pathway Activates IL-6 Gene Transcription and Human Breast Cancer Stem Cell Formation. Cancers 2019, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Youn, H.; Kwon, T.; Son, B.; Kang, J.; Yang, H.J.; Seong, K.M.; Kim, W.; Youn, B. PAK1 tyrosine phosphorylation is required to induce epithelial-mesenchymal transition and radioresistance in lung cancer cells. Cancer Res. 2014, 74, 5520–5531. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yi, P.; Wang, H.; Xia, L.; Han, Y.; Wang, H.; Zeng, B.; Tang, L.; Pan, Q.; Tian, Y.; et al. RAC1 Involves in the Radioresistance by Mediating Epithelial-Mesenchymal Transition in Lung Cancer. Front. Oncol. 2020, 10, 649. [Google Scholar] [CrossRef]
- Ito, M.; Codony-Servat, C.; Codony-Servat, J.; Llige, D.; Chaib, I.; Sun, X.; Miao, J.; Sun, R.; Cai, X.; Verlicchi, A.; et al. Targeting PKCiota-PAK1 signaling pathways in EGFR and KRAS mutant adenocarcinoma and lung squamous cell carcinoma. Cell Commun Signal 2019, 17, 137. [Google Scholar] [CrossRef]
- Chen, M.J.; Wu, D.W.; Wang, Y.C.; Chen, C.Y.; Lee, H. PAK1 confers chemoresistance and poor outcome in non-small cell lung cancer via beta-catenin-mediated stemness. Sci. Rep. 2016, 6, 34933. [Google Scholar] [CrossRef]
- Jin, R.; Wang, X.; Zang, R.; Liu, C.; Zheng, S.; Li, H.; Sun, N.; He, J. Desmoglein-2 modulates tumor progression and osimertinib drug resistance through the EGFR/Src/PAK1 pathway in lung adenocarcinoma. Cancer Lett. 2020, 483, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.W.; Wu, T.C.; Chen, C.Y.; Lee, H. PAK1 Is a Novel Therapeutic Target in Tyrosine Kinase Inhibitor-Resistant Lung Adenocarcinoma Activated by the PI3K/AKT Signaling Regardless of EGFR Mutation. Clin. Cancer Res. 2016, 22, 5370–5382. [Google Scholar] [CrossRef]
- Liu, W.W.; Hu, J.; Wang, R.; Han, Q.; Liu, Y.; Wang, S. Cytoplasmic P120ctn Promotes Gefitinib Resistance in Lung Cancer Cells by Activating PAK1 and ERK Pathway. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Baldwin, G.S.; Nikfarjam, M.; He, H. Antitumor effects of all-trans retinoic acid and its synergism with gemcitabine are associated with downregulation of p21-activated kinases in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G632–G640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jubb, A.M.; Lyle, K.; Xiao, Q.; Ong, C.C.; Desai, R.; Fu, L.; Gnad, F.; Song, Q.; Haverty, P.M.; et al. PAK1 mediates pancreatic cancer cell migration and resistance to MET inhibition. J. Pathol. 2014, 234, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Aruri, J.; Kapadia, R.; Mehr, H.; White, M.A.; Ganesan, A.K. RhoJ regulates melanoma chemoresistance by suppressing pathways that sense DNA damage. Cancer Res. 2012, 72, 5516–5528. [Google Scholar] [CrossRef]
- Babagana, M.; Johnson, S.; Slabodkin, H.; Bshara, W.; Morrison, C.; Kandel, E.S. P21-activated kinase 1 regulates resistance to BRAF inhibition in human cancer cells. Mol. Carcinog. 2017, 56, 1515–1525. [Google Scholar] [CrossRef]
- McGarry, D.J.; Castino, G.; Lilla, S.; Carnet, A.; Kelly, L.; Micovic, K.; Zanivan, S.; Olson, M.F. MICAL1 activation by PAK1 mediates actin filament disassembly. Cell Rep. 2022, 41, 111442. [Google Scholar] [CrossRef]
- Luo, H.; Yi, T.; Huang, D.; Chen, X.; Li, X.; Wan, Q.; Huang, H.; Huang, H.; Wei, H.; Song, Y.; et al. circ_PTN contributes to -cisplatin resistance in glioblastoma via PI3K/AKT signaling through the miR-542-3p/PIK3R3 pathway. Mol. Ther. Nucleic Acids 2021, 26, 1255–1269. [Google Scholar] [CrossRef]
- Liu, J.; Ren, G.; Li, K.; Liu, Z.; Wang, Y.; Chen, T.; Mu, W.; Yang, X.; Li, X.; Shi, A.; et al. The Smad4-MYO18A-PP1A complex regulates beta-catenin phosphorylation and pemigatinib resistance by inhibiting PAK1 in cholangiocarcinoma. Cell Death Differ 2022, 29, 818–831. [Google Scholar] [CrossRef]
- Advani, S.J.; Camargo, M.F.; Seguin, L.; Mielgo, A.; Anand, S.; Hicks, A.M.; Aguilera, J.; Franovic, A.; Weis, S.M.; Cheresh, D.A. Kinase-independent role for CRAF-driving tumour radioresistance via CHK2. Nat. Commun. 2015, 6, 8154. [Google Scholar] [CrossRef]
- Aoki, H.; Yokoyama, T.; Fujiwara, K.; Tari, A.M.; Sawaya, R.; Suki, D.; Hess, K.R.; Aldape, K.D.; Kondo, S.; Kumar, R.; et al. Phosphorylated Pak1 level in the cytoplasm correlates with shorter survival time in patients with glioblastoma. Clin. Cancer Res. 2007, 13, 6603–6609. [Google Scholar] [CrossRef]
- Venu, A.; Archana, B.; Kanumuri, R.; Vuttaradhi, V.K.; D’Cruze, L.; Murugan, S.; Ganesh, K.; Prathiba, D.; Dymova, M.A.; Rayala, S.K.; et al. Clinical Evaluation of P21 Activated Kinase 1 (PAK1) Activation in Gliomas and Its Effect on Cell Proliferation. Cancer Investig. 2021, 39, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, H.; Meng, L.; Song, H.; Zhou, Q.; Qu, C.; Zhao, P.; Li, Q.; Zou, C.; Liu, X.; et al. Hypoxia-induced acetylation of PAK1 enhances autophagy and promotes brain tumorigenesis via phosphorylating ATG5. Autophagy 2021, 17, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.K.; Lee, S.; Kang, G.W.; Lee, Y.R.; Park, S.Y.; Song, I.S.; Yun, J.W.; Lee, J.; Choi, Y.K.; Park, K.G. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jia, R.; Li, W.; Zhou, Y.; Guo, D.; Teng, Q.; Du, S.; Li, M.; Li, W.; Sun, T.; et al. PAK1 Mediates Bone Marrow Stromal Cell-Induced Drug Resistance in Acute Myeloid Leukemia via ERK1/2 Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 686695. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Fan, L.; Tang, H.; Zuo, X.; Gu, D.; Lu, X.; Li, Y.; Wu, J.; Qin, S.; et al. Exploring the significance of PAK1 through chromosome conformation signatures in ibrutinib-resistant chronic lymphocytic leukaemia. Mol. Oncol. 2022, 16, 2920–2935. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.; McKinney, M.S.; Love, C.; Liu, Q.; Fan, A.; Patel, A.; Smith, J.; Beaven, A.; Jima, D.D.; Dave, S.S. PAK1 mediates resistance to PI3K inhibition in lymphomas. Clin. Cancer Res. 2013, 19, 1106–1115. [Google Scholar] [CrossRef]
- Lin, X.J.; He, C.L.; Sun, T.; Duan, X.J.; Sun, Y.; Xiong, S.J. hsa-miR-485-5p reverses epithelial to mesenchymal transition and promotes cisplatin-induced cell death by targeting PAK1 in oral tongue squamous cell carcinoma. Int. J. Mol. Med. 2017, 40, 83–89. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Xu, L.; An, H.; Liu, W.; Liu, Y.; Lin, Z.; Xu, J. p21-activated kinase 1 determines stem-like phenotype and sunitinib resistance via NF-kappaB/IL-6 activation in renal cell carcinoma. Cell Death Dis. 2015, 6, e1637. [Google Scholar] [CrossRef]
- Knippler, C.M.; Saji, M.; Rajan, N.; Porter, K.; La Perle, K.M.D.; Ringel, M.D. MAPK- and AKT-activated thyroid cancers are sensitive to group I PAK inhibition. Endocr. Relat. Cancer 2019, 26, 699–712. [Google Scholar] [CrossRef]
- Xu, J.; Ma, X.; Yang, H.; Zhang, J.; Cai, G.; Yao, N. MiR-509-3p Induces Apoptosis and Affects the Chemosensitivity of Cervical Cancer Cells by Targeting the RAC1/PAK1/LIMK1/Cofilin Pathway. Chem. Pharm. Bull. 2021, 69, 325–332. [Google Scholar] [CrossRef]
- Huynh, N.; Shulkes, A.; Baldwin, G.; He, H. Up-regulation of stem cell markers by P21-activated kinase 1 contributes to 5-fluorouracil resistance of colorectal cancer. Cancer Biol Ther 2016, 17, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.; Wang, K.; Yim, M.; Dumesny, C.J.; Sandrin, M.S.; Baldwin, G.S.; Nikfarjam, M.; He, H. Depletion of p21-activated kinase 1 up-regulates the immune system of APC(∆14/+) mice and inhibits intestinal tumorigenesis. BMC Cancer 2017, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wester, L.; He, J.; Geiger, T.; Moerkens, M.; Siddappa, R.; Helmijr, J.A.; Timmermans, M.M.; Look, M.P.; van Deurzen, C.H.M.; et al. IGF1R signaling drives antiestrogen resistance through PAK2/PIX activation in luminal breast cancer. Oncogene 2018, 37, 1869–1884. [Google Scholar] [CrossRef]
- Chang, Y.; Park, K.H.; Lee, J.E.; Han, K.C. Phosphoproteomic analysis reveals PAK2 as a therapeutic target for lapatinib resistance in HER2-positive breast cancer cells. Biochem. Biophys. Res. Commun. 2018, 505, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, W.; Liu, K.; Zhu, F.; Malakhova, M.; Peng, C.; Li, T.; Kim, H.G.; Ma, W.; Cho, Y.Y.; et al. Phosphorylation of caspase-7 by p21-activated protein kinase (PAK) 2 inhibits chemotherapeutic drug-induced apoptosis of breast cancer cell lines. J. Biol. Chem. 2011, 286, 22291–22299. [Google Scholar] [CrossRef]
- Eron, S.J.; Raghupathi, K.; Hardy, J.A. Dual Site Phosphorylation of Caspase-7 by PAK2 Blocks Apoptotic Activity by Two Distinct Mechanisms. Structure 2017, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.X.; Ma, J.X.; Zhang, J.; Guo, Y.; Mueller, M.D.; Remick, S.C.; Yu, J.J. Prostasin may contribute to chemoresistance, repress cancer cells in ovarian cancer, and is involved in the signaling pathways of CASP/PAK2-p34/actin. Cell Death Dis. 2014, 5, e995. [Google Scholar] [CrossRef]
- Gupta, A.; Ajith, A.; Singh, S.; Panday, R.K.; Samaiya, A.; Shukla, S. PAK2-c-Myc-PKM2 axis plays an essential role in head and neck oncogenesis via regulating Warburg effect. Cell Death Dis. 2018, 9, 825. [Google Scholar] [CrossRef]
- Cho, H.J.; Baek, K.E.; Park, S.M.; Kim, I.K.; Choi, Y.L.; Cho, H.J.; Nam, I.K.; Hwang, E.M.; Park, J.Y.; Han, J.Y.; et al. RhoGDI2 expression is associated with tumor growth and malignant progression of gastric cancer. Clin. Cancer Res. 2009, 15, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, F. p21-Activated Kinase: Role in Gastrointestinal Cancer and Beyond. Cancers 2022, 14, 4736. [Google Scholar] [CrossRef]
- Yi, Y.; Li, P.; Huang, Y.; Chen, D.; Fan, S.; Wang, J.; Yang, M.; Zeng, S.; Deng, J.; Lv, X.; et al. P21-activated kinase 2-mediated beta-catenin signaling promotes cancer stemness and osimertinib resistance in EGFR-mutant non-small-cell lung cancer. Oncogene 2022, 41, 4318–4329. [Google Scholar] [CrossRef]
- Zuo, Z.; Ji, S.; He, L.; Zhang, Y.; Peng, Z.; Han, J. LncRNA TTN-AS1/miR-134-5p/PAK3 axis regulates the radiosensitivity of human large intestine cancer cells through the P21 pathway and AKT/GSK-3beta/beta-catenin pathway. Cell Biol. Int. 2020, 44, 2284–2292. [Google Scholar] [CrossRef]
- Quan, L.; Cheng, Z.; Dai, Y.; Jiao, Y.; Shi, J.; Fu, L. Prognostic significance of PAK family kinases in acute myeloid leukemia. Cancer Gene Ther. 2020, 27, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Li, Y.; Muqbil, I.; Aboukameel, A.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Philip, P.A.; Azmi, A.S. Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases 2019, 10, 367–377. [Google Scholar] [CrossRef]
- Tyagi, N.; Marimuthu, S.; Bhardwaj, A.; Deshmukh, S.K.; Srivastava, S.K.; Singh, A.P.; McClellan, S.; Carter, J.E.; Singh, S. p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett. 2016, 370, 260–267. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Dumesny, C.; Ang, C.S.; Dong, L.; Ma, Y.; Zeng, J.; Nikfarjam, M. A novel PAK4 inhibitor suppresses pancreatic cancer growth and enhances the inhibitory effect of gemcitabine. Transl. Oncol. 2022, 16, 101329. [Google Scholar] [CrossRef]
- Mpilla, G.; Aboukameel, A.; Muqbil, I.; Kim, S.; Beydoun, R.; Philip, P.A.; Mohammad, R.M.; Kamgar, M.; Shidham, V.; Senapedis, W.; et al. PAK4-NAMPT Dual Inhibition as a Novel Strategy for Therapy Resistant Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 1902. [Google Scholar] [CrossRef]
- Park, J.J.; Park, M.H.; Oh, E.H.; Soung, N.K.; Lee, S.J.; Jung, J.K.; Lee, O.J.; Yun, S.J.; Kim, W.J.; Shin, E.Y.; et al. The p21-activated kinase 4-Slug transcription factor axis promotes epithelial-mesenchymal transition and worsens prognosis in prostate cancer. Oncogene 2018, 37, 5147–5159. [Google Scholar] [CrossRef]
- Kim, H.; Woo, D.J.; Kim, S.Y.; Yang, E.G. p21-activated kinase 4 regulates HIF-1alpha translation in cancer cells. Biochem. Biophys. Res. Commun. 2017, 486, 270–276. [Google Scholar] [CrossRef]
- Fu, X.; Feng, J.; Zeng, D.; Ding, Y.; Yu, C.; Yang, B. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci. Rep. 2014, 34, e00094. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Zhu, J.; Li, Z.; Lorent, J.; Zhao, C.; Dahlman-Wright, K.; Stromblad, S. p21-activated kinase group II small compound inhibitor GNE-2861 perturbs estrogen receptor alpha signaling and restores tamoxifen-sensitivity in breast cancer cells. Oncotarget 2015, 6, 43853–43868. [Google Scholar] [CrossRef] [PubMed]
- Blankenstein, L.J.; Cordes, N.; Kunz-Schughart, L.A.; Vehlow, A. Targeting of p21-Activated Kinase 4 Radiosensitizes Glioblastoma Cells via Impaired DNA Repair. Cells 2022, 11, 2133. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, X.; He, Y.; Tian, Y.; Pan, W.; Xu, L.; Ma, Y.; Gao, Y.; Gao, J.; Qi, Y.; et al. P21-activated kinase 7 (PAK7) interacts with and activates Wnt/beta-catenin signaling pathway in breast cancer. J. Cancer 2018, 9, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Han, F.; Li, L.; Ge, Y.; Lin, W.; Wang, J.; Wu, L.; Zhao, G.; Deng, Y.; Zhang, J. Deubiquitinating enzyme 4 facilitates chemoresistance in glioblastoma by inhibiting P53 activity. Oncol. Lett. 2019, 17, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Li, Y.; Zhang, W.; Wang, F.; Wang, X.; Jin, Z.; Xing, Y.; Li, D.; Zhang, H.; Li, Y.; et al. A PAK5-DNPEP-USP4 axis dictates breast cancer growth and metastasis. Int. J. Cancer 2020, 146, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, Y.; Hu, B.; Han, F.; Zhao, X.; Zhang, H.; Li, Y.; Li, D.; Li, J.; Jin, F.; et al. PAK5-mediated AIF phosphorylation inhibits its nuclear translocation and promotes breast cancer tumorigenesis. Int. J. Biol. Sci. 2021, 17, 1315–1327. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, Y.; Wang, C.; Li, X.; Cui, X.; Tu, S.; You, L.; Fu, J.; Chen, Z.; Hu, W.; et al. PAK5 facilitates the proliferation, invasion and migration in colorectal cancer cells. Cancer Med. 2020, 9, 4777–4790. [Google Scholar] [CrossRef]
- Gong, W.; An, Z.; Wang, Y.; Pan, X.; Fang, W.; Jiang, B.; Zhang, H. P21-activated kinase 5 is overexpressed during colorectal cancer progression and regulates colorectal carcinoma cell adhesion and migration. Int. J. Cancer 2009, 125, 548–555. [Google Scholar] [CrossRef]
- Gu, J.; Li, K.; Li, M.; Wu, X.; Zhang, L.; Ding, Q.; Wu, W.; Yang, J.; Mu, J.; Wen, H.; et al. A role for p21-activated kinase 7 in the development of gastric cancer. FEBS J. 2013, 280, 46–55. [Google Scholar] [CrossRef]
- Li, D.; Yao, X.; Zhang, P. The overexpression of P21-activated kinase 5 (PAK5) promotes paclitaxel-chemoresistance of epithelial ovarian cancer. Mol. Cell Biochem. 2013, 383, 191–199. [Google Scholar] [CrossRef]
- He, S.; Feng, M.; Liu, M.; Yang, S.; Yan, S.; Zhang, W.; Wang, Z.; Hu, C.; Xu, Q.; Chen, L.; et al. P21-activated kinase 7 mediates cisplatin-resistance of esophageal squamous carcinoma cells with Aurora-A overexpression. PLoS ONE 2014, 9, e113989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Zhang, J.; Mao, L.L.; Wu, J.X.; Cao, W.J.; Zheng, J.N.; Pei, D.S. p21-Activated kinase 5 affects cisplatin-induced apoptosis and proliferation in hepatocellular carcinoma cells. Tumour. Biol. 2015, 36, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Gong, C.C.; Wu, X.J.; Ren, X.; Sedaka, R.S.; Chen, W.C.; Huo, F.C.; Chen, C.; Du, W.Q.; Pei, D.S. P21-activated kinase 5 potentiates the chemoresistant phenotype of liver cancer. Signal Transduct Target Ther. 2021, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.F.; Kong, L.T. Inhibiting p21-activated kinase (PAK7) enhances radiosensitivity in hepatocellular carcinoma. Hum. Exp. Toxicol. 2021, 40, 2202–2214. [Google Scholar] [CrossRef]
- Bao, Z.; Ji, W.; Yang, Y.; Chen, Z.; Li, Z.; Wang, K.; Lu, T.; Yu, Y.; Xia, W.; Lu, S. PAK5 promotes the cell stemness ability by phosphorylating SOX2 in lung squamous cell carcinomas. Exp. Cell Res. 2020, 395, 112187. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, B.; Zhou, W. Correlation between chemotherapy resistance in osteosarcoma patients and PAK5 and Ezrin gene expression. Oncol. Lett. 2018, 15, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, Y.; Huang, Y.; Zhang, P.; Fang, X. PAK5 Induces EMT and Promotes Cell Migration and Invasion by Activating the PI3K/AKT Pathway in Ovarian Cancer. Anal. Cell Pathol. 2018, 2018, 8073124. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.; Yan, D.; Cui, F.; Wang, X.; Yu, F.; Xue, Y.; Feng, X.; Wang, J.; Wang, X.; et al. PAK6 increase chemoresistance and is a prognostic marker for stage II and III colon cancer patients undergoing 5-FU based chemotherapy. Oncotarget 2015, 6, 355–367. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, W.; Li, T.; Hu, Y.; Chen, S.; Xi, S.; Wen, Y.; Huang, L.; Zhao, L.; Xiao, C.; et al. Prognostic and Predictive Value of p21-activated Kinase 6 Associated Support Vector Machine Classifier in Gastric Cancer Treated by 5-fluorouracil/Oxaliplatin Chemotherapy. EBioMedicine 2017, 22, 78–88. [Google Scholar] [CrossRef]
- Huang, W.; Han, Z.; Sun, Z.; Feng, H.; Zhao, L.; Yuan, Q.; Chen, C.; Yu, S.; Hu, Y.; Yu, J.; et al. PAK6 promotes homologous-recombination to enhance chemoresistance to oxaliplatin through ATR/CHK1 signaling in gastric cancer. Cell Death Dis. 2022, 13, 658. [Google Scholar] [CrossRef]
- Zhang, M.; Siedow, M.; Saia, G.; Chakravarti, A. Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate 2010, 70, 807–816. [Google Scholar] [CrossRef]
- Lee, S.R.; Ramos, S.M.; Ko, A.; Masiello, D.; Swanson, K.D.; Lu, M.L.; Balk, S.P. AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 2002, 16, 85–99. [Google Scholar] [CrossRef]
- Marchetti, C.; Zyner, K.G.; Ohnmacht, S.A.; Robson, M.; Haider, S.M.; Morton, J.P.; Marsico, G.; Vo, T.; Laughlin-Toth, S.; Ahmed, A.A.; et al. Targeting Multiple Effector Pathways in Pancreatic Ductal Adenocarcinoma with a G-Quadruplex-Binding Small Molecule. J. Med. Chem. 2018, 61, 2500–2517. [Google Scholar] [CrossRef]
- Zyner, K.G.; Simeone, A.; Flynn, S.M.; Doyle, C.; Marsico, G.; Adhikari, S.; Portella, G.; Tannahill, D.; Balasubramanian, S. G-quadruplex DNA structures in human stem cells and differentiation. Nat. Commun. 2022, 13, 142. [Google Scholar] [CrossRef]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-quadruplexes: A promising target for cancer therapy. Mol. Cancer 2021, 20, 40. [Google Scholar] [CrossRef]
- Alessandrini, I.; Recagni, M.; Zaffaroni, N.; Folini, M. On the Road to Fight Cancer: The Potential of G-quadruplex Ligands as Novel Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 5947. [Google Scholar] [CrossRef]
- von Mering, C.; Huynen, M.; Jaeggi, D.; Schmidt, S.; Bork, P.; Snel, B. STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 2003, 31, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Zorzan, E.; Elgendy, R.; Giantin, M.; Dacasto, M.; Sissi, C. Whole-Transcriptome Profiling of Canine and Human in Vitro Models Exposed to a G-Quadruplex Binding Small Molecule. Sci. Rep. 2018, 8, 17107. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.; Riou, J.F.; Teulade-Fichou, M.P.; Frickey, T.; Hartig, J.S. Bisquinolinium compounds induce quadruplex-specific transcriptome changes in HeLa S3 cell lines. BMC Res. Notes 2012, 5, 138. [Google Scholar] [CrossRef]
- Dhapola, P.; Chowdhury, S. QuadBase2: Web server for multiplexed guanine quadruplex mining and visualization. Nucleic Acids Res. 2016, 44, W277–W283. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. This Is the First Study Using Escalating Doses of PF-03758309, an Oral Compound, in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT00932126 (accessed on 4 January 2023).
- ClinicalTrials.gov. PAK4 and NAMPT in Patients with Solid Malignancies or NHL (PANAMA). Available online: https://clinicaltrials.gov/ct2/show/NCT02702492 (accessed on 4 January 2023).
- ClinicalTrials.gov. A Study of Evaluating Dual Inhibitor of PAK4 and NAMPT ATG-019 in Advanced Solid Tumors or Non-Hodgkin’s Lymphoma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04281420 (accessed on 4 January 2023).
- ClinicalTrials.gov. KPT-9274 in Patients With Relapsed and Refractory Acute Myeloid Leukemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04914845 (accessed on 4 January 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankaran, D.; Amjesh, R.; Paul, A.M.; George, B.; Kala, R.; Saini, S.; Kumar, R. Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity. Biomedicines 2023, 11, 462. https://doi.org/10.3390/biomedicines11020462
Sankaran D, Amjesh R, Paul AM, George B, Kala R, Saini S, Kumar R. Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity. Biomedicines. 2023; 11(2):462. https://doi.org/10.3390/biomedicines11020462
Chicago/Turabian StyleSankaran, Deivendran, Revikumar Amjesh, Aswathy Mary Paul, Bijesh George, Rajat Kala, Sunil Saini, and Rakesh Kumar. 2023. "Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity" Biomedicines 11, no. 2: 462. https://doi.org/10.3390/biomedicines11020462
APA StyleSankaran, D., Amjesh, R., Paul, A. M., George, B., Kala, R., Saini, S., & Kumar, R. (2023). Hyperactivation of p21-Activated Kinases in Human Cancer and Therapeutic Sensitivity. Biomedicines, 11(2), 462. https://doi.org/10.3390/biomedicines11020462




