P2X7 Receptor Modulation of the Gut Microbiota and the Inflammasome Determines the Severity of Toxoplasma gondii-Induced Ileitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study Ethics Statement
2.2. Animals
2.3. Maintenance of T. gondii Cysts
2.4. Experimental Design
2.5. Ileal Parasite Load
2.6. Aminotransferase Assays
2.7. Cytokine Measurements
2.8. Histological Analysis
2.9. Immunohistochemistry (IHC) and Apoptosis Assessment
2.10. Nitric Oxide (NO) Production and Myeloperoxidase Activity Assessment
2.11. Ileal IL-1β Measurement
2.12. RNA Isolation, cDNA Synthesis, and qRT-PCR
2.13. Contractile Activity of Longitudinal Smooth Muscle in the Ileum
2.14. Fecal Microbiota Analysis
2.15. Statistical Analysis
3. Results
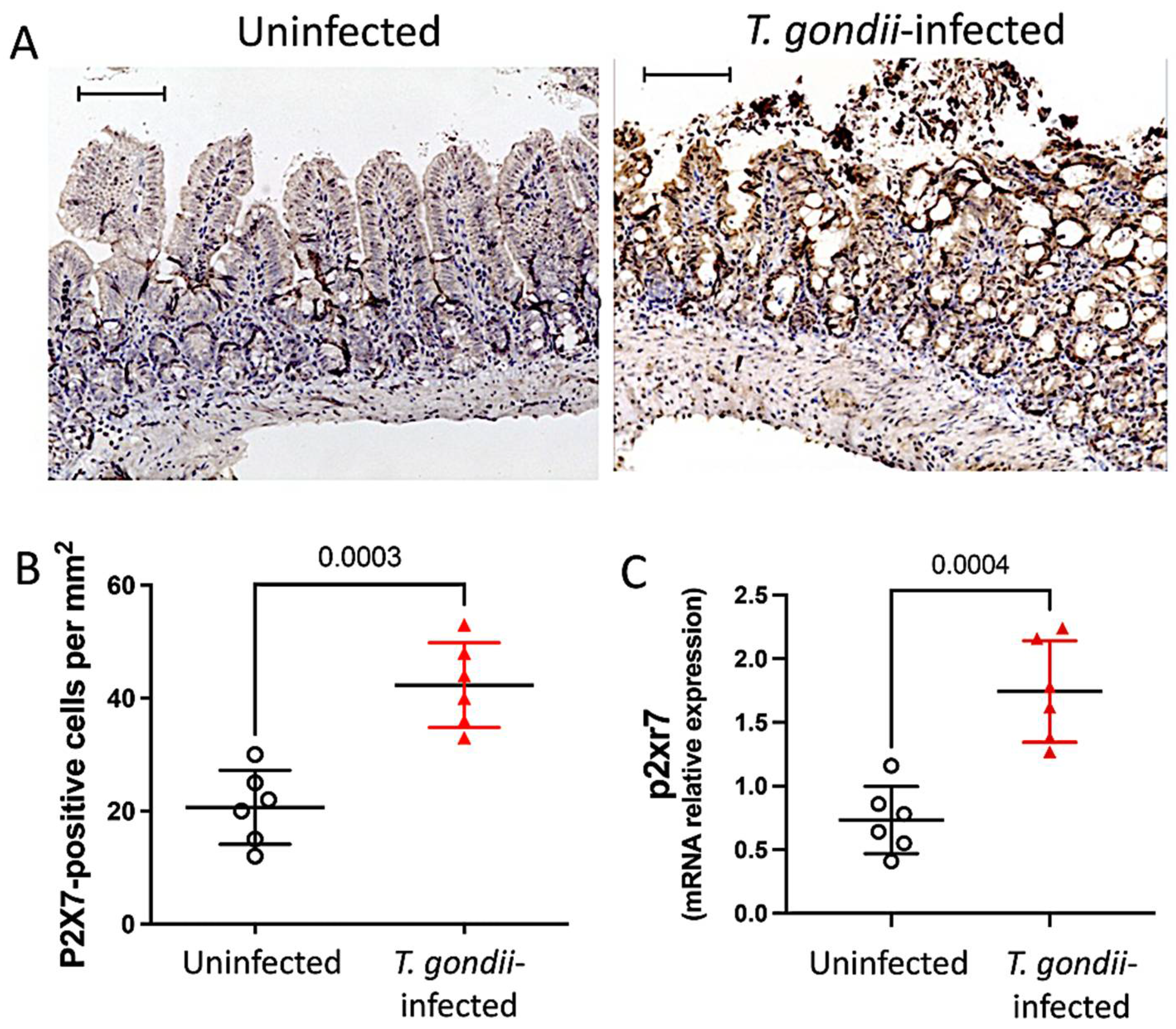
3.1. The P2X7 Receptor Is Overexpressed in the Ileum Following T. gondii Infection
3.2. The P2X7 Receptor Protects the Host against T. gondii Infection
3.2.1. Survival Curve
3.2.2. Tissue Damage and Parasite Loading
3.3. P2X7 Modulates Morbidity and the Inflammatory Response in T. gondii Infection
3.3.1. Body Weight and Liver Aminotransferases
3.3.2. Systemic Inflammatory Response
3.3.3. Mucosal Inflammatory Response and Caspase-1 Activation
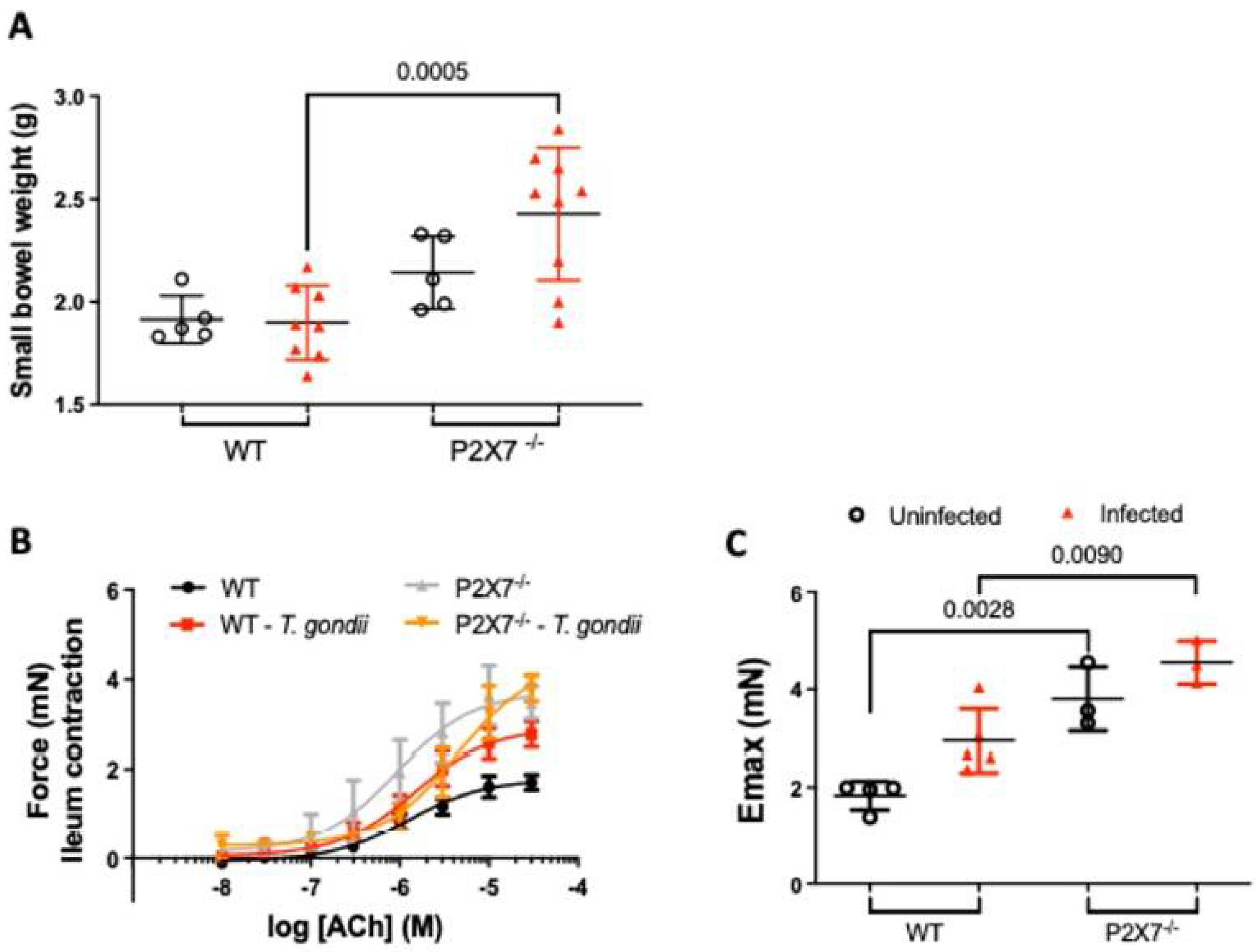
3.4. Increased Ileal Contractility Is Enhanced in the Absence of the P2X7 Receptor in T. gondii Infection
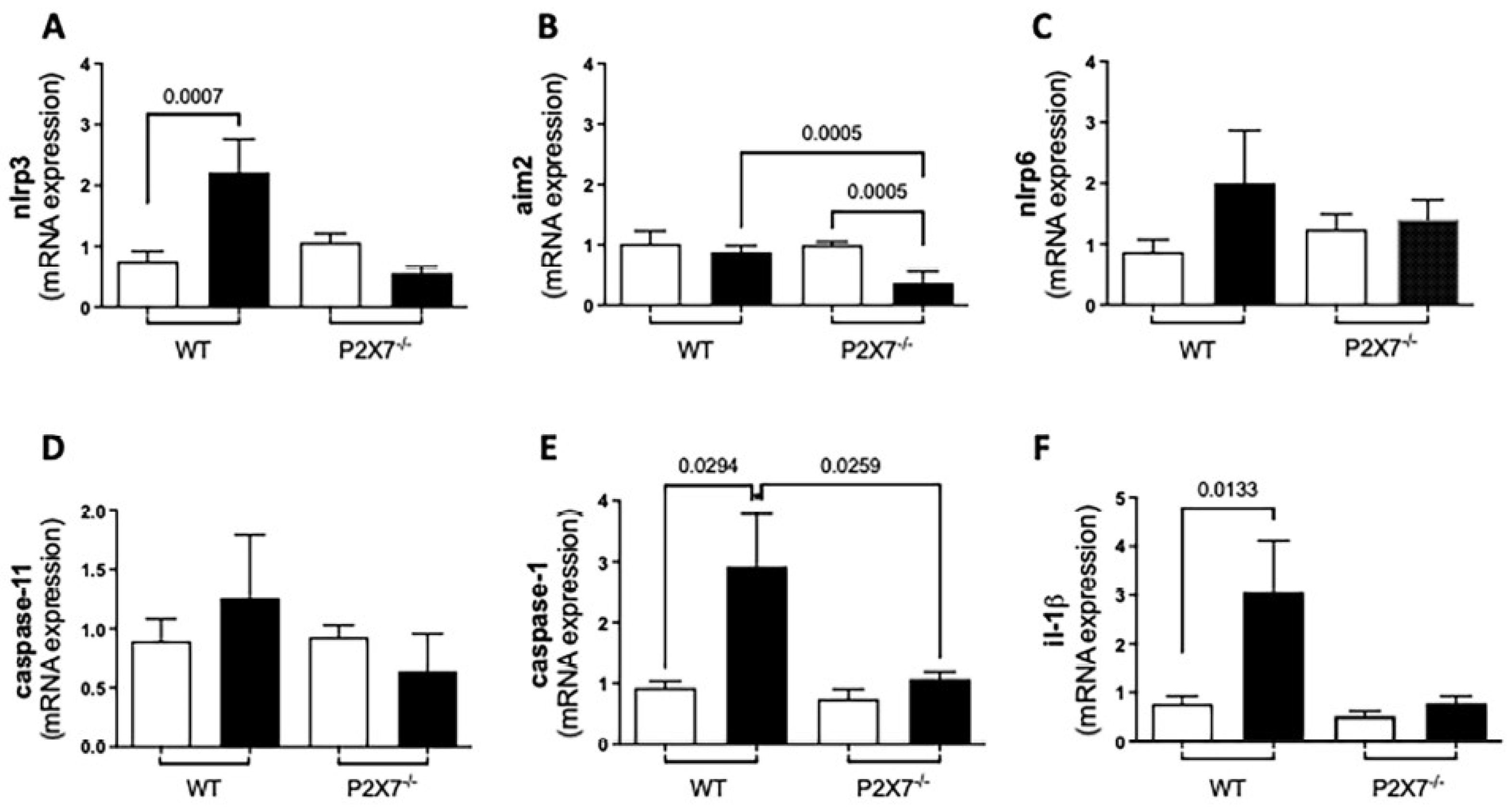
3.5. Inflammasome-Mediated Immune Response against T. gondii Infection
3.5.1. RT-qPCR
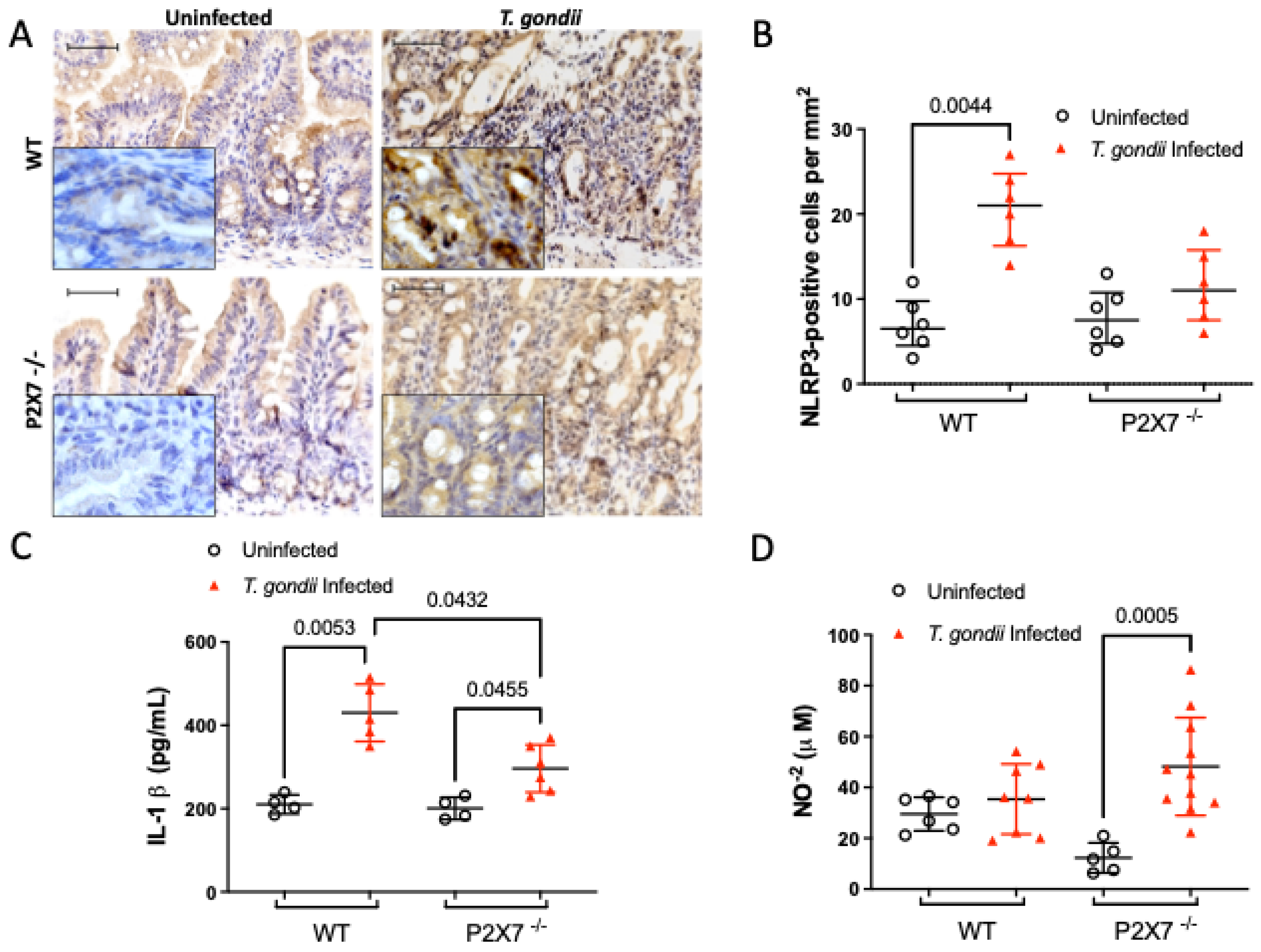
3.5.2. The P2X7 Receptor Modulates NLRP3 and IL-1β Upregulation and NO Downregulation after T. gondii Infection
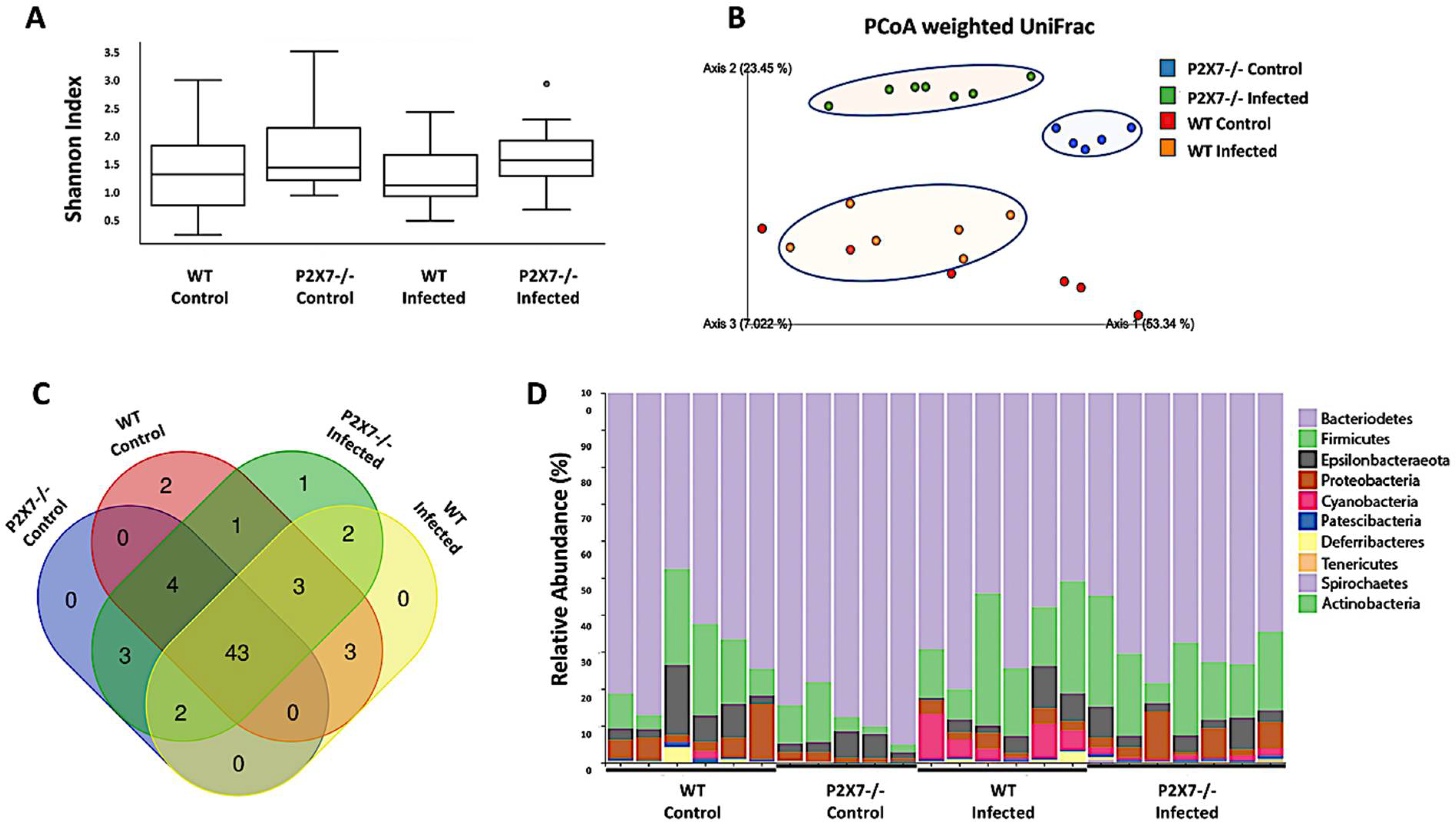
3.6. The P2X7 Receptor Modulates the Microbiota Associated with T. gondii-Induced Ileitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pittman, K.J.; Knoll, L.J. Long-Term Relationships: The Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol. Mol. Biol. Rev. 2015, 79, 387–401. [Google Scholar] [CrossRef]
- Lambert, H.; Barragan, A. Modelling parasite dissemination: Host cell subversion and immune evasion by Toxoplasma gondii. Cell Microbiol. 2010, 12, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Liesenfeld, O.; Heimesaat, M.M. Immunology of Toxoplasma gondii. Immunol. Rev. 2011, 240, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, F.; Arseneau, K.O.; Rodriguez-Palacios, A.; Pizarro, T.T. Uncovering Pathogenic Mechanisms of Inflammatory Bowel Disease Using Mouse Models of Crohn’s Disease-Like Ileitis: What is the Right Model? Cell Mol. Gastroenterol. Hepatol. 2017, 4, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Foureau, D.M.; Mielcarz, D.W.; Menard, L.C.; Schulthess, J.; Werts, C.; Vasseur, V.; Ryffel, B.; Kasper, L.H.; Buzoni-Gatel, D. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J. Immunol. 2010, 184, 7022–7029. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Jamieson, S.E.; Peixoto-Rangel, A.L.; Hargrave, A.C.; Roubaix, L.A.; Mui, E.J.; Boulter, N.R.; Miller, E.N.; Fuller, S.J.; Wiley, J.S.; Castellucci, L.; et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010, 11, 374–383. [Google Scholar] [CrossRef]
- Huang, S.W.; Walker, C.; Pennock, J.; Else, K.; Muller, W.; Daniels, M.J.; Pellegrini, C.; Brough, D.; Lopez-Castejon, G.; Cruickshank, S.M. P2X7 receptor-dependent tuning of gut epithelial responses to infection. Immunol. Cell Biol. 2017, 95, 178–188. [Google Scholar] [CrossRef]
- Quan, J.H.; Huang, R.; Wang, Z.; Huang, S.; Choi, I.W.; Zhou, Y.; Lee, Y.H.; Chu, J.Q. P2X7 receptor mediates NLRP3-dependent IL-1beta secretion and parasite proliferation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasit. Vectors 2018, 11, 1. [Google Scholar] [CrossRef]
- Moreira-Souza, A.C.A.; Almeida-da-Silva, C.L.C.; Rangel, T.P.; Rocha, G.D.C.; Bellio, M.; Zamboni, D.S.; Vommaro, R.C.; Coutinho-Silva, R. The P2X7 Receptor Mediates Toxoplasma gondii Control in Macrophages through Canonical NLRP3 Inflammasome Activation and Reactive Oxygen Species Production. Front. Immunol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Fischer, A.; Jahn, H.K.; Niebergall, J.; Freudenberg, M.; Blaut, M.; Liesenfeld, O.; Schumann, R.R.; Gobel, U.B.; Bereswill, S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut 2007, 56, 941–948. [Google Scholar] [CrossRef] [PubMed]
- von Klitzing, E.; Ekmekciu, I.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Intestinal, extra-intestinal and systemic sequelae of Toxoplasma gondii induced acute ileitis in mice harboring a human gut microbiota. PLoS ONE 2017, 12, e0176144. [Google Scholar] [CrossRef]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the Gut Microbiota Colonization Resistance and Enteric Pathogen Infection. Front. Cell Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Pickard, J.M.; Nunez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Laukens, D.; Brinkman, B.M.; Raes, J.; De Vos, M.; Vandenabeele, P. Heterogeneity of the gut microbiome in mice: Guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016, 40, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Souza, A.C.A.; Rangel, T.P.; Silva, S.; Figliuolo, V.R.; Savio, L.E.B.; Schmitz, F.; Takiya, C.M.; Wyse, A.T.S.; Vommaro, R.C.; Coutinho-Silva, R. Disruption of Purinergic Receptor P2X7 Signaling Increases Susceptibility to Cerebral Toxoplasmosis. Am. J. Pathol. 2019, 189, 730–738. [Google Scholar] [CrossRef]
- Neves, A.R.; Castelo-Branco, M.T.; Figliuolo, V.R.; Bernardazzi, C.; Buongusto, F.; Yoshimoto, A.; Nanini, H.F.; Coutinho, C.M.; Carneiro, A.J.; Coutinho-Silva, R.; et al. Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Liu, X.; Xiong, Y.; Ren, Y.; Chen, J.; Lu, N.; Guo, Y.; Bai, A. Extracellular ATP mediates inflammatory responses in colitis via P2X7 receptor signaling. Sci. Rep. 2016, 6, 19108. [Google Scholar] [CrossRef]
- Ohbori, K.; Fujiwara, M.; Ohishi, A.; Nishida, K.; Uozumi, Y.; Nagasawa, K. Prophylactic Oral Administration of Magnesium Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice through a Decrease of Colonic Accumulation of P2X7 Receptor-Expressing Mast Cells. Biol. Pharm. Bull. 2017, 40, 1071–1077. [Google Scholar] [CrossRef]
- Santos, A.; Costa, D.V.S.; Foschetti, D.A.; Duarte, A.S.G.; Martins, C.S.; Soares, P.M.G.; Castelucci, P.; Brito, G.A.C. P2X7 receptor blockade decreases inflammation, apoptosis, and enteric neuron loss during Clostridioides difficile toxin A-induced ileitis in mice. World J. Gastroenterol. 2022, 28, 4075–4088. [Google Scholar] [CrossRef]
- Miller, C.M.; Zakrzewski, A.M.; Robinson, D.P.; Fuller, S.J.; Walker, R.A.; Ikin, R.J.; Bao, S.J.; Grigg, M.E.; Wiley, J.S.; Smith, N.C. Lack of a Functioning P2X7 Receptor Leads to Increased Susceptibility to Toxoplasmic Ileitis. PLoS ONE 2015, 10, e0129048. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Fahmawi, A.; Christian, D.A.; Fang, Q.; Radaelli, E.; Chen, L.; Sullivan, M.C.; Misic, A.M.; Ellringer, J.A.; Zhu, X.Q.; et al. Infection-Induced Intestinal Dysbiosis Is Mediated by Macrophage Activation and Nitrate Production. mBio 2019, 10, e00935-19. [Google Scholar] [CrossRef] [PubMed]
- Correa, G.; Almeida Lindenberg, C.; Moreira-Souza, A.C.; Savio, L.E.; Takiya, C.M.; Marques-da-Silva, C.; Vommaro, R.C.; Coutinho-Silva, R. Inflammatory early events associated to the role of P2X7 receptor in acute murine toxoplasmosis. Immunobiology 2017, 222, 676–683. [Google Scholar] [CrossRef]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef]
- Ferezin, R.I.; Vicentino-Vieira, S.L.; Gois, M.B.; Araujo, E.J.; Melo, G.A.; Garcia, J.L.; Sant’Ana, D.M. Different inoculum loads of Toxoplasma gondii induce reduction of myenteric neurons of the rat colon. Rev. Bras. Parasitol. Vet. 2017, 26, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.J.; Zaniolo, L.M.; Vicentino, S.L.; Gois, M.B.; Zanoni, J.N.; da Silva, A.V.; Sant’Ana Dde, M. Toxoplasma gondii causes death and plastic alteration in the jejunal myenteric plexus. World J. Gastroenterol. 2015, 21, 4829–4839. [Google Scholar] [CrossRef]
- Trevizan, A.R.; Vicentino-Vieira, S.L.; da Silva Watanabe, P.; Gois, M.B.; de Melo Gde, A.; Garcia, J.L.; Jose de Almeida Araujo, E.; Sant’Ana Dde, M. Kinetics of acute infection with Toxoplasma gondii and histopathological changes in the duodenum of rats. Exp. Parasitol. 2016, 165, 22–29. [Google Scholar] [CrossRef]
- Diezmos, E.F.; Bertrand, P.P.; Liu, L. Purinergic Signaling in Gut Inflammation: The Role of Connexins and Pannexins. Front. Neurosci. 2016, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.C. Purinergic mechanisms in the control of gastrointestinal motility. Purinergic Signal 2008, 4, 197–212. [Google Scholar] [CrossRef]
- Antonioli, L.; Giron, M.C.; Colucci, R.; Pellegrini, C.; Sacco, D.; Caputi, V.; Orso, G.; Tuccori, M.; Scarpignato, C.; Blandizzi, C.; et al. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS ONE 2014, 9, e116253. [Google Scholar] [CrossRef] [PubMed]
- Pego, B.; Martinusso, C.A.; Bernardazzi, C.; Ribeiro, B.E.; de Araujo Cunha, A.F.; de Souza Mesquita, J.; Nanini, H.F.; Machado, M.P.; Castelo-Branco, M.T.L.; Cavalcanti, M.G.; et al. Schistosoma mansoni Coinfection Attenuates Murine Toxoplasma gondii-Induced Crohn’s-Like Ileitis by Preserving the Epithelial Barrier and Downregulating the Inflammatory Response. Front. Immunol. 2019, 10, 442. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Kanneganti, T.D.; Dubyak, G.R.; Nunez, G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J. Biol. Chem. 2007, 282, 18810–18818. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Q.; Shi, G.; Fan, Y.M.; Choi, I.W.; Cha, G.H.; Zhou, Y.; Lee, Y.H.; Quan, J.H. Production of IL-1beta and Inflammasome with Up-Regulated Expressions of NOD-Like Receptor Related Genes in Toxoplasma gondii-Infected THP-1 Macrophages. Korean J. Parasitol. 2016, 54, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.Q.; Gao, F.F.; Wu, W.; Li, C.; Pan, Z.; Sun, J.; Wang, H.; Huang, C.; Lee, S.H.; Quan, J.H.; et al. Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasites Vectors 2021, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; Cao, Y.; Shen, J.; Yu, L. Insight Into Inflammasome Signaling: Implications for Toxoplasma gondii Infection. Front. Immunol. 2020, 11, 583193. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, O.; Kang, H.; Park, D.; Nguyen, T.A.; Parkhe, C.V.; Watanabe, H.; Abo, T.; Sher, A.; Remington, J.S.; Suzuki, Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999, 21, 365–376. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [CrossRef]
- Yan, X.; Han, W.; Jin, X.; Sun, Y.; Gao, J.; Yu, X.; Guo, J. Study on the effect of koumiss on the intestinal microbiota of mice infected with Toxoplasma gondii. Sci. Rep. 2022, 12, 1271. [Google Scholar] [CrossRef]
- Egan, C.E.; Maurer, K.J.; Cohen, S.B.; Mack, M.; Simpson, K.W.; Denkers, E.Y. Synergy between intraepithelial lymphocytes and lamina propria T cells drives intestinal inflammation during infection. Mucosal Immunol. 2011, 4, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Fuchs, A.; Sibley, L.D. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect. Immun. 2010, 78, 1564–1570. [Google Scholar] [CrossRef]
- Egan, C.E.; Cohen, S.B.; Denkers, E.Y. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol. Cell Biol. 2012, 90, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Raetz, M.; Hwang, S.H.; Wilhelm, C.L.; Kirkland, D.; Benson, A.; Sturge, C.R.; Mirpuri, J.; Vaishnava, S.; Hou, B.; Defranco, A.L.; et al. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat. Immunol. 2013, 14, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.; Araujo, A.; Lopez-Yglesias, A.; Rajala, M.W.; Geng, L.; Levine, B.; Hooper, L.V.; Burstein, E.; Yarovinsky, F. Loss of Paneth Cell Autophagy Causes Acute Susceptibility to Toxoplasma gondii-Mediated Inflammation. Cell Host Microbe 2018, 23, 177–190 e174. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. Trefoil Factor Family (TFF) Peptides and their Different Roles in the Mucosal Innate Immune Defense and More: An Update. Curr. Med. Chem. 2021, 28, 7387–7399. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakamura, K.; Yoshii, A.; Yokoi, Y.; Kikuchi, M.; Shinozaki, R.; Nakamura, S.; Ohira, S.; Sugimoto, R.; Ayabe, T. Paneth cell alpha-defensin misfolding correlates with dysbiosis and ileitis in Crohn’s disease model mice. Life Sci. Alliance 2020, 3, e201900592. [Google Scholar] [CrossRef]
- Pereira, F.C.; Wasmund, K.; Cobankovic, I.; Jehmlich, N.; Herbold, C.W.; Lee, K.S.; Sziranyi, B.; Vesely, C.; Decker, T.; Stocker, R.; et al. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat. Commun. 2020, 11, 5104. [Google Scholar] [CrossRef]
- Bernardazzi, C.; Castelo-Branco, M.T.L.; Pego, B.; Ribeiro, B.E.; Rosas, S.L.B.; Santana, P.T.; Machado, J.C.; Leal, C.; Thompson, F.; Coutinho-Silva, R.; et al. The P2X7 Receptor Promotes Colorectal Inflammation and Tumorigenesis by Modulating Gut Microbiota and the Inflammasome. Int. J. Mol. Sci. 2022, 23, 4616. [Google Scholar] [CrossRef]
- Liu, G.H.; Zhuo, X.C.; Huang, Y.H.; Liu, H.M.; Wu, R.C.; Kuo, C.J.; Chen, N.H.; Chuang, L.P.; Lin, S.W.; Chen, Y.L.; et al. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology 2022, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Bereswill, S.; Kuhl, A.A.; Alutis, M.; Fischer, A.; Mohle, L.; Struck, D.; Liesenfeld, O.; Gobel, U.B.; Dunay, I.R.; Heimesaat, M.M. The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. 2014, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Dominguez Rieg, J.A.; Thomas, L.; White, J.R.; Rieg, T. Intestine-Specific NHE3 Deletion in Adulthood Causes Microbial Dysbiosis. Front. Cell Infect. Microbiol. 2022, 12, 896309. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.; Pifer, R.; Behrendt, C.L.; Hooper, L.V.; Yarovinsky, F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 2009, 6, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P.; Hooper, L.V.; Yarovinsky, F. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glockner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Phylogenetic pattern and the quantification of organismal biodiversity. Philos. Trans. R Soc. Lond B Biol. Sci. 1994, 345, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Souza, A.C.A.; Nanini, H.F.; Rangel, T.P.; da Silva, S.R.B.; Damasceno, B.P.; Ribeiro, B.E.; Cascabulho, C.M.; Thompson, F.; Leal, C.; Santana, P.T.; et al. P2X7 Receptor Modulation of the Gut Microbiota and the Inflammasome Determines the Severity of Toxoplasma gondii-Induced Ileitis. Biomedicines 2023, 11, 555. https://doi.org/10.3390/biomedicines11020555
Moreira-Souza ACA, Nanini HF, Rangel TP, da Silva SRB, Damasceno BP, Ribeiro BE, Cascabulho CM, Thompson F, Leal C, Santana PT, et al. P2X7 Receptor Modulation of the Gut Microbiota and the Inflammasome Determines the Severity of Toxoplasma gondii-Induced Ileitis. Biomedicines. 2023; 11(2):555. https://doi.org/10.3390/biomedicines11020555
Chicago/Turabian StyleMoreira-Souza, Aline Cristina Abreu, Hayandra Ferreira Nanini, Thuany Prado Rangel, Sthefani Rodrigues Batista da Silva, Beatriz Pêgo Damasceno, Beatriz Elias Ribeiro, Cynthia M. Cascabulho, Fabiano Thompson, Camille Leal, Patrícia Teixeira Santana, and et al. 2023. "P2X7 Receptor Modulation of the Gut Microbiota and the Inflammasome Determines the Severity of Toxoplasma gondii-Induced Ileitis" Biomedicines 11, no. 2: 555. https://doi.org/10.3390/biomedicines11020555
APA StyleMoreira-Souza, A. C. A., Nanini, H. F., Rangel, T. P., da Silva, S. R. B., Damasceno, B. P., Ribeiro, B. E., Cascabulho, C. M., Thompson, F., Leal, C., Santana, P. T., Rosas, S. L. B., de Andrade, K. Q., Silva, C. L. M., Vommaro, R. C., de Souza, H. S. P., & Coutinho-Silva, R. (2023). P2X7 Receptor Modulation of the Gut Microbiota and the Inflammasome Determines the Severity of Toxoplasma gondii-Induced Ileitis. Biomedicines, 11(2), 555. https://doi.org/10.3390/biomedicines11020555






