An Array SPRi Biosensor for the Determination on PARP-1 in Blood Plasma
Abstract
:1. Introduction
2. Experimental
2.1. Material and Reagents
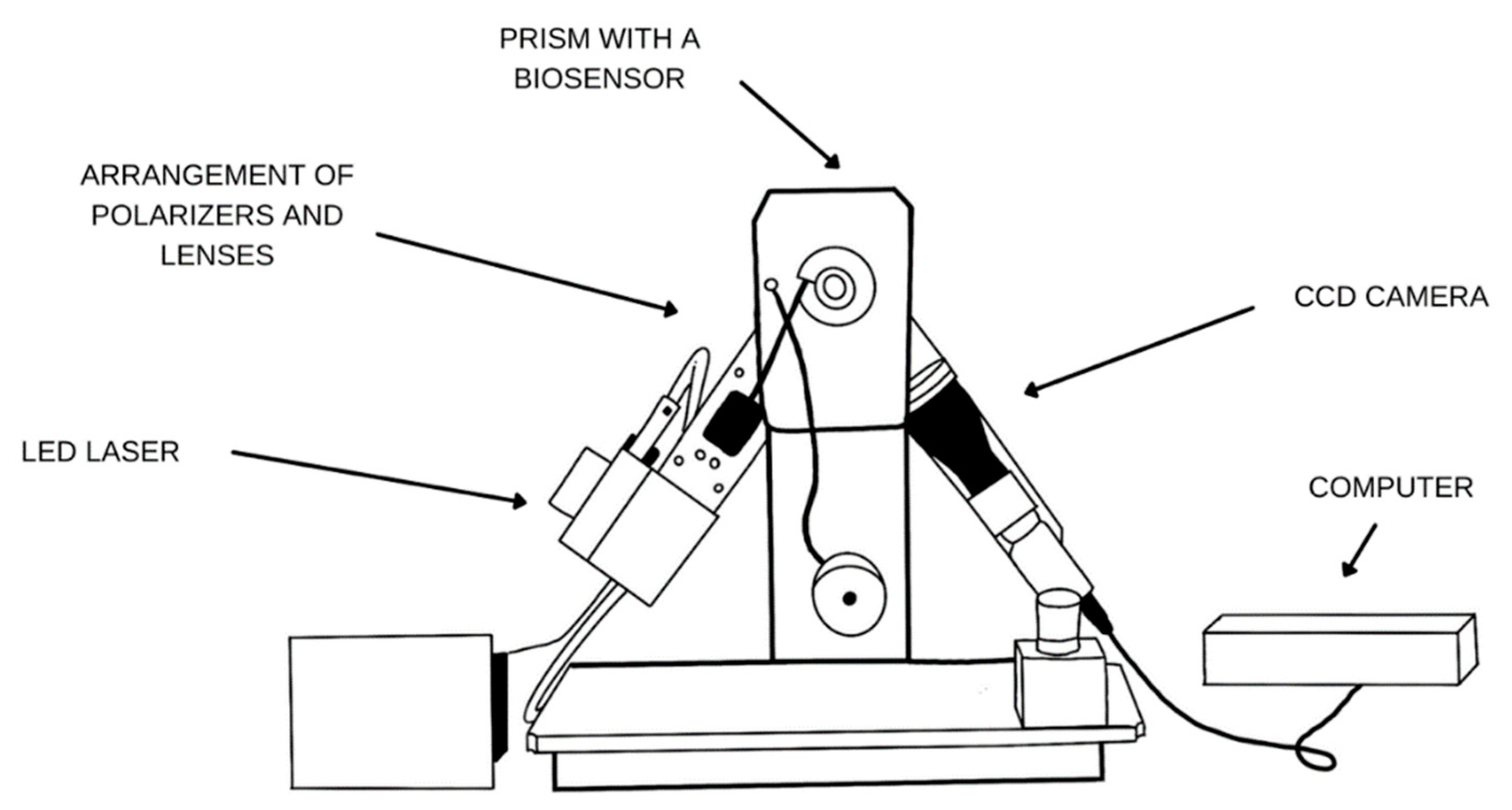
2.2. Apparatus
2.3. Biological Material
2.4. Procedures
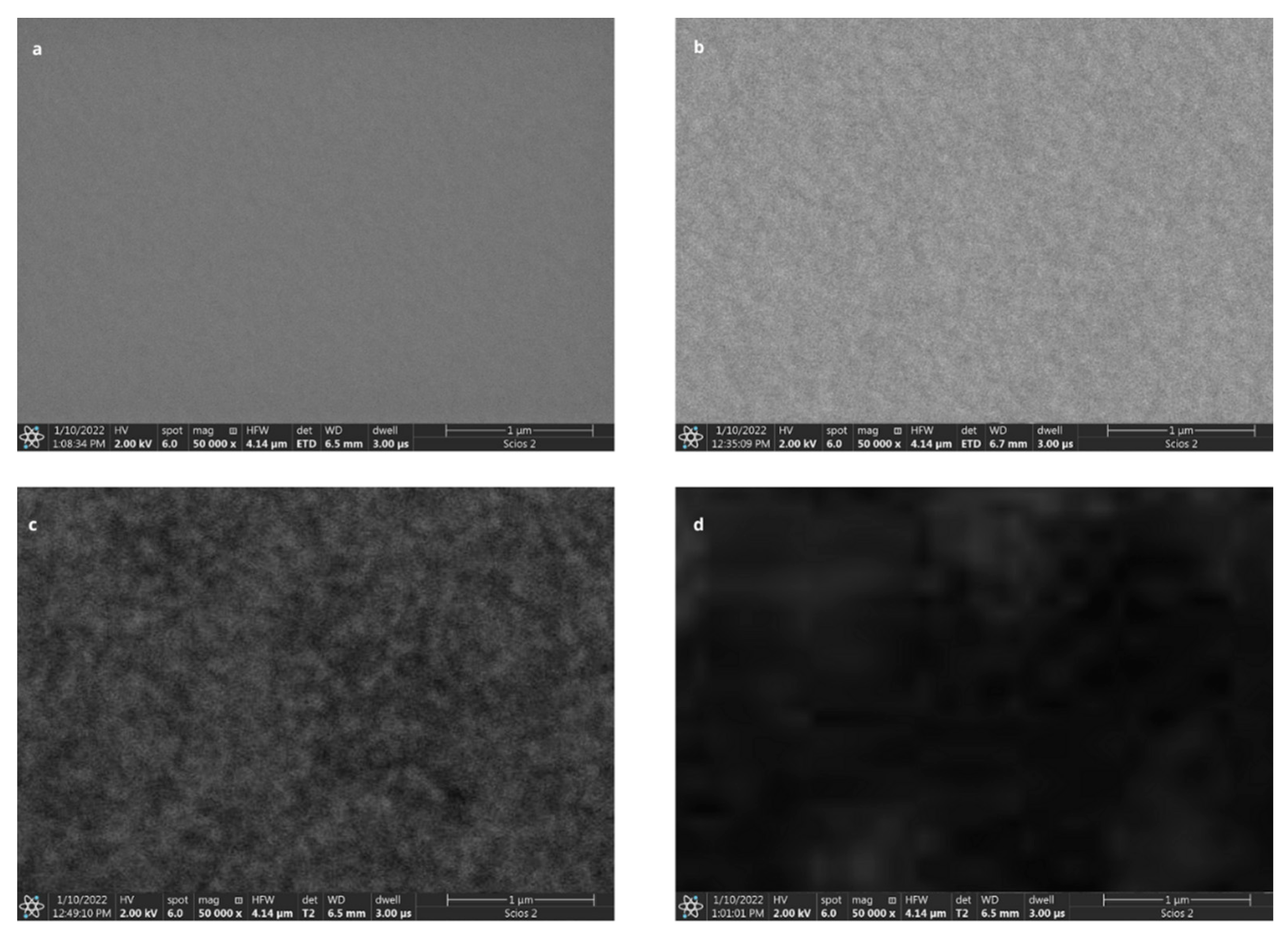
2.4.1. Chip Preparation and Ligand Immobilization
2.4.2. SPRi Measurement
2.4.3. ELISA Measurement
2.4.4. Preparation of Biological Samples
3. Results and Discussion
3.1. Optimization of the Concentration of the Ligand (Antibody) Monolayer
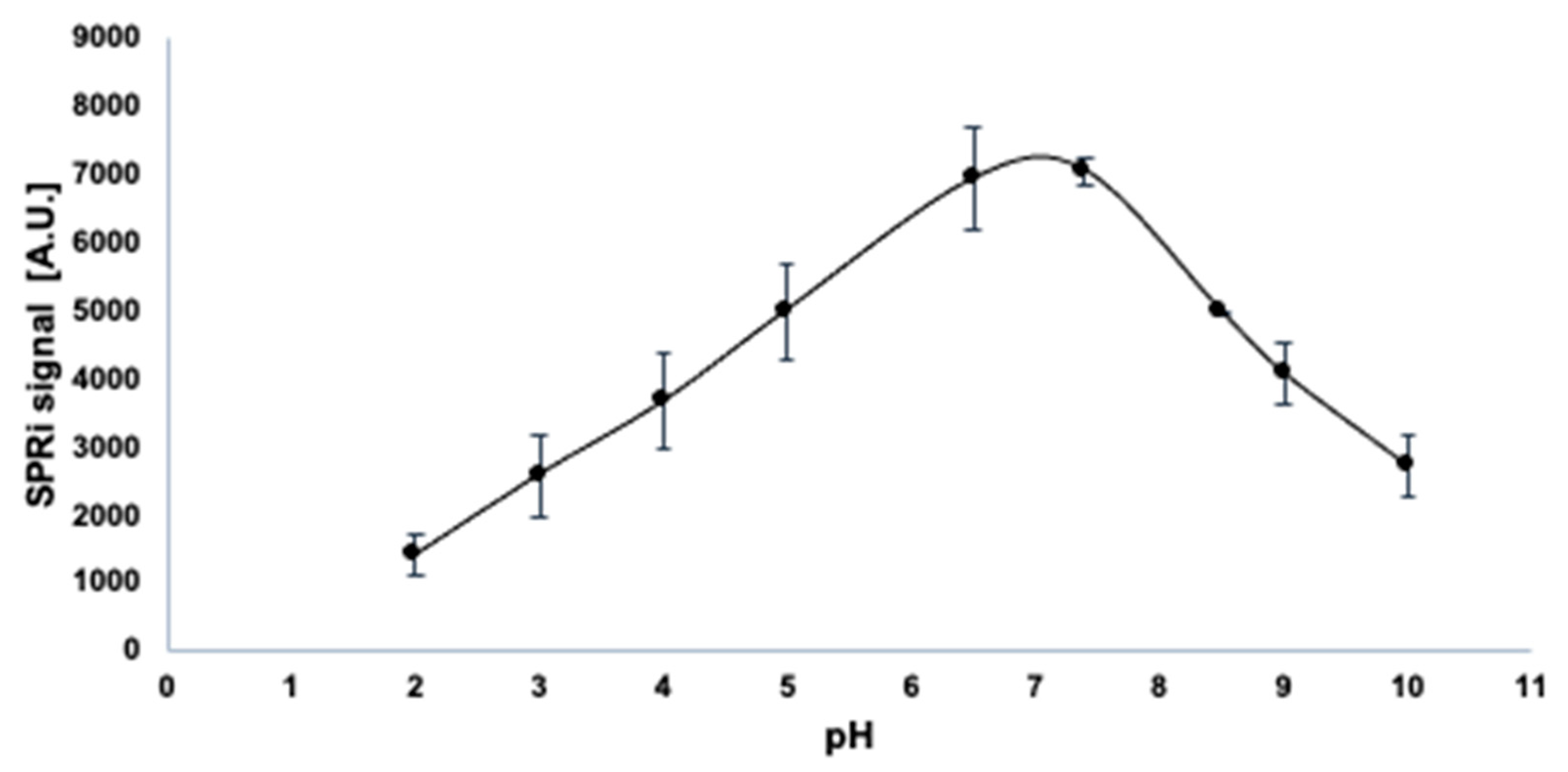
3.2. Influence of pH on the Activity of PARP-1
3.3. Analytical Response to PARP-1 Concentration
- LOD—limit of detection
- LOQ—limit of quantification
- SD—standard deviation of the blank
- a—slope of the calibration curve

3.4. Precision and Accuracy of the Determination of PARP-1 Concentration
3.5. The Influence of Interferents on the Analytical Signal
3.6. PARP-1 Determination in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A




| Patients Group | ||
|---|---|---|
| Number of Sample | SPRi | ELISA |
| Concentration of PARP-1 [ng∙mL−1] | Concentration of PARP-1 [ng∙mL−1] | |
| 1A | 11.046 | 11.140 |
| 2A | 18.024 | 18.350 |
| 3A | 7.679 | 7.744 |
| 4A | 13.011 | 13.490 |
| 5A | 20.576 | 20.360 |
| 6A | 15.294 | 15.390 |
| 7A | 9.459 | 9.750 |
| 8A | 13.875 | 13.780 |
| 9A | 10.233 | 10.180 |
| Control group | ||
| Number of sample | SPRi | ELISA |
| Concentration of PARP-1 [ng∙mL−1] | Concentration of PARP-1 [ng∙mL−1] | |
| 1 | 0.197 | 0.196 |
| 2 | 0.163 | 0.160 |
| 3 | 0.113 | 0.141 |
| 4 | 0.140 | 0.131 |
| 5 | 0.155 | 0.160 |
| 6 | 0.144 | 0.115 |
| 7 | 0.209 | 0.222 |
| 8 | 0.369 | 0.386 |
| 9 | 0.427 | 0.451 |
References
- Otto, H.; Reche, P.A.; Bazan, F.; Dittmar, K.; Haag, F.; Koch-Nolte, F. In silico characterization of the family of PARP-like poly(ADP-ribosyl)transferases (PARTs). BMC Genom. 2005, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Donizy, P.; Pietrzyk, G.; Halon, A.; Kozyra, C.; Gansukh, T.; Lage, H.; Surowiak, P.; Matowski, R. Nuclear-cytoplasmic PARP-1 expression as an unfavorable prognostic marker in lymph node-negative early breast cancer: 15-year follow-up. Oncol. Rep. 2014, 31, 1777–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotova, E.; Jarnik, M.; Tulin, A.V. Poly (ADP-Ribose) Polymerase 1 Is Required for Protein Localization to Cajal Body. PLoS Genet. 2009, 5, e1000387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Fan, J.; Shangguan, L.; Liu, Y.; Wei, Y.; Wei, W.; Liu, S. Ultrasensitive electrochemical detection of poly (ADP-ribose) polymerase-1 via polyaniline deposition. Talanta 2018, 180, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, M.; Noguchi, S.; Yamashita, R.; Ogura, T.; Sugimura, T.; Gill, D.M.; Miwa, M. The zinc fingers of human poly(ADP-ribose) polymerase are differentially required for the recognition of DNA breaks and nicks and the consequent enzyme activation. Other structures recognize intact DNA. J. Biol. Chem. 1990, 265, 21907–21913. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl, G.; Ménissier de Murcia, J.M.; Molinete, M.; Simonin, F.; Koken, M.; Hoeijmakers, J.H.; de Murcia, G. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc. Natl. Acad. Sci. USA 1990, 87, 2990–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Gao, P.; Hoffman, D.W.; Liu, H.W. Domain C of human poly(ADP-ribose) polymerase-1 is important for enzyme activity and contains a novel zinc-ribbon motif. Biochemistry 2008, 47, 5804–5813. [Google Scholar] [CrossRef]
- Kilianska, Z.M.; Żołnierczyk, J.; Węsierska-Gądek, J. Biological activity of poly(ADP-ribose)polymerase-1. Postepy High Med. Dosw. 2010, 64, 344–363. [Google Scholar]
- Wiśnik, E.; Ryksa, M.; Koter-Michalak, M. Inhibitory PARP1: Współczesne próby zastosowania w terapii przeciwnowotworowej i perspektywy na przyszłość. Postepy High Med. Dosw. 2016, 70, 280–294. [Google Scholar] [CrossRef]
- Vyas, S.; Matic, I.; Uchima, L. Family-wide analysis of poly(ADP-ribose) polymerase activity. Nat. Commun. 2014, 5, 4426. [Google Scholar] [CrossRef] [Green Version]
- Mao, K.; Zhang, G. The role of PARP1 in neurodegenerative diseases and aging. FEBS J. 2022, 289, 2013–2024. [Google Scholar] [CrossRef]
- Rulten, S.L.; Rotheray, A.; Green, R.L.; Grundy, G.J.; Moore, D.A.Q.; Gómez-Herreros, F.; Hafezparast, M.; Caldecott, K.W. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res. 2014, 42, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Guo, W.; Mitra, J.; Hegde, P.M.; Vandoorne, T.; Eckelmann, B.J.; Mitra, S.; Tomkinson, A.E.; Van Den Bosch, L.; Hegde, M.L. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat. Commun. 2018, 9, 3683. [Google Scholar] [CrossRef] [Green Version]
- Pazzaglia, S.; Pioli, C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnik, P.; Kacperczyk-Bartnik, J.; Goławski, K.; Sierdziński, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; et al. Plasma and Peritoneal Fluid ZEB Levels in Patients with Endometriosis and Infertility. Biomedicines 2022, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk-Bartnik, J.; Bartnik, P.; Goławski, K.; Sierdziński, J.; Mańka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczyński, R.; Piekarski, P.; et al. Plasma and Peritoneal Poly (ADP-Ribose) Polymerase Levels in Patients with Endometriosis. Biomedicines 2022, 10, 2451. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Ly, D.H.; Rosenthal, D.S.; Konopka, G.; Luo, R.; Wang, Z.Q.; Schultz, P.G.; Smulson, M.E. Misregulation of gene expression in primary fibroblasts lacking poly(ADP-ribose)polymerase. Proc. Natl. Acad. Sci. USA 2000, 97, 11274–11279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znajewska-Szulc, K.; Wileński, S. Niraparib—A chance for ovarian cancer patients without BRCA mutation? Farmacja Współczesna 2021, 14, 55–63. [Google Scholar]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar]
- Musacchio, L.; Caruso, G.; Pisano, C.; Cecere, S.C.; Di Napoli, M.; Attademo, L.; Tambaro, R.; Russo, D.; Califano, D.; Palaia, I.; et al. PARP inhibitors in endometrial cancer: Current status and perspectives. Cancer Manag. Res. 2020, 12, 6123–6135. [Google Scholar] [CrossRef]
- Yang, H.; Li, P.; Wang, D.; Liu, Y.; Wei, W.; Zhang, Y.; Liu, S. Quartz crystal microbalance detection of Poly(ADP-ribose) polymerase-1 based on gold nanorods signal amplification. Anal. Chem. 2019, 91, 11038–11044. [Google Scholar] [CrossRef] [PubMed]
- Moonen, H.J.J.; Geraets, L.; Vaarhorst, A.; Bast, A.; Wouters, E.F.M.; Hageman., G.J. Theophylline prevents NAD+ depletion via PARP-1 inhibition in human pulmonary epithelial cells. Biochem. Biophys. Res. Commun. 2005, 338, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Sablinskas, V.; Hübner, A.; Kuhne, C.; Salzer, R. Surface plasmon resonance imaging of microstructured monolayers. J. Mol. Struct. 1999, 509, 265–273. [Google Scholar] [CrossRef]
- Laudański, P.; Gorodkiewicz, E.; Ramotowska, B.; Charkiewicz, R.; Kuzmicki, M.; Szamatowicz, J. Determination of cathepsins B, D and G concentration in eutopicproliferative endometrium of women with endometriosis by surface plasmon resonance imaging (SPRI) technique. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Sankiewicz, A.; Romanowicz, L.; Laudanski, P.; Zelazowska-Rutkowska, B.; Puzan, B.; Cylwik, B.; Gorodkiewicz, E. SPR imaging biosensor for determination of laminin-5 as a potential cancer marker in biological material. Anal. Bioanal. Chem. 2016, 408, 5269–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorodkiewicz, E.; Sankiewicz, A.; Laudański, P. Surface plasmon resonance imaging biosensors for aromatase based on a potent inhibitor and a specific antibody: Sensor development and application for biological material. Cent. Eur. J. Chem. 2014, 12, 557–567. [Google Scholar] [CrossRef]
- Pattnaik, P. Surface plasmon resonance: Applications in understanding receptor-ligand interaction. Appl. Biochem. Biotechnol. 2005, 126, 79–92. [Google Scholar] [CrossRef]
- Steiner, G. Surface plasmon resonance imaging. Anal. Bioanal. Chem. 2004, 379, 328–331. [Google Scholar] [CrossRef]
- Gorodkiewicz, E.; Sieńczyk, M.; Regulska, E.; Grzywa, R.; Pietrusewicz, E.; Lesner, A.; Łukaszewski, Z. Surface plasmon resonance imaging biosensor for cathepsin G based on a potent inhibitor: Development and applications. Anal. Biochem. 2012, 423, 218–223. [Google Scholar] [CrossRef]
- Konopsky, V.; Mitko, T.; Aldarov, K.; Alieva, E.; Basmanov, D.; Moskalets, A.; Matveeva, A.; Morozova, O.; Klinov, D. Photonic crystal surface mode imaging for multiplexed and high-throughput label-free biosensing. Biosens. Bioelectron. 2020, 168, 112575. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Lukaszewski, Z.; Trojanowska, K.; Gorodkiewicz, E. Determination of collagen type IV by Surface Plasmon Resonance Imaging using a specific biosensor. Anal. Biochem. 2016, 515, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Barreta, A.; Sarian, L.O.; Ferracini, A.C.; Costa, L.B.E.; Mazzola, P.G.; de Angelo Andrade, L.; Derchain, S. Immunohistochemistry expression of targeted therapies biomarkers in ovarian clear cell and endometrioid carcinomas (type I) and endometriosis. Hum. Pathol. 2019, 85, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Postawski, K.; Monist, M.; Keith, G. PARP-1 activity in normal and cancerous human endometrium and its relationship with quantity of abasic sites (AP). Ginekol. Pol. 2011, 82, 16–21. [Google Scholar] [PubMed]



| Ctheoretical [ng∙mL−1] | Cfound [ng∙mL−1] | SD [ng∙mL−1] | Recovery [%] | RSD [%] |
|---|---|---|---|---|
| 0.010 | 0.099 | 0.0001 | 99 | 0.0101 |
| 0.500 | 0.5007 | 0.0127 | 100.14 | 0.0254 |
| 1.000 | 1.0335 | 0.0398 | 103.35 | 0.0385 |
| Potential Interferent | CPARP-1 vs. Cinterferent | Cfound [ng∙mL−1] | Recovery [%] |
|---|---|---|---|
| Human albumin | 1:1 | 0.517 | 103 |
| 1:10 | 0.512 | 102 | |
| 1:100 | 0.500 | 100 | |
| PARP-2 protein | 1:1 | 0.527 | 105 |
| 1:10 | 0.507 | 101 | |
| 1:100 | 0.499 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielinska, Z.; Oldak, L.; Kacperczyk-Bartnik, J.; Koc-Żórawska, E.; Żórawski, M.; Laudanski, P.; Gorodkiewicz, E. An Array SPRi Biosensor for the Determination on PARP-1 in Blood Plasma. Biomedicines 2023, 11, 602. https://doi.org/10.3390/biomedicines11020602
Zielinska Z, Oldak L, Kacperczyk-Bartnik J, Koc-Żórawska E, Żórawski M, Laudanski P, Gorodkiewicz E. An Array SPRi Biosensor for the Determination on PARP-1 in Blood Plasma. Biomedicines. 2023; 11(2):602. https://doi.org/10.3390/biomedicines11020602
Chicago/Turabian StyleZielinska, Zuzanna, Lukasz Oldak, Joanna Kacperczyk-Bartnik, Ewa Koc-Żórawska, Marcin Żórawski, Piotr Laudanski, and Ewa Gorodkiewicz. 2023. "An Array SPRi Biosensor for the Determination on PARP-1 in Blood Plasma" Biomedicines 11, no. 2: 602. https://doi.org/10.3390/biomedicines11020602





