Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Experiments
2.3. Analyses
2.4. Statistics
3. Results
3.1. Glucose Controls
3.2. GIP Receptor Antagonism
3.3. GLP-1 Receptor Antagonism
3.4. Combination of GIP and GLP-1 Receptor Antagonism
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, N.; Holdsworth, C.D.; Turner, D.S. New interpretation of oral glucose tolerance. Lancet 1964, 2, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Elrick, H.; Stimmler, L.; Hlad, C.J.; Arai, Y. Plasma insulin responses to oral and intravenous glucose administration. J. Clin. Endocrinol. Metab. 1964, 24, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, W. The incretin concept today. Diabetologia 1979, 16, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Homberger, E.; Siegel, E.G.; Allen, R.C.; Eaton, R.P.; Ebert, R.; Creutzfeldt, W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986, 63, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Jensen, M.H.; Gabe, M.B.N.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; et al. Separate and combined gluco-metabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 in healthy individuals. Diabetes 2019, 68, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Alsalim, W.; Lindgren, O.; Ahrén, B. Glucose-dependent insulinotropic polypeptide and glucagon-like peptie-1 secretion in humans: Characteristics and regulation. J. Diabetes Investig. 2023; in press. [Google Scholar]
- Ahrén, B. Glucose-dependent insulinotropic polypeptide secretion after oral macronutrient ingestion: The human literature revisited and a systematic study in model experiments in mice. J. Diabetes Investig. 2022, 13, 1655–1665. [Google Scholar] [CrossRef]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Gasbjerg, L.S.; Helsted, M.M.; Hartmann, B.; Sparre-Ulrich, A.H.; Veedfald, S.; Stensen, S.; Lanng, A.R.; Bergmann, N.C.; Christensen, M.B.; Vilsbøll, T.; et al. GIP and GLP-1 receptor antagonism during a meal in healthy individuals. J. Clin. Endocrinol. Metab. 2020, 105, dgz175. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Sörhede Winzell, M.; Pacini, G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J. Endocrinol. 2008, 197, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ahlkvist, L.; Vikman, J.; Pacini, G.; Ahrén, B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul. Pept. 2012, 178, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacini, G.; Ahrén, B. Glucagon-like peptide-1 and glucose-dependent insulinotropic peptide: Effects alone and in combination on insulin secretion and glucose disappearance in mice. Physiol. Rep. 2017, 5, e13280. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The incretin effect in female mice with double deletion of GLP-1 and GIP receptors. J. Endocr. Soc. 2020, 4, bvz036. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. Islet adaptation in GIP receptor knockout mice. Peptides 2020, 125, 170152. [Google Scholar] [CrossRef]
- Ahrén, B.; Yamada, Y.; Seino, Y. The mediation by GLP-1 receptors of glucagon-induced insulin secretion revisited in GLP-1 receptor knockout mice. Peptides 2021, 135, 170434. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Christensen, M.B.; Hartmann, B.; Lanng, A.R.; Sparre-Ulrich, A.H.; Gabe, M.B.N.; Dela, F.; Vilsbøll, T.; Holst, J.J.; Rosenkilde, M.M.; et al. GIP(3-30)NH2 is an efficacious GIP receptor antagonist in humans: A randomised, double-blinded, placebo-controlled, crossover study. Diabetologia 2018, 61, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.A.; Craig, S.L.; Ng, M.T.; Gault, V.A.; Flatt, P.R.; Irwin, N. Characterization of glucose-dependent insulinotropic polypeptide receptor antagonists in rodent pancreatic beta cells and mice. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 31548798. [Google Scholar] [CrossRef] [Green Version]
- West, J.A.; Tsakmaki, A.; Ghosh, S.S.; Parkes, D.G.; Grønlund, R.V.; Pedersen, P.J.; Maggs, D.; Rajahopalan, H.; Bewick, G.A. Chronic peptide-based GIP receptor inhibition exhibits glucose metabolic changes in mice when administered either alone or combined with GLP-1 agonism. PLoS ONE 2021, 16, e024239. [Google Scholar] [CrossRef]
- Ovlund, T.; Pacini, G.; Ahrén, B. Impact of incretin hormone receptors on insulin-independent glucose disposal in model experiments in mice. Front. Endocrinol. 2021, 12, 680153. [Google Scholar] [CrossRef] [PubMed]
- Gasbjerg, L.S.; Bari, E.J.; Christensen, M.; Knop, F.K. Exendin (9-39)NH2: Recommendations for clinical use based in a systematic literature review. Diabetes Obes. Metab. 2021, 23, 2419–2436. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Val, T.P.; D´Alessio, D.A. Regulation of islet hormone release and gastric emptying by endogenous glucagon-like peptide 1 after glucose ingestion. J. Clin. Endocrinol. Metab. 2008, 93, 4909–4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.M.; Hoselton, A.L.; Krishna, R.; Slentz, C.A.; D´Alessio, D.A. GLP-1 receptor blockade reduces stimulated insulin secretion in fasted subjects with low circulating GLP-1. J. Clin. Endocrinol. Metab. 2022, 107, 2500–2510. [Google Scholar] [CrossRef]
- Sparre-Ulrich, A.H.; Gabe, M.N.; Gasbjerg, L.S.; Christiansen, C.B.; Svendsen, B.; Hartmann, B.; Holst, J.J.; Rosenkilde, M.M. GIP(3-30)NH2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochem. Pharmacol. 2017, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Stöckmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.J. The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Miyawaki, K.; Yamada, Y.; Yano, H.; Niwa, H.; Ban, N.; Ihara, Y.; Kubota, A.; Fujimoto, S.; Kajikawa, M.; Kuroe, A.; et al. Glucose intolerance caused by a defect in the entero-insular axis: A study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14843–14847. [Google Scholar] [CrossRef] [Green Version]
- Pamir, N.; Lynn, F.C.; Buchan, A.M.; Ehses, J.; Hinke, S.A.; Pospisilik, J.A.; Miyawaki, K.; Yamada, Y.; Seino, Y.; McIntosh, C.H.S.; et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E931–E939. [Google Scholar] [CrossRef] [Green Version]
- Preitner, F.; Ibberson, M.; Franklin, I.; Binnert, C.; Pende, M.; Gijnovci, A.; Hansotia, T.; Drucker, D.J.; Wollheim, C.; Burcelin, R.; et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J. Clin. Investig. 2004, 113, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Scrocchi, L.A.; Brown, T.J.; MaClusky, N.; Brubaker, P.L.; Auerbach, A.B.; Joyner, A.L.; Drucker, D.J. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 1996, 2, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Scrocchi, L.A.; Marshall, B.A.; Cook, S.M.; Brubaker, P.L.; Drucker, D.J. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 1998, 47, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B.; Yamada, Y.; Seino, Y. The insulin response to oral glucose in GIP and GLP-1 receptor knockout mice: Review of the literature and stepwise glucose dose response studies in female mice. Front. Endocrinol. 2021, 12, 665537. [Google Scholar] [CrossRef] [PubMed]
- Hansotia, T.; Baggio, L.L.; Delmeire, D.; Hinke, S.A.; Yamada, Y.; Tsukiyama, K.; Seino, Y.; Holst, J.J.; Schuit, F.; Drucker, D.J. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes 2004, 53, 1326–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
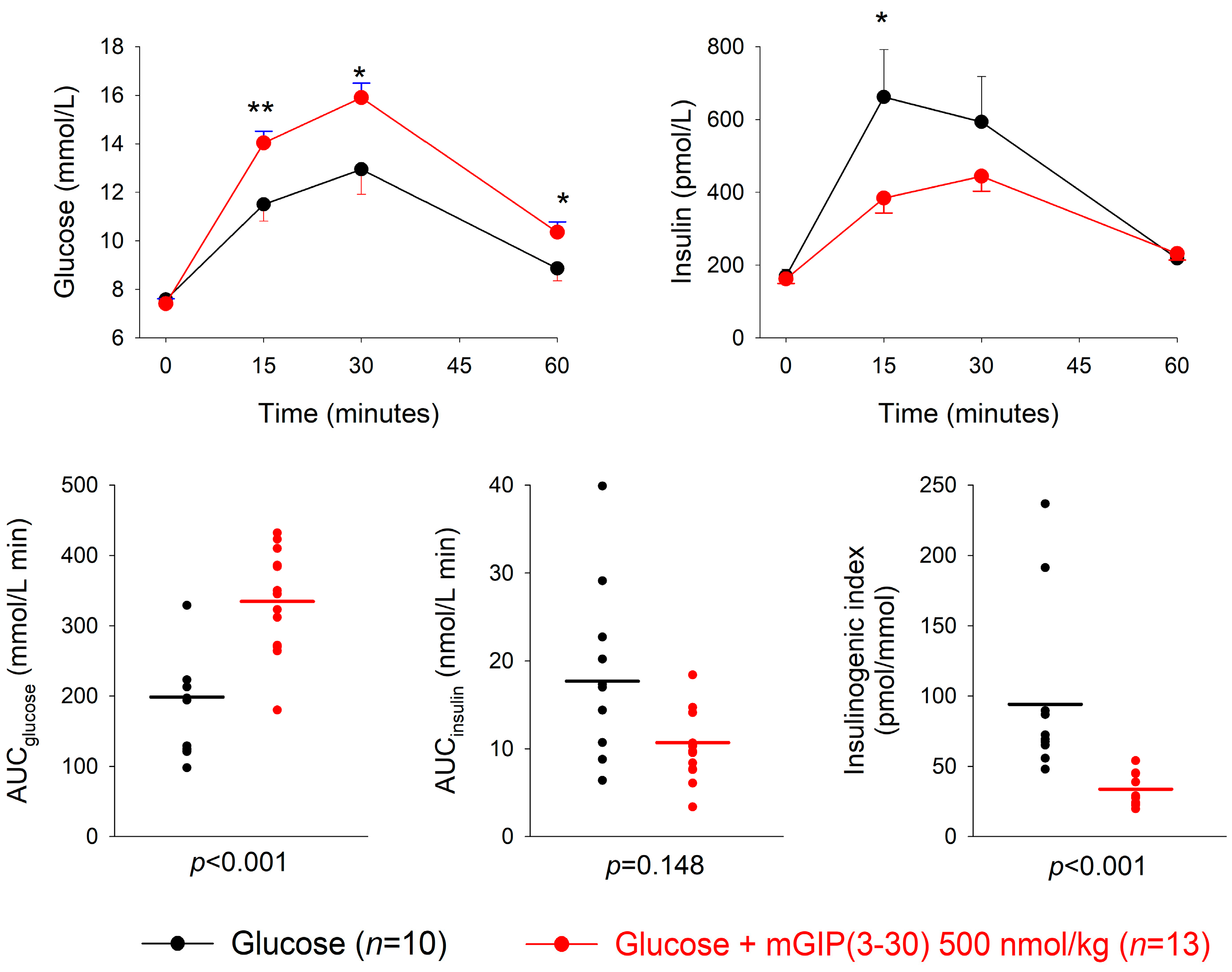
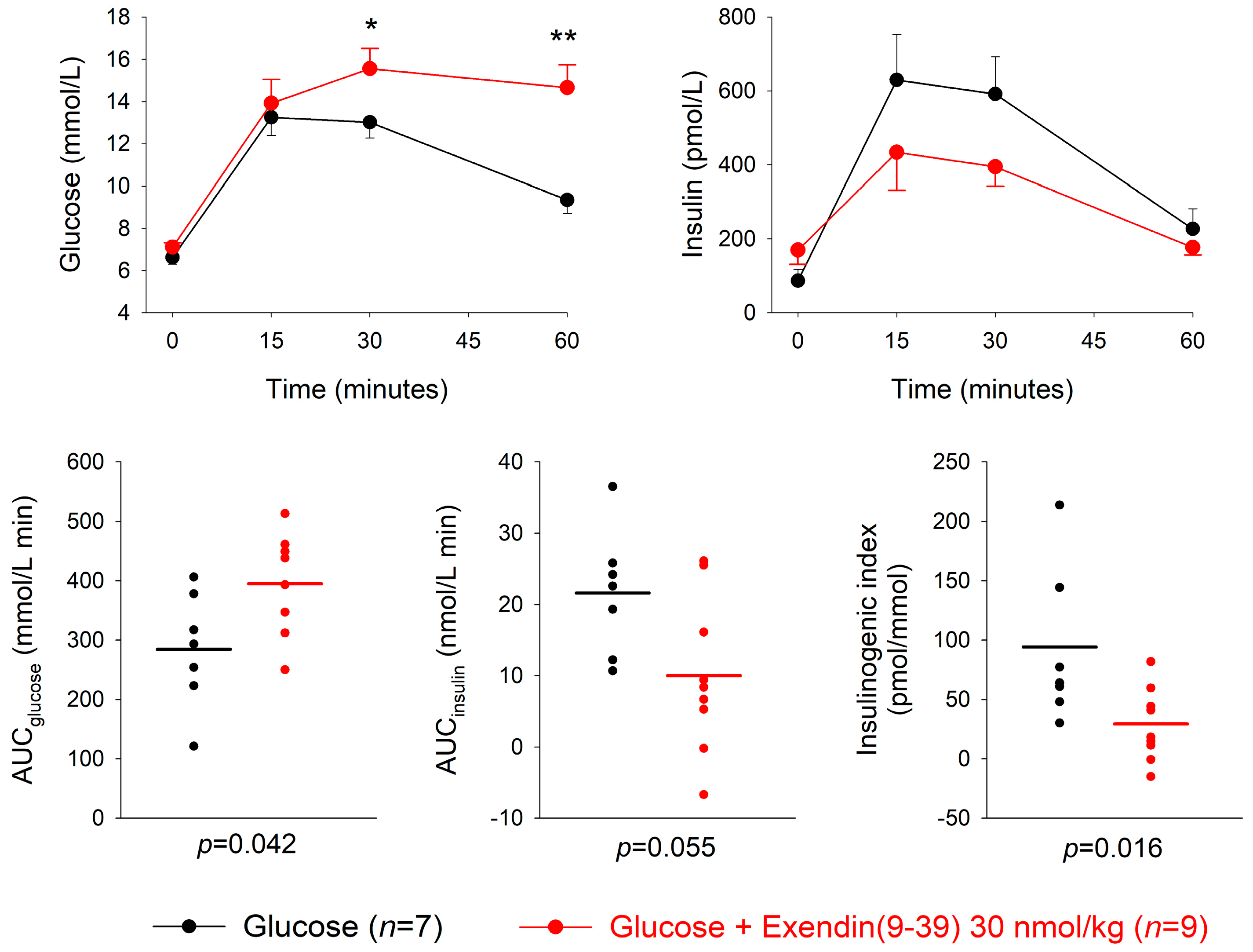
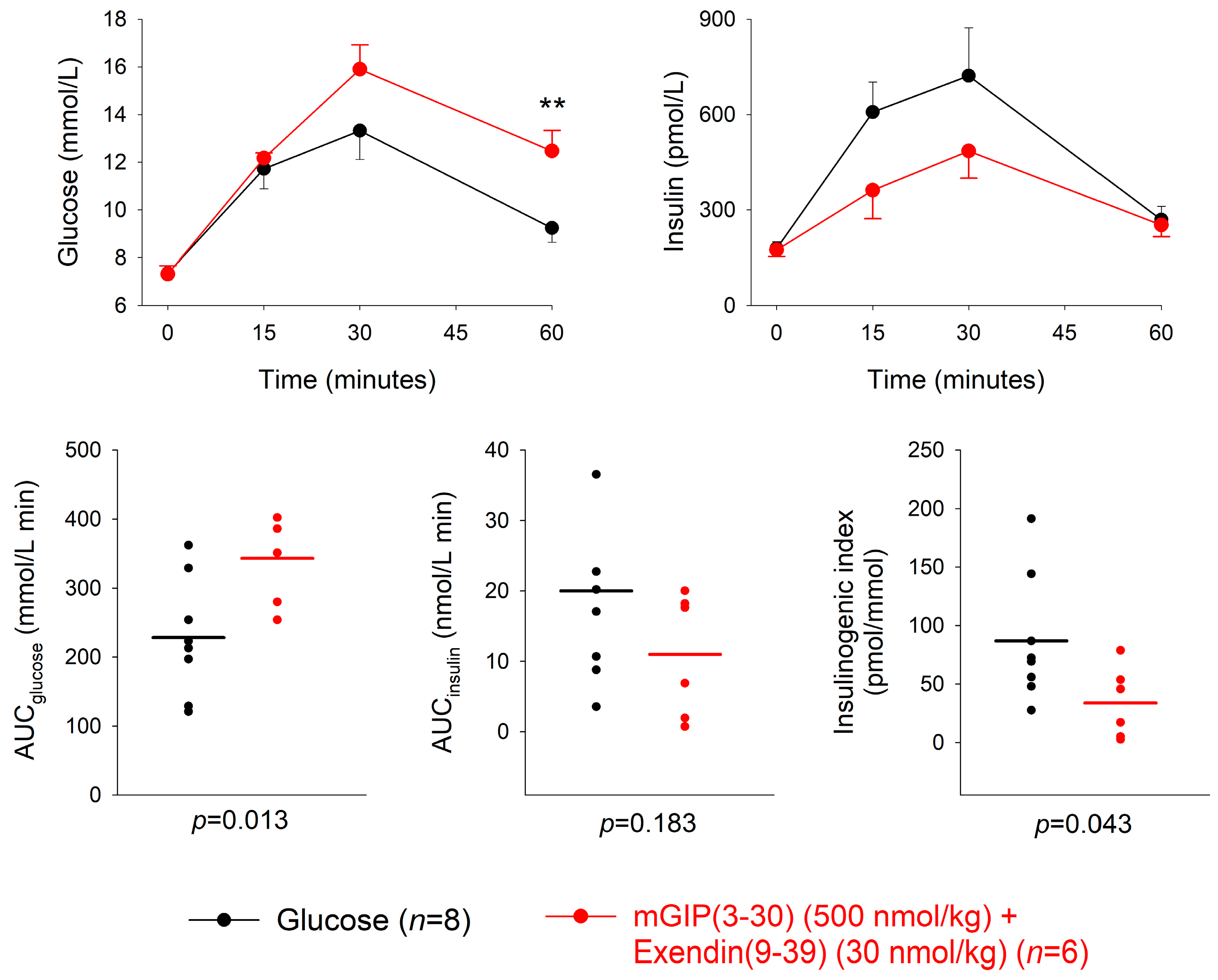
| Dose of Antagonist (nmol/kg) | n | AUCglucose mmol/L min | p | AUCinsulin nmol/L min | p | Insulinogenic Index (pmol/ mmol) | p | |
|---|---|---|---|---|---|---|---|---|
| mGIP(3-30) + glucose | 50 | 14 | 258.5 ± 15.5 | 0.45 | 17.4 ± 1.7 | 0.32 | 71.5 ± 8.6 | 0.076 |
| Glucose alone | 12 | 233.1 ± 30.9 | 20.1 ± 1.9 | 107.2 ± 16.1 | ||||
| mGIP(3-30) + glucose | 500 | 13 | 334.7 ± 20.6 | <0.001 | 10.7 ± 1.1 | 0.148 | 33.8 ± 4.3 | <0.001 |
| Glucose alone | 10 | 198.8 ± 28.1 | 17.7 ± 3.6 | 94.2 ± 21.0 | ||||
| Exendin(9-39) + glucose | 3 | 8 | 272.3 ± 33.9 | 0.80 | 18.0 ± 3.4 | 0.44 | 71.1 ± 12.0 | 0.44 |
| Glucose alone | 8 | 260.5 ± 30.6 | 14.8 ± 3.9 | 53.8 ± 12.8 | ||||
| Exendin(9-39) + glucose | 30 | 9 | 394.6 ± 32.8 | 0.042 | 10.0 ± 3.6 | 0.055 | 29.4 ± 10.2 | 0.016 |
| Glucose | 7 | 284.3 ± 36.6 | 21.6 ± 3.3 | 91.1 ± 24.6 | ||||
| mGIP(3-30) + Exendin(9-39) + glucose | 500 (mGIP) 30 (exendin) | 6 | 343.1 ± 25.2 | 0.013 | 11.0 ± 3.6 | 0.183 | 33.8 ± 12.4 | 0.043 |
| Glucose alone | 8 | 228.4 ± 30.2 | 20.0 ± 4.6 | 86.9 ± 19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrén, B. Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice. Biomedicines 2023, 11, 591. https://doi.org/10.3390/biomedicines11020591
Ahrén B. Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice. Biomedicines. 2023; 11(2):591. https://doi.org/10.3390/biomedicines11020591
Chicago/Turabian StyleAhrén, Bo. 2023. "Contribution of GIP and GLP-1 to the Insulin Response to Oral Administration of Glucose in Female Mice" Biomedicines 11, no. 2: 591. https://doi.org/10.3390/biomedicines11020591





