The Role of Pericytes in Regulation of Innate and Adaptive Immunity
Abstract
1. Introduction
2. Pericytes as Macrophage-like Cells
3. Pericytes in the Regulation of the Innate Immune System
3.1. PC in Inflammatory Responses
3.2. PC in Innate Immunity
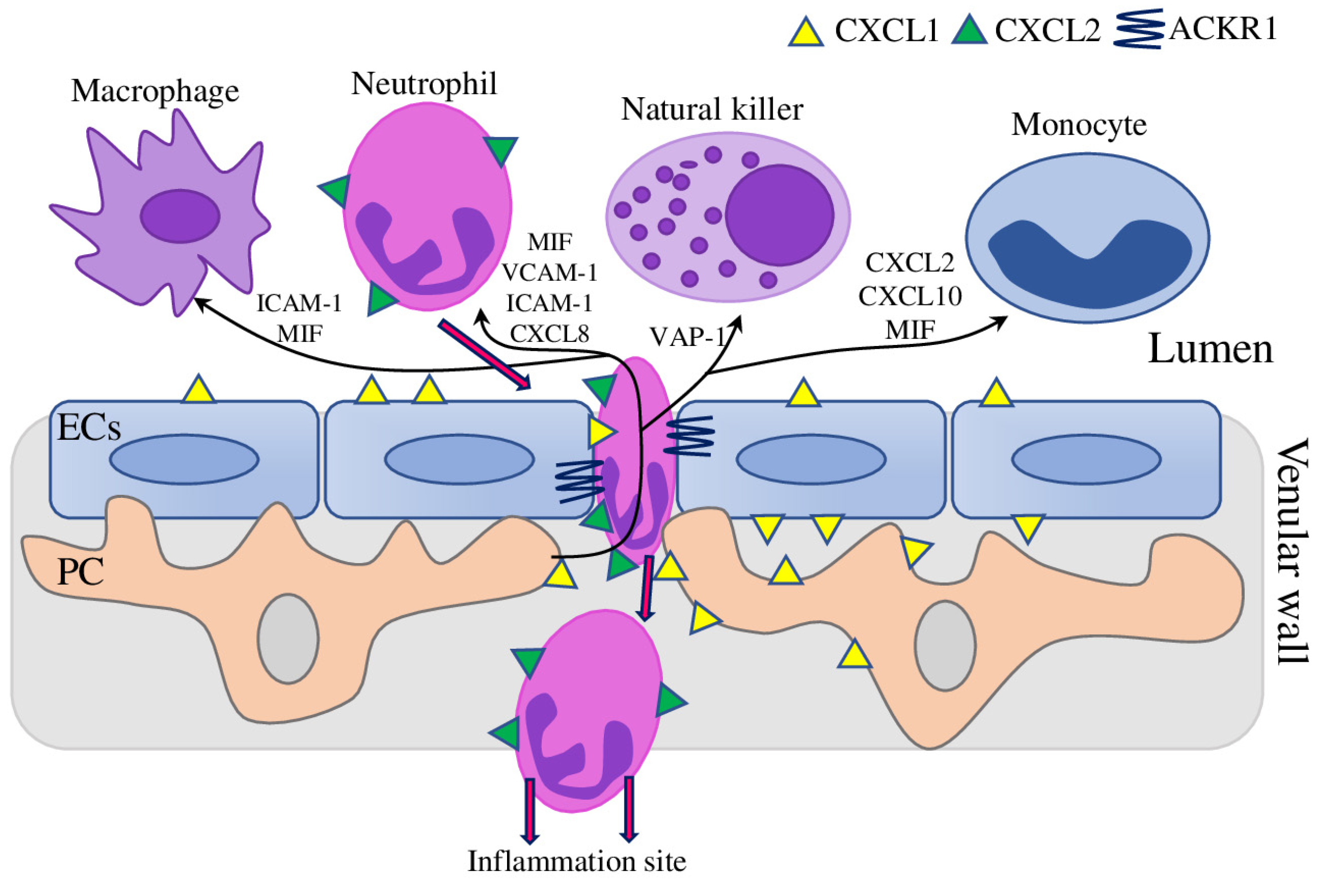
3.3. PC in the Regulation of Immune Cell Trafficking
4. Pericytes in the Regulation of the Adaptive Immune System
PC in Allergic Asthma and Pulmonary Fibrosis
5. Cancer Evokes Immunosuppressive Function in PC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACKR1 | Atypical chemokine receptor 1 |
| Ang-1/Tie-2 | Angiopoietin 1/TEK Receptor Tyrosine Kinase |
| APC | Antigen-presenting cell |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| C/EBP | CCAAT enhancer binding protein |
| C1q | Complement C1q Chain A |
| C5aR1 | Complement Component 5a Receptor 1 |
| CD105 | Endoglin |
| CD11b | Alpha chain of the integrin Mac-1/CR3 |
| CD13 | Aminopeptidase N |
| CD163 | Macrophage-Associated Antigen |
| CD4 | T Cell Surface Glycoprotein CD4 |
| CD45 | Leukocyte-common antigen |
| CD68 | Macrophage Antigen CD68 |
| CMA | Chaperone-mediated autophagy |
| COX2 | Cyclooxygenase-2 |
| DAMP | Damage-associated molecular patterns |
| EAE | Experimental auto-immune encephalomyelitis |
| ECs | Endothelial cells |
| FGFb | Basic fibroblast growth factor (b) |
| FOXP3 | Forkhead box P3 |
| FSP | Fibroblast Secretory Protein |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating Factor |
| GROa/b/g | Growth-regulated protein alpha/beta/gamma |
| HMGB1 | High mobility group box 1 |
| HPSCs | Human pluripotent stem cells |
| I/R | Ischaemia-reperfusion |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| IFN-γ | Interferon Gamma |
| Jag1 | Jagged Canonical Notch Ligand 1 |
| LCFAs | Free long-chain fatty acids |
| LPS | Lipopolysaccharide |
| MHC | Major histocompatibility complex |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MCSF | Macrophage colony-stimulating factor |
| MDSC | Myeloid-derived suppressor cells |
| MIF | Macrophage migration–inhibitory factor |
| MIP-1α | Macrophage Inflammatory Protein-1-Alpha |
| MMP2 | Matrix Metallopeptidase 2 |
| MSCs | Multipotent mesenchymal stem cells |
| NG2 | Nerveglial antigen-2/chondroitin sulfate proteoglycan 4 |
| NLRs | NOD-like receptors |
| NOD1 | Nucleotide Binding Oligomerization Domain Containing 1 |
| Notch3 | Notch Receptor 3 |
| NOX4 | NADPH Oxidase 4 |
| PC | Pericytes |
| PDGF-BB | Platelet-derived growth factor-BB |
| PDGFR-β | Platelet-derived growth factor receptor β |
| PD-L1 | Programmed Cell Death 1 Ligand 1 |
| PGN | Peptidoglycan |
| PLGF | Placental growth factor |
| PRR | Pattern-recognition receptors |
| RGS5 | The regulator of G-protein signaling-5 |
| RIPK2 | Receptor Interacting Serine/Threonine Kinase 2 |
| SDF-1a | Stromal cell-derived factor 1 alpha |
| SMAD2/3 | Mad-Related Protein |
| SMCs | Smooth muscle cells |
| SOD2 | Superoxide Dismutase 2 |
| TAM | Tumour-associated macrophages |
| TEM | Effector memory T cells |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| TJ | Tight junction |
| TLRs | Toll-like receptors |
| TNF-α | Tumour Necrosis Factor-Alpha |
| Tregs | Regulatory T cells |
| uNK | Uterine natural killer |
| VAP-1 | Vascular Adhesion Protein-1 |
| VCAM1 | Vascular Cell Adhesion Protein-1 |
| VEGF | Vascular endothelial growth factor |
| αSMA | α-smooth muscle actin |
| βNGF | Nerve growth factor beta |
References
- Meijer, M.E.M.; van Dijk, C.G.; Kramann, R.; Verhaar, M.C.; Cheng, C. Implementation of Pericytes in Vascular Regeneration Strategies. Tissue Eng. Part B: Rev. 2022, 28, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; James, A.W.; Wang, Y.; Tavian, M.; Crisan, M.; Péault, B.M. Blood Vessel Resident Human Stem Cells in Health and Disease. STEM CELLS Transl. Med. 2022, 11, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B. The NG2 Proteoglycan in Pericyte Biology. In Pericyte Biology—Novel Concepts. Advances in Experimental Medicine and Biology; Birbrair, A., Ed.; Springer International Publishing: Cham, Swizterland, 2018; Volume 1109, pp. 5–19. ISBN 978-3-030-02600-4. [Google Scholar]
- Baek, S.-H.; Maiorino, E.; Kim, H.; Glass, K.; Raby, B.A.; Yuan, K. Single Cell Transcriptomic Analysis Reveals Organ Specific Pericyte Markers and Identities. Front. Cardiovasc. Med. 2022, 9, 876591. [Google Scholar] [CrossRef] [PubMed]
- Wobma, H.; Satwani, P. Mesenchymal stromal cells: Getting ready for clinical primetime. Transfus. Apher. Sci. 2021, 60, 103058. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.C.; Rustenhoven, J.; Scotter, E.L.; Schweder, P.; Faull, R.L.; Park, T.I.; Dragunow, M. Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 2018, 92, 48–60. [Google Scholar] [CrossRef]
- Sato, S.; Tang, Y.J.; Wei, Q.; Hirata, M.; Weng, A.; Han, I.; Okawa, A.; Takeda, S.; Whetstone, H.; Nadesan, P.; et al. Mesenchymal Tumors Can Derive from Ng2/Cspg4-Expressing Pericytes with β-Catenin Modulating the Neoplastic Phenotype. Cell Rep. 2016, 16, 917–927. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kılıç, K.; Can, A.; Di Polo, A.; Dalkara, T. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 2018, 7, e34861. [Google Scholar] [CrossRef]
- Muñoz-Fernández, R.; de la Mata, C.; Prados, A.; Perea, A.; Ruiz-Magaña, M.J.; Llorca, T.; Fernández-Rubio, P.; Blanco, O.; Abadía-Molina, A.C.; Olivares, E.G. Human predecidual stromal cells have distinctive characteristics of pericytes: Cell contractility, chemotactic activity, and expression of pericyte markers and angiogenic factors. Placenta 2018, 61, 39–47. [Google Scholar] [CrossRef]
- Berger, M.; Bergers, G.; Arnold, B.; Hämmerling, G.J.; Ganss, R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 2005, 105, 1094–1101. [Google Scholar] [CrossRef]
- Hung, C.; Linn, G.; Chow, Y.-H.; Kobayashi, A.; Mittelsteadt, K.; Altemeier, W.A.; Gharib, S.A.; Schnapp, L.M.; Duffield, J.S. Role of Lung Pericytes and Resident Fibroblasts in the Pathogenesis of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2013, 188, 820–830. [Google Scholar] [CrossRef]
- Gomez, I.G.; Duffield, J.S. The FOXD1 lineage of kidney perivascular cells and myofibroblasts: Functions and responses to injury. Kidney Int. Suppl. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, B.; De Meyer, G.R.; Martinet, W. Intraplaque neovascularization as a novel therapeutic target in advanced atherosclerosis. Expert Opin. Ther. Targets 2016, 20, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.; Pugach, I.; Gordon, D.; Orekhov, A. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell 1998, 30, 127–135. [Google Scholar] [CrossRef]
- Rekhter, M.D.; Andreeva, E.R.; Mironov, A.A.; Orekhov, A.N. Three-dimensional cytoarchitecture of normal and atherosclerotic intima of human aorta. Am. J. Pathol. 1991, 138, 569–580. [Google Scholar] [PubMed]
- Andreeva, E.; Serebryakov, V.; Orekhov, A. Gap junctional communication in primary culture of cells derived from human aortic intima. Tissue Cell 1995, 27, 591–597. [Google Scholar] [CrossRef]
- Lin, P.P. Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 2020, 9, 1539. [Google Scholar] [CrossRef]
- Liu, X.-D.; Hoang, A.; Zhou, L.; Kalra, S.; Yetil, A.; Sun, M.; Ding, Z.; Zhang, X.; Bai, S.; German, P.; et al. Resistance to Antiangiogenic Therapy Is Associated with an Immunosuppressive Tumor Microenvironment in Metastatic Renal Cell Carcinoma. Cancer Immunol. Res. 2015, 3, 1017–1029. [Google Scholar] [CrossRef]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef]
- Ciciola, P.; Cascetta, P.; Bianco, C.; Formisano, L.; Bianco, R. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents. J. Clin. Med. 2020, 9, 675. [Google Scholar] [CrossRef]
- Ribatti, D.; Solimando, A.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, F.; Errede, M.; Bizzoca, A.; Virgintino, D.; Ribatti, D. Central Nervous System Pericytes Contribute to Health and Disease. Cells 2022, 11, 1707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, M.; Ying, Y.; Wu, Q.; Huang, Z.; Ni, W.; Wang, X.; Xu, H.; Bennett, S.; Xiao, J.; et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022, 10, 30. [Google Scholar] [CrossRef]
- Rajendran, S.; Seetharaman, S.; Dharmarajan, A.; Kuppan, K. Microvascular cells: A special focus on heterogeneity of pericytes in diabetes associated complications. Int. J. Biochem. Cell Biol. 2021, 134, 105971. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Markin, A.M.; Andreeva, E.R.; Eremin, I.I.; Orekhov, A.N.; Melnichenko, A.A. Molecular Mechanisms Underlying Pathological and Therapeutic Roles of Pericytes in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 11663. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Markin, A.M.; Andreeva, E.R.; Eremin, I.I.; Orekhov, A.N.; Melnichenko, A.A. Emerging role of pericytes in therapy of cardiovascular diseases. Biomed. Pharmacother. 2022, 156, 113928. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Bechmann, I. CNS pericytes: Concepts, misconceptions, and a way out. Glia 2010, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Pieper, C.; Marek, J.J.; Unterberg, M.; Schwerdtle, T.; Galla, H.-J. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014, 1550, 1–8. [Google Scholar] [CrossRef]
- Domev, H.; Milkov, I.; Itskovitz-Eldor, J.; Dar, A. Immunoevasive Pericytes from Human Pluripotent Stem Cells Preferentially Modulate Induction of Allogeneic Regulatory T Cells. STEM CELLS Transl. Med. 2014, 3, 1169–1181. [Google Scholar] [CrossRef]
- Chen, R.; Ganesan, A.; Okoye, I.; Arutyunova, E.; Elahi, S.; Lemieux, M.J.; Barakat, K. Targeting B7-1 in immunotherapy. Med. Res. Rev. 2020, 40, 654–682. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Muramatsu, M.; Azuma, E.; Ikutani, M.; Nagai, Y.; Sagara, H.; Koo, B.-N.; Kita, S.; O’Donnell, E.; Osawa, T.; et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep. 2017, 7, 3855. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Nalbandian, A.; Uchida, Y.; Li, W.; Arnold, T.D.; Kubota, Y.; Yamamoto, S.; Ema, M.; Mukouyama, Y.-S. Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-β Signaling in Developing Skin Vasculature. Cell Rep. 2017, 18, 2991–3004. [Google Scholar] [CrossRef]
- Kittikulsuth, W.; Nakano, D.; Kitada, K.; Suzuki, N.; Yamamoto, M.; Nishiyama, A. Renal NG2-expressing cells have a macrophage-like phenotype and facilitate renal recovery after ischemic injury. Am. J. Physiol. Physiol. 2021, 321, F170–F178. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Muñoz, I.; Compte, M.; Álvarez-Cienfuegos, A.; Álvarez-Vallina, L.; Sanz, L. Lipopolysaccharide Activates Toll-like Receptor 4 (TLR4)-mediated NF-κB Signaling Pathway and Proinflammatory Response in Human Pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef]
- Stark, K.; Eckart, A.; Haidari, S.; Tirniceriu, A.; Lorenz, M.; von Brühl, M.-L.; Gärtner, F.; Khandoga, A.G.; Legate, K.R.; Pless, R.; et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 2013, 14, 41–51. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-Wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Aalderink, M.; Scotter, E.L.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Graham, E.S.; Faull, R.L.M.; Curtis, M.A.; Park, T.I.-H.; et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J. Neuroinflamm. 2016, 13, 37. [Google Scholar] [CrossRef]
- Gaceb, A.; Özen, I.; Padel, T.; Barbariga, M.; Paul-Visse, G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J. Cereb. Blood Flow Metab. 2018, 38, 45–57. [Google Scholar] [CrossRef]
- Shen, J.; Xu, G.; Zhu, R.; Yuan, J.; Ishii, Y.; Hamashima, T.; Matsushima, T.; Yamamoto, S.; Takatsuru, Y.; Nabekura, J.; et al. PDGFR-β restores blood-brain barrier functions in a mouse model of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2019, 39, 1501–1515. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Y.; Chen, M.; Li, L.; Zhang, W. Pericytes for Therapeutic Approaches to Ischemic Stroke. Front. Neurosci. 2021, 15, 629297. [Google Scholar] [CrossRef]
- Park, T.I.-H.; Feisst, V.; Brooks, A.E.S.; Rustenhoven, J.; Monzo, H.J.; Feng, S.X.; Mee, E.W.; Bergin, P.S.; Oldfield, R.; Graham, E.S.; et al. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci. Rep. 2016, 6, 26587. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, L.; Yang, L.; Zhang, Q.; Wang, X. C/EBPγ is a critical negative regulator of LPS-/IgG immune complex-induced acute lung injury through the downregulation of C/EBPβ-/C/EBPδ-dependent C/EBP transcription activation. FASEB J. 2020, 34, 13696–13710. [Google Scholar] [CrossRef]
- Banerjee, S.; Fu, Q.; Shah, S.K.; Melnyk, S.B.; Sterneck, E.; Hauer-Jensen, M.; Pawar, S.A. C/EBPδ protects from radiation-induced intestinal injury and sepsis by suppression of inflammatory and nitrosative stress. Sci. Rep. 2019, 9, 13953. [Google Scholar] [CrossRef] [PubMed]
- Rustenhoven, J.; Scotter, E.L.; Jansson, D.; Kho, D.T.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.M.; Curtis, M.A.; Graham, S.E.; et al. An anti-inflammatory role for C/EBPδ in human brain pericytes. Sci. Rep. 2015, 5, 12132. [Google Scholar] [CrossRef] [PubMed]
- Dolasia, K.; Bisht, M.K.; Pradhan, G.; Udgata, A.; Mukhopadhyay, S. TLRs/NLRs: Shaping the landscape of host immunity. Int. Rev. Immunol. 2018, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Sheikh, B.N.; Guhathakurta, S.; Tsang, T.H.; Schwabenland, M.; Renschler, G.; Herquel, B.; Bhardwaj, V.; Holz, H.; Stehle, T.; Bondareva, O.; et al. Neural metabolic imbalance induced by MOF dysfunction triggers pericyte activation and breakdown of vasculature. Nat. Cell Biol. 2020, 22, 828–841. [Google Scholar] [CrossRef]
- Navarro, R.; Delgado-Wicke, P.; Nuñez-Prado, N.; Compte, M.; Blanco-Toribio, A.; Nuñez, G.; Álvarez-Vallina, L.; Sanz, L. Role of nucleotide-binding oligomerization domain 1 (NOD1) in pericyte-mediated vascular inflammation. J. Cell. Mol. Med. 2016, 20, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Nyúl-Tóth, Á.; Kozma, M.; Nagyőszi, P.; Nagy, K.; Fazakas, C.; Haskó, J.; Molnár, K.; Farkas, A.E.; Végh, A.G.; Váró, G.; et al. Expression of pattern recognition receptors and activation of the non-canonical inflammasome pathway in brain pericytes. Brain Behav. Immun. 2017, 64, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Mittelsteadt, K.L.; Brauer, R.; McKinney, B.L.; Hallstrand, T.S.; Parks, W.C.; Chen, P.; Schnapp, L.M.; Liles, W.C.; Duffield, J.S.; et al. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L556–L567. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.; Sahu, R.K.; Landes, S.G.; Yu, J.; Taylor, R.P.; Ayyadevara, S.; Megyesi, J.; Stallcup, W.B.; Duffield, J.S.; Reis, E.S.; et al. Pericytes and immune cells contribute to complement activation in tubulointerstitial fibrosis. Am. J. Physiol. Renal Physiol. 2017, 312, F516–F532. [Google Scholar] [CrossRef]
- Sahu, R.K.; Xavier, S.; Chauss, D.; Wang, L.; Chew, C.; Taylor, R.; Stallcup, W.B.; Ma, J.Z.; Kazemian, M.; Afzali, B.; et al. Folic acid-mediated fibrosis is driven by C5a receptor 1-mediated activation of kidney myeloid cells. Am. J. Physiol. Renal Physiol. 2022, 322, F597–F610. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Renkawitz, J. Principles of Leukocyte Migration Strategies. Trends Cell Biol. 2020, 30, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.; Venturini, C.; Stirling, D.; Turner, C.; Tezera, L.B.; Ercoli, G.; Baker, T.; Best, K.; Brown, J.S.; Noursadeghi, M. Pericyte derived chemokines amplify neutrophil recruitment across the cerebrovascular endothelial barrier. Front. Immunol. 2022, 13, 935798. [Google Scholar] [CrossRef]
- Török, O.; Schreiner, B.; Schaffenrath, J.; Tsai, H.-C.; Maheshwari, U.; Stifter, S.A.; Welsh, C.; Amorim, A.; Sridhar, S.; Utz, S.G.; et al. Pericytes regulate vascular immune homeostasis in the CNS. Proc. Natl. Acad. Sci. USA 2021, 118, e2016587118. [Google Scholar] [CrossRef]
- Ulusoy, C.; Sekerdag, E.; Yilmaz, V.; Yilmaz, A.B.; Atak, D.; Vural, A.; Kucukali, C.I.; Karaaslan, Z.; Kurtuncu, M.; Gursoy-Ozdemir, Y.; et al. Impact of autoimmune demyelinating brain disease sera on pericyte survival. Arch. Neuropsychiatry 2020, 58, 83–86. [Google Scholar] [CrossRef]
- Joulia, R.; Guerrero-Fonseca, I.M.; Girbl, T.; Coates, J.A.; Stein, M.; Vázquez-Martínez, L.; Lynam, E.; Whiteford, J.; Schnoor, M.; Voehringer, D.; et al. Neutrophil breaching of the blood vessel pericyte layer during diapedesis requires mast cell-derived IL-17A. Nat. Commun. 2022, 13, 7029. [Google Scholar] [CrossRef]
- Lauridsen, H.M.; Pellowe, A.S.; Ramanathan, A.; Liu, R.; Miller-Jensen, K.; McNiff, J.M.; Pober, J.S.; Gonzalez, A.L. Tumor Necrosis Factor-α and IL-17A Activation Induces Pericyte-Mediated Basement Membrane Remodeling in Human Neutrophilic Dermatoses. Am. J. Pathol. 2017, 187, 1893–1906. [Google Scholar] [CrossRef]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M.; et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018, 49, 1062–1076.e6. [Google Scholar] [CrossRef] [PubMed]
- Gharanei, S.; Fishwick, K.; Durairaj, R.P.; Jin, T.; Siamantouras, E.; Liu, K.-K.; Straube, A.; Lucas, E.S.; Weston, C.J.; Rantakari, P.; et al. Vascular Adhesion Protein-1 Determines the Cellular Properties of Endometrial Pericytes. Front. Cell Dev. Biol. 2021, 8, 621016. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.M.; Laschke, M.W.; Rössler, O.G.; Huang, W.; Scheller, A.; Menger, M.D.; Ampofo, E. Nerve/glial antigen (NG) 2 is a crucial regulator of intercellular adhesion molecule (ICAM)-1 expression. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Liao, K.; Hu, G.; Sil, S.; Callen, S.; Guo, M.-L.; Yang, L.; Buch, S. Cocaine-induced release of CXCL10 from pericytes regulates monocyte transmigration into the CNS. J. Cell Biol. 2019, 218, 700–721. [Google Scholar] [CrossRef]
- Sil, S.; Niu, F.; Tom, E.; Liao, K.; Periyasamy, P.; Buch, S. Cocaine Mediated Neuroinflammation: Role of Dysregulated Autophagy in Pericytes. Mol. Neurobiol. 2019, 56, 3576–3590. [Google Scholar] [CrossRef]
- Minervina, A.; Pogorelyy, M.; Mamedov, I. T-cell receptor and B-cell receptor repertoire profiling in adaptive immunity. Transpl. Int. 2019, 32, 1111–1123. [Google Scholar] [CrossRef]
- Maier, C.L.; Pober, J.S. Human Placental Pericytes Poorly Stimulate and Actively Regulate Allogeneic CD4 T Cell Responses. Arter. Thromb. Vasc. Biol. 2011, 31, 183–189. [Google Scholar] [CrossRef]
- Tu, Z.; Li, Y.; Smith, D.S.; Sheibani, N.; Huang, S.; Kern, T.; Lin, F. Retinal Pericytes Inhibit Activated T Cell Proliferation. Investig. Opthalmology Vis. Sci. 2011, 52, 9005–9010. [Google Scholar] [CrossRef]
- Pober, J.S.; Merola, J.; Liu, R.; Manes, T.D. Antigen Presentation by Vascular Cells. Front. Immunol. 2017, 8, 1907. [Google Scholar] [CrossRef]
- Tomé, D. Amino acid metabolism and signalling pathways: Potential targets in the control of infection and immunity. Nutr. Diabetes 2021, 11, 20. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Rossini, S.; Suvieri, C.; Coletti, A.; Orabona, C.; Macchiarulo, A.; Volpi, C.; Grohmann, U. Indoleamine 2,3-dioxygenase 1 (IDO1): An up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 2022, 289, 6099–6118. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Liu, R.; Merola, J.; Manes, T.D.; Qin, L.; Tietjen, G.T.; López-Giráldez, F.; Broecker, V.; Fang, C.; Xie, C.; Chen, P.-M.; et al. Interferon-γ converts human microvascular pericytes into negative regulators of alloimmunity through induction of indoleamine 2,3-dioxygenase 1. JCI Insight 2018, 3, e97881. [Google Scholar] [CrossRef]
- Papamichael, M.; Katsardis, C.; Sarandi, E.; Georgaki, S.; Frima, E.-S.; Varvarigou, A.; Tsoukalas, D. Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment. Metabolites 2021, 11, 251. [Google Scholar] [CrossRef]
- Hough, K.; Curtiss, M.L.; Blain, T.J.; Liu, R.-M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Johnson, J.R.; Folestad, E.; Rowley, J.E.; Noll, E.M.; Walker, S.A.; Lloyd, C.M.; Rankin, S.M.; Pietras, K.; Eriksson, U.; Fuxe, J. Pericytes contribute to airway remodeling in a mouse model of chronic allergic asthma. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L658–L671. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirai, S.; Tanaka, Y.; Sumi, T.; Tada, M.; Takahashi, H.; Watanabe, A.; Sakuma, Y. Pericyte-myofibroblast transition in the human lung. Biochem. Biophys. Res. Commun. 2020, 528, 269–275. [Google Scholar] [CrossRef]
- Hannan, R.T.; Miller, A.E.; Hung, R.-C.; Sano, C.; Peirce, S.M.; Barker, T.H. Extracellular matrix remodeling associated with bleomycin-induced lung injury supports pericyte-to-myofibroblast transition. Matrix Biol. Plus 2021, 10, 100056. [Google Scholar] [CrossRef]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg-Riethmacher, E.; Miehe, M.; Riethmacher, D. Periostin in Allergy and Inflammation. Front. Immunol. 2021, 12, 722170. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.K.; Jonker, M.R.; Berg, M.; Hacken, N.T.H.T.; Meyer, K.B.; Berge, M.V.D.; Nawijn, M.C.; Heijink, I.H. Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 2021, 57, 2001286. [Google Scholar] [CrossRef] [PubMed]
- Bignold, R.E.; Johnson, J.R. Matricellular Protein Periostin Promotes Pericyte Migration in Fibrotic Airways. Front. Allergy 2021, 2, 786034. [Google Scholar] [CrossRef] [PubMed]
- Palikhe, N.S.; Mackenzie, C.A.; Licskai, C.; Kim, R.B.; Vliagoftis, H.; Cameron, L. The CRTh2 polymorphism rs533116 G > A associates with asthma severity in older females. Front. Med. 2022, 9, 970495. [Google Scholar] [CrossRef]
- Singh, D.; Ravi, A.; Southworth, T. CRTH2 antagonists in asthma: Current perspectives. Clin. Pharmacol. Adv. Appl. 2017, 9, 165–173. [Google Scholar] [CrossRef]
- Fowler, A.; Koenen, R.; Hilbert, J.; Blatchford, J.; Kappeler, D.; Benediktus, E.; Wood, C.; Gupta, A. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Novel CRTH2 Antagonist BI 1021958 at Single Oral Doses in Healthy Men and Multiple Oral Doses in Men and Women with Well-Controlled Asthma. J. Clin. Pharmacol. 2017, 57, 1444–1453. [Google Scholar] [CrossRef]
- Miller, D.; Wood, C.; Bateman, E.; LaForce, C.; Blatchford, J.; Hilbert, J.; Gupta, A.; Fowler, A. A randomized study of BI 671800, a CRTH2 antagonist, as add-on therapy in poorly controlled asthma. Allergy Asthma Proc. 2017, 38, 157–164. [Google Scholar] [CrossRef]
- Rajapaksa, K.S.; Huang, T.; Sharma, N.; Liu, S.; Solon, M.; Reyes, A.; Paul, S.; Yee, A.; Tao, J.; Chalasani, S.; et al. Preclinical Safety Profile of a Depleting Antibody against CRTh2 for Asthma: Well Tolerated Despite Unexpected CRTh2 Expression on Vascular Pericytes in the Central Nervous System and Gastric Mucosa. Toxicol. Sci. 2016, 152, 72–84. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Okamoto, O.K. Combined Effects of Pericytes in the Tumor Microenvironment. Stem Cells Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Picoli, C.C.; Gonçalves, B.; Santos, G.S.; Rocha, B.G.; Costa, A.C.; Resende, R.R.; Birbrair, A. Pericytes cross-talks within the tumor microenvironment. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188608. [Google Scholar] [CrossRef] [PubMed]
- Sena, I.F.G.; Paiva, A.E.; Prazeres, P.H.D.M.; Azevedo, P.O.; Lousado, L.; Bhutia, S.K.; Salmina, A.B.; Mintz, A.; Birbrair, A. Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med. 2018, 7, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Tobin, N.P.; Rundqvist, H.; Li, T.; Lavergne, M.; García-Ibáñez, Y.; Qin, H.; Paulsson, J.; Zeitelhofer, M.; Adzemovic, M.Z.; et al. Role of Tumor Pericytes in the Recruitment of Myeloid-Derived Suppressor Cells. JNCI: J. Natl. Cancer Inst. 2015, 107, djv209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Andersson, P.; Hosaka, K.; Zhang, Y.; Cao, R.; Iwamoto, H.; Yang, X.; Nakamura, M.; Wang, J.; Zhuang, R.; et al. The PDGF-BB-SOX7 axis-modulated IL-33 in pericytes and stromal cells promotes metastasis through tumour-associated macrophages. Nat. Commun. 2016, 7, 11385. [Google Scholar] [CrossRef]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive mechanisms in glioblastoma: Figure 1. Neuro-Oncol. 2015, 17, vii9–vii14. [Google Scholar] [CrossRef]
- Valdor, R.; García-Bernal, D.; Bueno, C.; Ródenas, M.; Moraleda, J.M.; Macian, F.; Martínez, S. Glioblastoma progression is assisted by induction of immunosuppressive function of pericytes through interaction with tumor cells. Oncotarget 2017, 8, 68614–68626. [Google Scholar] [CrossRef]
- Molina, M.L.; García-Bernal, D.; Martinez, S.; Valdor, R. Autophagy in the Immunosuppressive Perivascular Microenvironment of Glioblastoma. Cancers 2019, 12, 102. [Google Scholar] [CrossRef]
- Kirchner, P.; Bourdenx, M.; Madrigal-Matute, J.; Tiano, S.; Diaz, A.; Bartholdy, B.A.; Will, B.; Cuervo, A.M. Proteome-wide analysis of chaperone-mediated autophagy targeting motifs. PLoS Biol. 2019, 17, e3000301. [Google Scholar] [CrossRef]
- Arias, E.; Cuervo, A.M. Pros and Cons of Chaperone-Mediated Autophagy in Cancer Biology. Trends Endocrinol. Metab. 2020, 31, 53–66. [Google Scholar] [CrossRef]
- Valdor, R.; García-Bernal, D.; Riquelme, D.; Martinez, C.M.; Moraleda, J.M.; Cuervo, A.M.; Macian, F.; Martinez, S. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc. Natl. Acad. Sci. USA 2019, 116, 20655–20665. [Google Scholar] [CrossRef]
- Molina, M.L.; García-Bernal, D.; Salinas, M.D.; Rubio, G.; Aparicio, P.; Moraleda, J.M.; Martínez, S.; Valdor, R. Chaperone-Mediated Autophagy Ablation in Pericytes Reveals New Glioblastoma Prognostic Markers and Efficient Treatment Against Tumor Progression. Front. Cell Dev. Biol. 2022, 10, 797945. [Google Scholar] [CrossRef]
- Blervaque, L.; Passerieux, E.; Pomiès, P.; Catteau, M.; Héraud, N.; Blaquière, M.; Bughin, F.; Ayoub, B.; Molinari, N.; Cristol, J.-P.; et al. Impaired training-induced angiogenesis process with loss of pericyte-endothelium interactions is associated with an abnormal capillary remodelling in the skeletal muscle of COPD patients. Respir. Res. 2019, 20, 278. [Google Scholar] [CrossRef]
- Blervaque, L.; Pomiès, P.; Rossi, E.; Catteau, M.; Blandinières, A.; Passerieux, E.; Blaquière, M.; Ayoub, B.; Molinari, N.; Mercier, J.; et al. COPD is deleterious for pericytes: Implications during training-induced angiogenesis in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1142–H1151. [Google Scholar] [CrossRef]
- Jerome, J.A.; Wenzel, S.E.; Bittar, H.E.T. Digital Imaging Analysis Reveals Reduced Alveolar α-Smooth Muscle Actin Expression in Severe Asthma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 506–512. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.Q.; Lim, C.M.; Sinha, A.K.; Tan, C.S.; Chan, G.H.J.; Huang, Y.; Kumarakulasinghe, N.B.; Sundar, R.; Jeyasekharan, A.D.; Loh, W.S.; et al. Integration of Antiangiogenic Therapy with Cisplatin and Gemcitabine Chemotherapy in Patients with Nasopharyngeal Carcinoma. Clin. Cancer Res. 2020, 26, 5320–5328. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, A.; Rushing, E.J.; Kirschenbaum, D.; Mangana, J.; Mittmann, C.; Moch, H.; Lugassy, C.; Barnhill, R.L.; Mihic-Probst, D. Eight autopsy cases of melanoma brain metastases showing angiotropism and pericytic mimicry. Implications for extravascular migratory metastasis. J. Cutan. Pathol. 2019, 46, 570–578. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Calimeri, T.; Ponzoni, M.; Curnis, F.; Conte, G.M.; Scarano, E.; Rrapaj, E.; De Lorenzo, D.; Cattaneo, D.; Fallanca, F.; et al. Improving the antitumor activity of R-CHOP with NGR-hTNF in primary CNS lymphoma: Final results of a phase 2 trial. Blood Adv. 2020, 4, 3648–3658. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Morimoto, Y.; Kosugi, K.; Sato, M.; Oishi, Y.; Ueda, R.; Kikuchi, R.; Nagashima, H.; Hikichi, T.; Noji, S.; et al. Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma—A case series. BMC Cancer 2020, 20, 196. [Google Scholar] [CrossRef]


| Marker | Location | Function | Other Cell Types/Tissues/Organs | References |
|---|---|---|---|---|
| NG2 | Arteriolar and capillary pericytes | Pericyte/endothelial cell interaction in tumour angiogenesis | Cancer cells, Oesophagus, Placenta, Uterus and others | [7] |
| αSMA | Capillary pericytes | Regulates contraction/relaxation | Smooth muscle cells | [8] |
| PDGFR-β | Brain pericytes | Pericytes recruitment during embryogenic angiogenesis | Fibroblasts and smooth muscle cells | [9] |
| RGS5 | Brain pericytes in mouse embryogenic development | Tumour and embryogenic angiogenesis | Abundantly expressed in blood vessels, heart, lung, skeletal muscle, and small intestine | [10] |
| FOXD1+ - progeny | Lung and kidney pericytes | Contributes to the myofibroblast pool in kidney and pulmonary fibrosis | Lung and kidney perivascular cells and myofibroblasts | [11,12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Andreeva, E.R.; Eremin, I.I.; Markin, A.M.; Nadelyaeva, I.I.; Orekhov, A.N.; Melnichenko, A.A. The Role of Pericytes in Regulation of Innate and Adaptive Immunity. Biomedicines 2023, 11, 600. https://doi.org/10.3390/biomedicines11020600
Dabravolski SA, Andreeva ER, Eremin II, Markin AM, Nadelyaeva II, Orekhov AN, Melnichenko AA. The Role of Pericytes in Regulation of Innate and Adaptive Immunity. Biomedicines. 2023; 11(2):600. https://doi.org/10.3390/biomedicines11020600
Chicago/Turabian StyleDabravolski, Siarhei A., Elena R. Andreeva, Ilya I. Eremin, Alexander M. Markin, Irina I. Nadelyaeva, Alexander N. Orekhov, and Alexandra A. Melnichenko. 2023. "The Role of Pericytes in Regulation of Innate and Adaptive Immunity" Biomedicines 11, no. 2: 600. https://doi.org/10.3390/biomedicines11020600
APA StyleDabravolski, S. A., Andreeva, E. R., Eremin, I. I., Markin, A. M., Nadelyaeva, I. I., Orekhov, A. N., & Melnichenko, A. A. (2023). The Role of Pericytes in Regulation of Innate and Adaptive Immunity. Biomedicines, 11(2), 600. https://doi.org/10.3390/biomedicines11020600







