Electric Fields Regulate In Vitro Surface Phosphatidylserine Exposure of Cancer Cells via a Calcium-Dependent Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Electric Field Exposure Set-Up
2.3. Flow Cytometry Analysis
2.4. Immunofluorescence Staining
2.5. Cell Cycle Arrest and Synchronization
2.6. SDS Page and Western Blots
2.7. Statistical Analysis
3. Results
3.1. Electric Field Stimulation Is Safe and Does Not Affect Cell Viability
3.2. Electric Field Modulates the Non-Apoptotic PS Exposure in Cancer Cells
3.3. EF-Induced PS Exposure in Cancer Cells Regulate through Cytosolic Ca2+
3.4. Dual Effect of Electric Field on F-Actin Polymerization and Corresponding EF-Induced PS Exposure in Cancer Cells Provide Mechanistic Insight into EF-Mediated PS Regulation
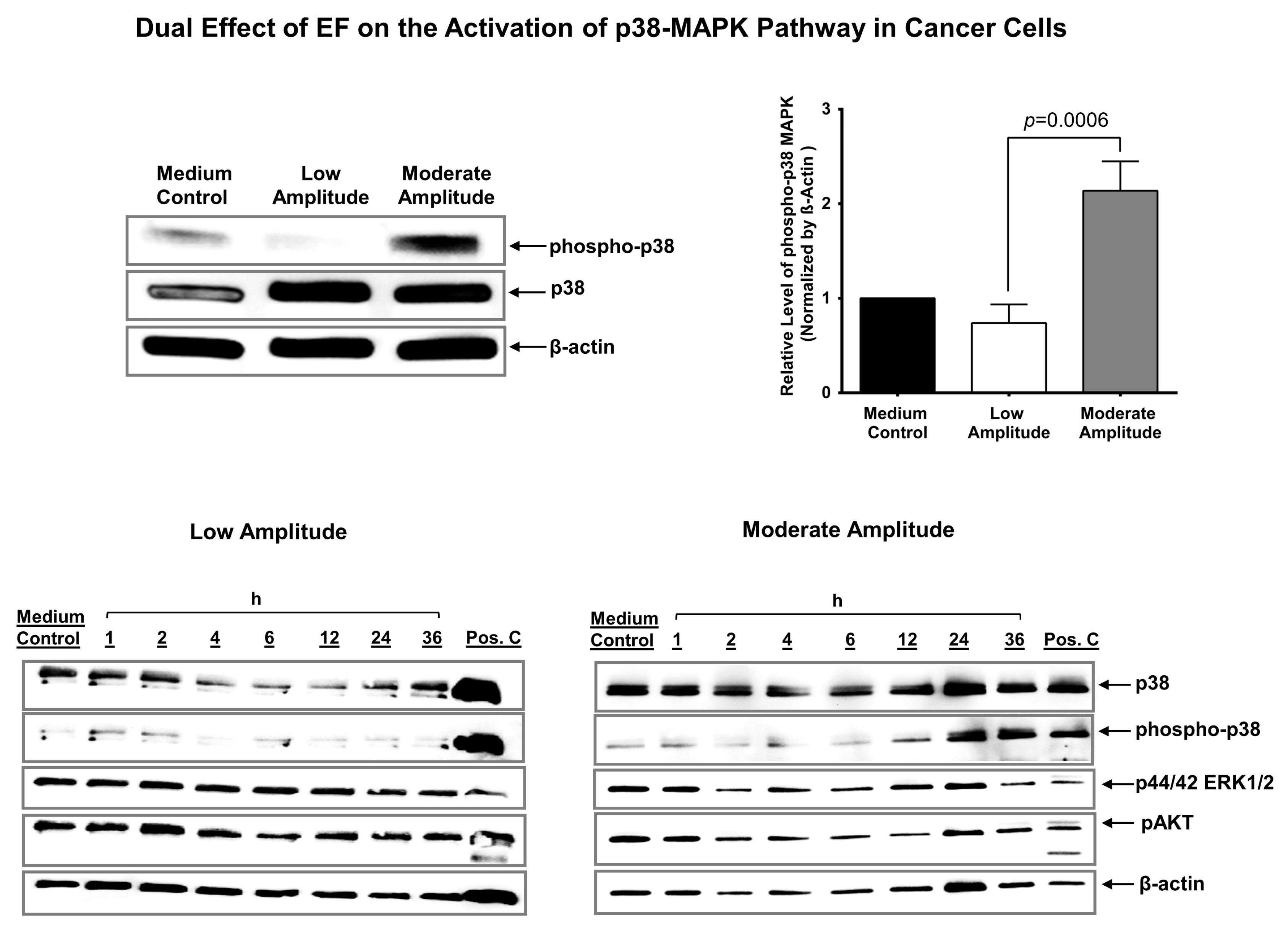
3.5. Electric Field Has a Dual Effect on the Activation of p38 MAPK in Cancer Cells
3.6. Electric Field Stimulation Leads to Cell Cycle Arrest in Cancer Cells
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teissie, J.; Tsong, T.Y. Electric field induced transient pores in phospholipid bilayer vesicles. Biochemistry 1981, 20, 1548–1554. [Google Scholar] [CrossRef]
- Graybill, P.M.; Davalos, R.V. Cytoskeletal Disruption after Electroporation and Its Significance to Pulsed Electric Field Therapies. Cancers 2020, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Russano, F.; Del Fiore, P.; Di Prata, C.; Pasqual, A.; Marconato, R.; Campana, L.G.; Spina, R.; Gianesini, C.M.; Collodetto, A.; Tropea, S.; et al. The Role of Electrochemotherapy in the Cutaneous and Subcutaneous Metastases From Breast Cancer: Analysis of Predictive Factors to Treatment From an Italian Cohort of Patients. Front. Oncol. 2021, 11, 772144. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; De Vincentiis, M.; Greco, A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment. Oncol. Lett. 2018, 16, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Sieni, E.; Dettin, M.; De Robertis, M.; Bazzolo, B.; Conconi, M.T.; Zamuner, A.; Marino, R.; Keller, F.; Campana, L.G.; Signori, E. The Efficiency of Gene Electrotransfer in Breast-Cancer Cell Lines Cultured on a Novel Collagen-Free 3D Scaffold. Cancers 2020, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Vissing, M.; Ploen, J.; Pervan, M.; Vestergaard, K.; Schnefeldt, M.; Frandsen, S.K.; Rafaelsen, S.R.; Lindhardt, C.L.; Jensen, L.H.; Rody, A.; et al. Study protocol designed to investigate tumour response to calcium electroporation in cancers affecting the skin: A non-randomised phase II clinical trial. BMJ Open 2021, 11, e046779. [Google Scholar] [CrossRef]
- Ágoston, D.; Baltás, E.; Ócsai, H.; Rátkai, S.; Lázár, P.G.; Korom, I.; Varga, E.; Németh, I.B.; Dósa-Rácz Viharosné, É.; Gehl, J.; et al. Evaluation of Calcium Electroporation for the Treatment of Cutaneous Metastases: A Double Blinded Randomised Controlled Phase II Trial. Cancers 2020, 12, 179. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation -A Novel Cancer Treatment Modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef]
- Giladi, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Munster, M.; Blat, R.; Sherbo, S.; Bomzon, Z.; Urman, N.; Itzhaki, A.; et al. Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells. Sci. Rep. 2015, 5, 18046. [Google Scholar] [CrossRef]
- Blatt, R.; Davidi, S.; Munster, M.; Shteingauz, A.; Cahal, S.; Zeidan, A.; Marciano, T.; Bomzon, Z.; Haber, A.; Giladi, M.; et al. In Vivo Safety of Tumor Treating Fields (TTFields) Applied to the Torso. Front. Oncol. 2021, 11, 670809. [Google Scholar] [CrossRef]
- Karanam, N.K.; Srinivasan, K.; Ding, L.; Sishc, B.; Saha, D.; Story, M.D. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017, 8, e2711. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, V.J.; Macarulla, T.; Philip, P.A.; Becerra, C.R.; Dragovich, T. PANOVA-3: A phase III study of tumor treating fields with nab-paclitaxel and gemcitabine for front-line treatment of locally advanced pancreatic adenocarcinoma (LAPC). J. Clin. Oncol. 2020, 38, TPS792. [Google Scholar] [CrossRef]
- Mumblat, H.; Martinez-Conde, A.; Braten, O.; Munster, M.; Dor-On, E.; Schneiderman, R.S.; Porat, Y.; Voloshin, T.; Davidi, S.; Blatt, R.; et al. Tumor Treating Fields (TTFields) downregulate the Fanconi Anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer 2021, 160, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; von Moos, R.; Manso, L.; Van Nieuwenhuysen, E.; Concin, N.; Sessa, C. Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: Results of the INNOVATE pilot study. Gynecol. Oncol. 2018, 150, 471–477. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Ram, Z.; Kim, C.-Y.; Hottinger, A.F.; Idbaih, A.; Nicholas, G.; Zhu, J.-J. Efficacy and Safety of Tumor Treating Fields (TTFields) in Elderly Patients with Newly Diagnosed Glioblastoma: Subgroup Analysis of the Phase 3 EF-14 Clinical Trial. Front. Oncol. 2021, 11, 671972. [Google Scholar] [CrossRef]
- Liu, D. Cancer biomarkers for targeted therapy. Biomark. Res. 2019, 7, 25. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.D.; Blanco, V.M.; Sulaiman, M.K.; Vallabhapurapu, S.L.; Chu, Z.; Franco, R.S.; Qi, X. Variation in human cancer cell external phosphatidylserine is regulated by flippase activity and intracellular calcium. Oncotarget 2015, 6, 34375–34388. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef]
- N’Guessan, K.F.; Davis, H.W.; Chu, Z.; Vallabhapurapu, S.D.; Lewis, C.S.; Franco, R.S.; Olowokure, O.; Ahmad, S.A.; Yeh, J.J.; Bogdanov, V.Y.; et al. Enhanced Efficacy of Combination of Gemcitabine and Phosphatidylserine-Targeted Nanovesicles against Pancreatic Cancer. Mol. Ther. 2020, 28, 1876–1886. [Google Scholar] [CrossRef]
- Blanco, V.M.; Curry, R.; Qi, X. SapC-DOPS nanovesicles: A novel targeted agent for the imaging and treatment of glioblastoma. Oncoscience 2015, 2, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Fa, H.; Xiao, D.; Wang, J. Targeting phosphatidylserine for Cancer therapy: Prospects and challenges. Theranostics 2020, 10, 9214–9229. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liou, B.; Chu, Z.; Fannin, V.; Blackwood, R.; Peng, Y.; Grabowski, G.A.; Davis, H.W.; Qi, X. Systemic enzyme delivery by blood-brain barrier-penetrating SapC-DOPS nanovesicles for treatment of neuronopathic Gaucher disease. EBioMedicine 2020, 55, 102735. [Google Scholar] [CrossRef]
- Desai, A.K.; Li, C.; Rosenberg, A.S.; Kishnani, P.S. Immunological challenges and approaches to immunomodulation in Pompe disease: A literature review. Ann. Transl. Med. 2019, 7, 285. [Google Scholar] [CrossRef]
- Chu, Z.; Abu-Baker, S.; Palascak, M.B.; Ahmad, S.A.; Franco, R.S.; Qi, X. Targeting and cytotoxicity of SapC-DOPS nanovesicles in pancreatic cancer. PLoS ONE 2013, 8, e75507. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chu, Z.; Mahller, Y.Y.; Stringer, K.F.; Witte, D.P.; Cripe, T.P. Cancer-selective targeting and cytotoxicity by liposomal-coupled lysosomal saposin C protein. Clin. Cancer Res. 2009, 15, 5840–5851. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.W.; Vallabhapurapu, S.D.; Chu, Z.; Vallabhapurapu, S.L.; Franco, R.S.; Mierzwa, M.; Kassing, W.; Barrett, W.L.; Qi, X. Enhanced phosphatidylserine-selective cancer therapy with irradiation and SapC-DOPS nanovesicles. Oncotarget 2019, 10, 856–868. [Google Scholar] [CrossRef]
- Valerie, N.C.K.; Dziegielewska, B.; Hosing, A.S.; Augustin, E.; Gray, L.S.; Brautigan, D.L.; Larner, J.M.; Dziegielewski, J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharmacol. 2013, 85, 888–897. [Google Scholar] [CrossRef]
- Latour, I.; Louw, D.F.; Beedle, A.M.; Hamid, J.; Sutherland, G.R.; Zamponi, G.W. Expression of T-type calcium channel splice variants in human glioma. Glia 2004, 48, 112–119. [Google Scholar] [CrossRef]
- Schnipper, J.; Dhennin-Duthille, I.; Ahidouch, A.; Ouadid-Ahidouch, H. Ion Channel Signature in Healthy Pancreas and Pancreatic Ductal Adenocarcinoma. Front. Pharmacol. 2020, 11, 568993. [Google Scholar] [CrossRef]
- Sasaki, N.; Gomi, F.; Hasegawa, F.; Hirano, K.; Fujiwara, M.; Toyoda, M.; Ishiwata, T. Characterization of the metastatic potential of the floating cell component of MIA PaCa-2, a human pancreatic cancer cell line. Biochem. Biophys. Res. Commun. 2020, 522, 881–888. [Google Scholar] [CrossRef]
- Kalli, M.; Li, R.; Mills, G.B.; Stylianopoulos, T.; Zervantonakis, I.K. Mechanical Stress Signaling in Pancreatic Cancer Cells Triggers p38 MAPK- and JNK-Dependent Cytoskeleton Remodeling and Promotes Cell Migration via Rac1/cdc42/Myosin II. Mol. Cancer Res. 2022, 20, 485–497. [Google Scholar] [CrossRef]
- Halasi, M.; Pandit, B.; Wang, M.; Nogueira, V.; Hay, N.; Gartel, A.L. Combination of oxidative stress and FOXM1 inhibitors induces apoptosis in cancer cells and inhibits xenograft tumor growth. Am. J. Pathol. 2013, 183, 257–265. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Taghian, T.; Hemingway, B.; Cho, H.; Kogan, A.B.; Narmoneva, D.A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 2013, 10, 20120548. [Google Scholar] [CrossRef]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 20150153. [Google Scholar] [CrossRef]
- Lim, B.; Kim, H.B.; Jeong, S.; Kim, S.H.; Kang, J.M.; Park, Y.; Won, D.-S.; Kim, J.W.; Ryu, D.S.; Kim, Y.; et al. Novel platinum bipolar electrode for irreversible electroporation in prostate cancer: Preclinical study in the beagle prostate. Sci. Rep. 2021, 11, 17194. [Google Scholar] [CrossRef]
- Jourabchi, N.; Beroukhim, K.; Tafti, B.A.; Kee, S.T.; Lee, E.W. Irreversible electroporation (NanoKnife) in cancer treatment. Gastrointest. Interv. 2014, 3, 8–18. [Google Scholar] [CrossRef]
- Wang, R.C.; Wang, Z. Synchronization of Cultured Cells to G1, S, G2, and M Phases by Double Thymidine Block. In Cell-Cycle Synchronization: Methods and Protocols; Wang, Z., Ed.; Springer: New York, NY, USA, 2022; pp. 61–71. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G. DNA content measurement for DNA ploidy and cell cycle analysis. Curr. Protoc. Cytom. 2001, 1, 7.5.1–7.5.24. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Davies, K.P.; Allen, J.; Zhu, L.; Pestell, R.G.; Zagzag, D.; Kalpana, G.V. Cell cycle arrest and repression of cyclin D1 transcription by INI1/hSNF5. Mol. Cell Biol. 2002, 22, 5975–5988. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma—phosphatidylserine in non-apoptotic cell death. Cell Commun. Signal. 2019, 17, 139. [Google Scholar] [CrossRef]
- Li, C.; Fultz, M.E.; Parkash, J.; Rhoten, W.B.; Wright, G.L. Ca2+-dependent actin remodeling in the contracting A7r5 cell. J. Muscle Res. Cell Motil. 2001, 22, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Prudent, J.; Popgeorgiev, N.; Gadet, R.; Deygas, M.; Rimokh, R.; Gillet, G. Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016, 6, 36570. [Google Scholar] [CrossRef] [PubMed]
- Canovas, B.; Nebreda, A.R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.; Houle, F.; Marceau, F.; Landry, J. Oxidative Stress-Induced Actin Reorganization Mediated by the p38 Mitogen-Activated Protein Kinase/Heat Shock Protein 27 Pathway in Vascular Endothelial Cells. Circ. Res. 1997, 80, 383–392. [Google Scholar] [CrossRef]
- Takeda, K.; Matsuzawa, A.; Nishitoh, H.; Tobiume, K.; Kishida, S.; Ninomiya-Tsuji, J.; Matsumoto, K.; Ichijo, H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004, 5, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhang, W.; Chu, D.; Liu, T.; Xie, Y.; Fu, E.; Jin, F. The role of calcium, P38 MAPK in dihydroartemisinin-induced apoptosis of lung cancer PC-14 cells. Cancer Chemother. Pharmacol. 2008, 61, 639–645. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–51. [Google Scholar] [CrossRef]
- Calianese, D.C.; Birge, R.B. Biology of phosphatidylserine (PS): Basic physiology and implications in immunology, infectious disease, and cancer. Cell Commun. Signal. 2020, 18, 41. [Google Scholar] [CrossRef]
- Kaynak, A.; Davis, H.W.; Kogan, A.B.; Lee, J.H.; Narmoneva, D.A.; Qi, X. Phosphatidylserine: The Unique Dual-Role Biomarker for Cancer Imaging and Therapy. Cancers 2022, 14, 2536. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Ostrovidov, S.; Camci-Unal, G.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. A contactless electrical stimulator: Application to fabricate functional skeletal muscle tissue. Biomed. Microdevices 2013, 15, 109–115. [Google Scholar] [CrossRef]
- Hartig, M.; Joos, U.; Wiesmann, H.P. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur. Biophys. J. 2000, 29, 499–506. [Google Scholar] [CrossRef]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J. Cell Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef]
- Craviso, G.L.; Choe, S.; Chatterjee, P.; Chatterjee, I.; Vernier, P.T. Nanosecond electric pulses: A novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels. Cell Mol. Neurobiol. 2010, 30, 1259–1265. [Google Scholar] [CrossRef]
- Sun, Z.-C.; Ge, J.-L.; Guo, B.; Guo, J.; Hao, M.; Wu, Y.-C.; Lin, Y.-A.; La, T.; Yao, P.-T.; Mei, Y.-A. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci. Rep. 2016, 6, 21774. [Google Scholar] [CrossRef]
- Wood, A.; Karipidis, K. Radiofrequency Fields and Calcium Movements Into and Out of Cells. Radiat. Res. 2021, 195, 101–113. [Google Scholar] [CrossRef]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Morotomi-Yano, K.; Akiyama, H.; Yano, K.-i. Nanosecond pulsed electric fields activate MAPK pathways in human cells. Arch. Biochem. Biophys. 2011, 515, 99–106. [Google Scholar] [CrossRef]
- Raghupathy, R.; Anilkumar, A.A.; Polley, A.; Singh, P.P.; Yadav, M.; Johnson, C.; Suryawanshi, S.; Saikam, V.; Sawant, S.D.; Panda, A.; et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 2015, 161, 581–594. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaynak, A.; N’Guessan, K.F.; Patel, P.H.; Lee, J.-H.; Kogan, A.B.; Narmoneva, D.A.; Qi, X. Electric Fields Regulate In Vitro Surface Phosphatidylserine Exposure of Cancer Cells via a Calcium-Dependent Pathway. Biomedicines 2023, 11, 466. https://doi.org/10.3390/biomedicines11020466
Kaynak A, N’Guessan KF, Patel PH, Lee J-H, Kogan AB, Narmoneva DA, Qi X. Electric Fields Regulate In Vitro Surface Phosphatidylserine Exposure of Cancer Cells via a Calcium-Dependent Pathway. Biomedicines. 2023; 11(2):466. https://doi.org/10.3390/biomedicines11020466
Chicago/Turabian StyleKaynak, Ahmet, Kombo F. N’Guessan, Priyankaben H. Patel, Jing-Huei Lee, Andrei B. Kogan, Daria A. Narmoneva, and Xiaoyang Qi. 2023. "Electric Fields Regulate In Vitro Surface Phosphatidylserine Exposure of Cancer Cells via a Calcium-Dependent Pathway" Biomedicines 11, no. 2: 466. https://doi.org/10.3390/biomedicines11020466
APA StyleKaynak, A., N’Guessan, K. F., Patel, P. H., Lee, J.-H., Kogan, A. B., Narmoneva, D. A., & Qi, X. (2023). Electric Fields Regulate In Vitro Surface Phosphatidylserine Exposure of Cancer Cells via a Calcium-Dependent Pathway. Biomedicines, 11(2), 466. https://doi.org/10.3390/biomedicines11020466





