Racial Differences in Androgen Receptor (AR) and AR Splice Variants (AR-SVs) Expression in Treatment-Naïve Androgen-Dependent Prostate Cancer
Abstract
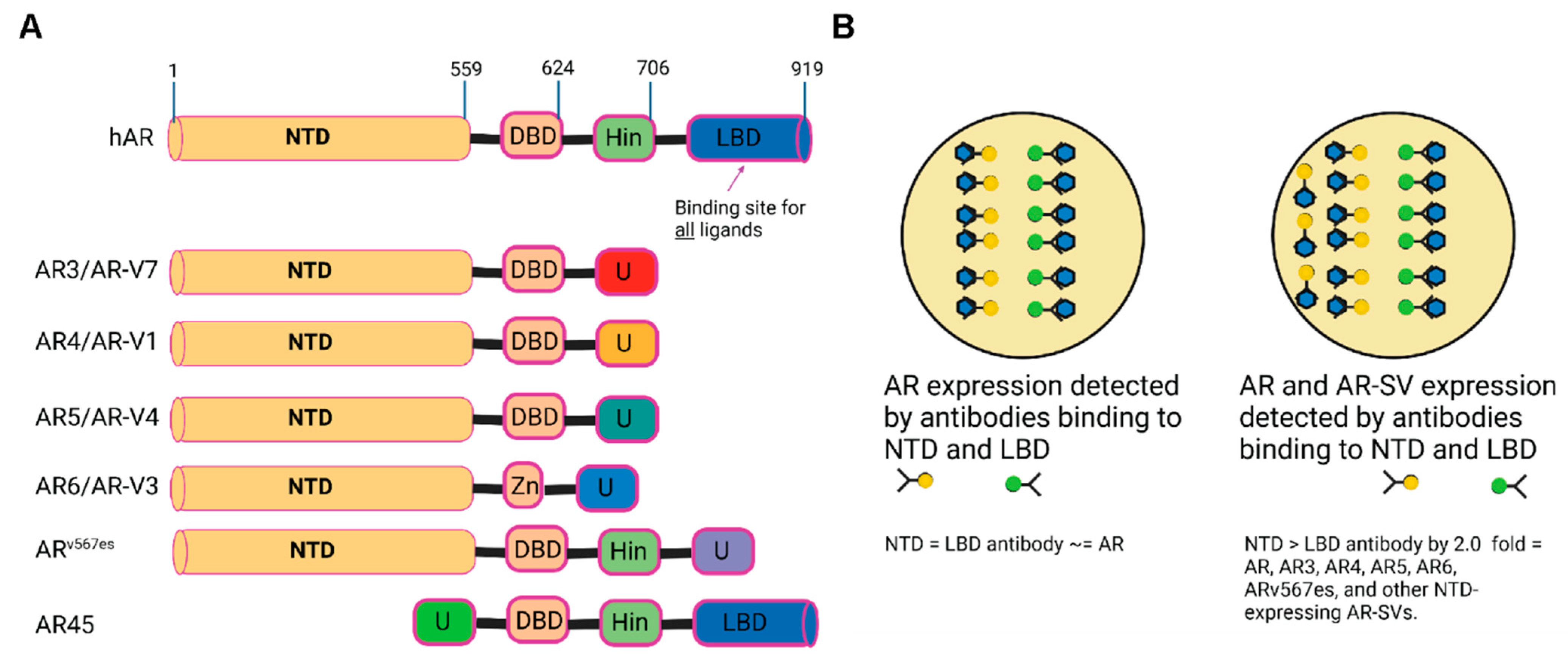
:1. Introduction
2. Materials and Methods
2.1. TMA Creation and Staining
2.2. Statistical Analysis
2.3. Patient-Derived Xenograft
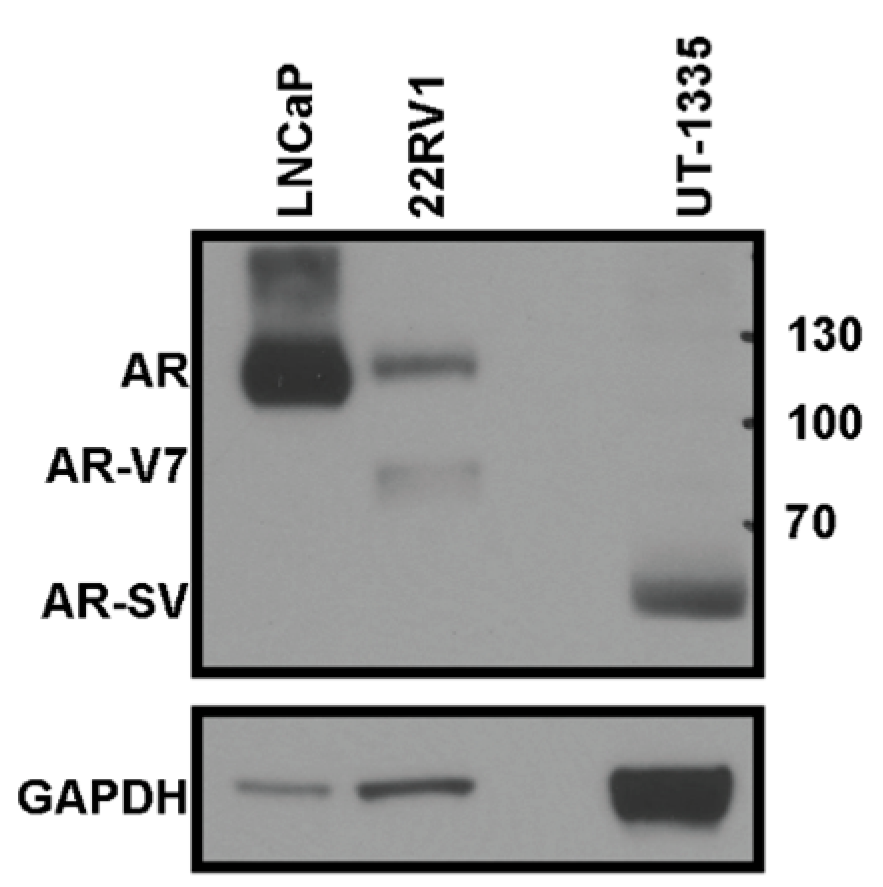
2.4. Western Blot
3. Results
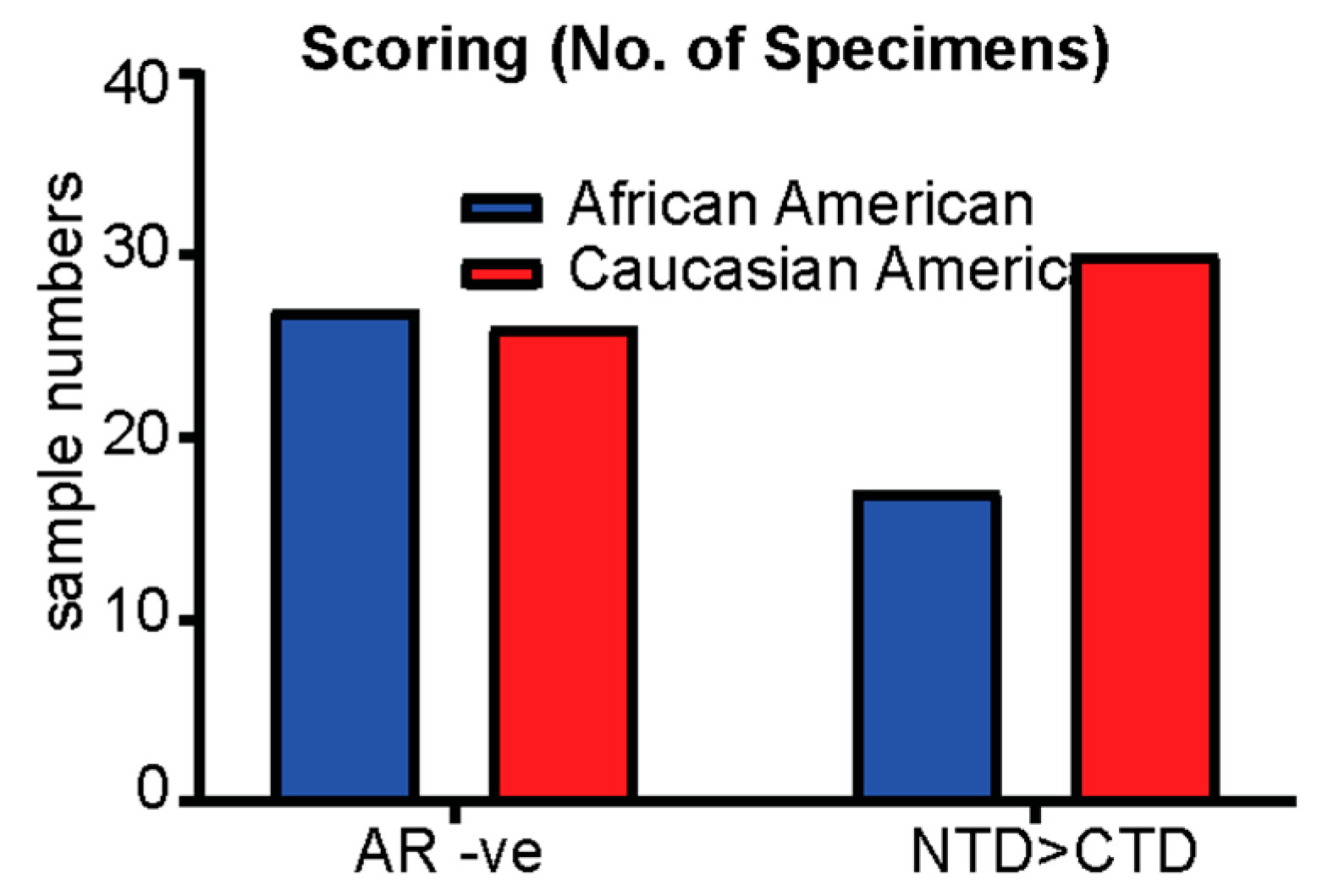
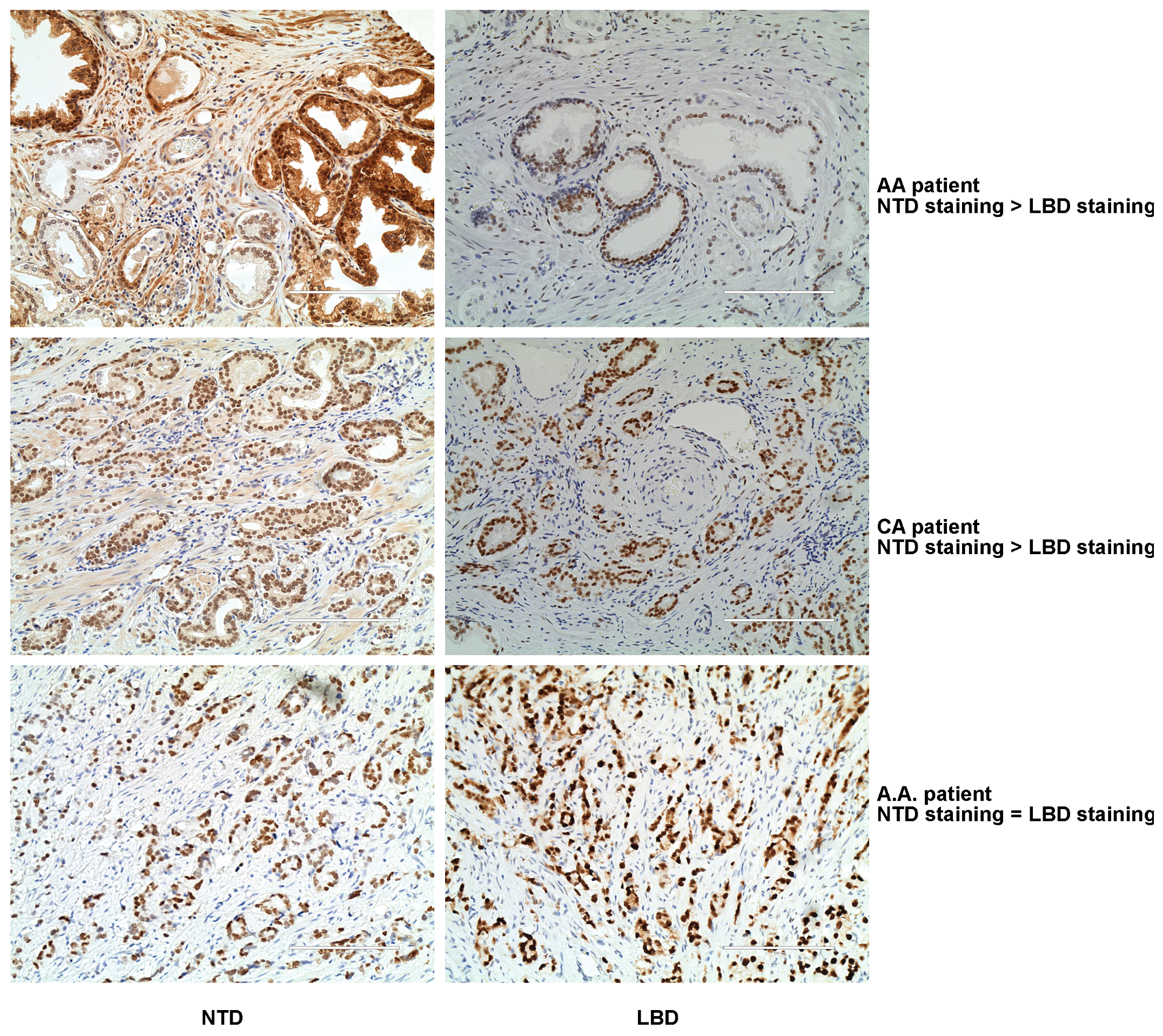
3.1. Patient Characteristics and Demographics
3.2. Treatment-Naïve Primary PCa Specimens Express AR-SVs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials, C., Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E.; et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef]
- Li, Y.; Chan, S.C.; Brand, L.J.; Hwang, T.H.; Silverstein, K.A.; Dehm, S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013, 73, 483–489. [Google Scholar] [CrossRef]
- Nyquist, M.D.; Li, Y.; Hwang, T.H.; Manlove, L.S.; Vessella, R.L.; Silverstein, K.A.; Voytas, D.F.; Dehm, S.M. TALEN-engineered AR gene rearrangements reveal endocrine uncoupling of androgen receptor in prostate cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 17492–17497. [Google Scholar] [CrossRef]
- Hornberg, E.; Ylitalo, E.B.; Crnalic, S.; Antti, H.; Stattin, P.; Widmark, A.; Bergh, A.; Wikstrom, P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE 2011, 6, e19059. [Google Scholar] [CrossRef]
- Kong, D.; Sethi, S.; Li, Y.; Chen, W.; Sakr, W.A.; Heath, E.; Sarkar, F.H. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015, 75, 161–174. [Google Scholar] [CrossRef]
- Lu, J.; Van der Steen, T.; Tindall, D.J. Are androgen receptor variants a substitute for the full-length receptor? Nat. Rev. Urol. 2015, 12, 137–144. [Google Scholar] [CrossRef]
- Dehm, S.M.; Tindall, D.J. Alternatively spliced androgen receptor variants. Endocr. Relat. Cancer 2011, 18, R183–R196. [Google Scholar] [CrossRef]
- Haile, S.; Sadar, M.D. Androgen receptor and its splice variants in prostate cancer. Cell Mol. Life Sci. 2011, 68, 3971–3981. [Google Scholar] [CrossRef]
- Crawford, E.D.; Eisenberger, M.A.; McLeod, D.G.; Spaulding, J.T.; Benson, R.; Dorr, F.A.; Blumenstein, B.A.; Davis, M.A.; Goodman, P.J. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med. 1989, 321, 419–424. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef]
- Sun, S.; Sprenger, C.C.; Vessella, R.L.; Haugk, K.; Soriano, K.; Mostaghel, E.A.; Page, S.T.; Coleman, I.M.; Nguyen, H.M.; Sun, H.; et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010, 120, 2715–2730. [Google Scholar] [CrossRef]
- Chan, S.C.; Li, Y.; Dehm, S.M. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J. Biol. Chem. 2012, 287, 19736–19749. [Google Scholar] [CrossRef]
- Sharp, A.; Coleman, I.; Yuan, W.; Sprenger, C.; Dolling, D.; Rodrigues, D.N.; Russo, J.W.; Figueiredo, I.; Bertan, C.; Seed, G.; et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Investig. 2019, 129, 192–208. [Google Scholar] [CrossRef]
- Nakata, D.; Nakao, S.; Nakayama, K.; Araki, S.; Nakayama, Y.; Aparicio, S.; Hara, T.; Nakanishi, A. The RNA helicase DDX39B and its paralog DDX39A regulate androgen receptor splice variant AR-V7 generation. Biochem. Biophys. Res. Commun. 2017, 483, 271–276. [Google Scholar] [CrossRef]
- Cao, H.; Wang, D.; Gao, R.; Chen, L.; Feng, Y. Down regulation of U2AF1 promotes ARV7 splicing and prostate cancer progression. Biochem. Biophys. Res. Commun. 2021, 541, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Lock, J.G.; Ma, Y.; Harman, D.G.; de Souza, P.; Chua, W.; Balakrishnar, B.; Scott, K.F.; Becker, T.M. Choice of antibody is critical for specific and sensitive detection of androgen receptor splice variant-7 in circulating tumor cells. Sci. Rep. 2022, 12, 16159. [Google Scholar] [CrossRef] [PubMed]
- Welti, J.; Rodrigues, D.N.; Sharp, A.; Sun, S.; Lorente, D.; Riisnaes, R.; Figueiredo, I.; Zafeiriou, Z.; Rescigno, P.; de Bono, J.S.; et al. Analytical Validation and Clinical Qualification of a New Immunohistochemical Assay for Androgen Receptor Splice Variant-7 Protein Expression in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2016, 70, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Crossley-May, H.; Vigneau, F.D.; Brown, K.; Banerjee, M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control 2003, 14, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.X.; Li, F.P.; Hankey, B.F.; Chu, K.; Edwards, B.K. Cancer survival among US whites and minorities: A SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch. Intern. Med. 2002, 162, 1985–1993. [Google Scholar] [CrossRef]
- Cohen, J.H.; Schoenbach, V.J.; Kaufman, J.S.; Talcott, J.A.; Schenck, A.P.; Peacock, S.; Symons, M.; Amamoo, M.A.; Carpenter, W.R.; Godley, P.A. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States). Cancer Causes Control 2006, 17, 803–811. [Google Scholar] [CrossRef]
- Fowke, J.H.; Schlundt, D.; Signorello, L.B.; Ukoli, F.A.; Blot, W.J. Prostate cancer screening between low-income African-American and Caucasian men. Urol. Oncol. 2005, 23, 333–340. [Google Scholar] [CrossRef]
- Powell, I.J.; Bock, C.H.; Ruterbusch, J.J.; Sakr, W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J. Urol. 2010, 183, 1792–1796. [Google Scholar] [CrossRef]
- Fowke, J.H.; Signorello, L.B.; Chang, S.S.; Matthews, C.E.; Buchowski, M.S.; Cookson, M.S.; Ukoli, F.M.; Blot, W.J. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer 2006, 107, 2361–2367. [Google Scholar] [CrossRef]
- Gaines, A.R.; Turner, E.L.; Moorman, P.G.; Freedland, S.J.; Keto, C.J.; McPhail, M.E.; Grant, D.J.; Vidal, A.C.; Hoyo, C. The association between race and prostate cancer risk on initial biopsy in an equal access, multiethnic cohort. Cancer Causes Control 2014, 25, 1029–1035. [Google Scholar] [CrossRef]
- Sakr, W.A.; Billis, A.; Ekman, P.; Wilt, T.; Bostwick, D.G. Epidemiology of high-grade prostatic intraepithelial neoplasia. Scand. J. Urol. Nephrol. Suppl. 2000, 34, 11–18. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Aronson, W.J.; Presti, J.C., Jr.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Freedland, S.J. Race, biochemical disease recurrence, and prostate-specific antigen doubling time after radical prostatectomy: Results from the SEARCH database. Cancer 2007, 110, 2202–2209. [Google Scholar] [CrossRef]
- Andersen, R.J.; Mawji, N.R.; Wang, J.; Wang, G.; Haile, S.; Myung, J.K.; Watt, K.; Tam, T.; Yang, Y.C.; Banuelos, C.A.; et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 2010, 17, 535–546. [Google Scholar] [CrossRef]
- Hwang, D.J.; He, Y.; Ponnusamy, S.; Mohler, M.L.; Thiyagarajan, T.; McEwan, I.J.; Narayanan, R.; Miller, D.D. New Generation of Selective Androgen Receptor Degraders: Our Initial Design, Synthesis, and Biological Evaluation of New Compounds with Enzalutamide-Resistant Prostate Cancer Activity. J. Med. Chem. 2019, 62, 491–511. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Coss, C.C.; Thiyagarajan, T.; Watts, K.; Hwang, D.J.; He, Y.; Selth, L.A.; McEwan, I.J.; Duke, C.B.; Pagadala, J.; et al. Novel Selective Agents for the Degradation of Androgen Receptor Variants to Treat Castration-Resistant Prostate Cancer. Cancer Res. 2017, 77, 6282–6298. [Google Scholar] [CrossRef]
- Ponnusamy, S.; He, Y.; Hwang, D.J.; Thiyagarajan, T.; Houtman, R.; Bocharova, V.; Sumpter, B.G.; Fernandez, E.; Johnson, D.; Du, Z.; et al. Orally Bioavailable Androgen Receptor Degrader, Potential Next-Generation Therapeutic for Enzalutamide-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 6764–6780. [Google Scholar] [CrossRef]
- Zhang, X.; Morrissey, C.; Sun, S.; Ketchandji, M.; Nelson, P.S.; True, L.D.; Vakar-Lopez, F.; Vessella, R.L.; Plymate, S.R. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE 2011, 6, e27970. [Google Scholar] [CrossRef]
- Cato, L.; Shomali, M. AR Structural Variants and Prostate Cancer. Adv. Exp. Med. Biol. 2022, 1390, 195–211. [Google Scholar]
| Characteristics | African American | Caucasian American |
|---|---|---|
| n | 118 | 115 |
| Age | 59.65 | 62.12 |
| BMI | 29.02 | 28.05 |
| PSA (ng/mL) | 6.2 (n = 67) | 4.7 (n = 90) |
| Gleason score (n) | ||
| 6 | 61 | 61 |
| 3 + 4 | 29 | 35 |
| 4 + 3 | 17 | 11 |
| 8 | 3 | 6 |
| 9 | 4 | 4 |
| AR-V7 | 2 | 0 |
| AR NTD antibody (+ve) n | 56 | 67 |
| AR NTD antibody (+ve) (intensity) | 30 | 30 |
| AR NTD antibody (−ve) n | 47 | 48 |
| AR LBD antibody (+ve) n | 73 | 67 |
| AR LBD antibody (+ve) (intensity) | 30 | 30 |
| AR LBD antibody (−ve) n | 60 | 100 |
| AR NTD and LBD antibody (−ve) n | 27 | 26 |
| Gleason score (NTD-H-score) mean | ||
| 6 | 34 | 25.5 |
| 3 + 4 | 39.7 | 32.9 |
| 4 + 3 | 15.6 | 42.3 |
| 8 | 1.7 | 8.3 |
| 9 | 20 | 50 |
| Gleason score (CTD-H-score) mean | ||
| 6 | 59.4 | 52.2 |
| 3 + 4 | 103.8 | 68.2 |
| 4 + 3 | 65.9 | 70 |
| 8 | 8.3 | 10 |
| 9 | 62.5 | 103.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Anelo, O.M.; Sadiq, Q.; Effah, W.; Price, G.; Johnson, D.L.; Ponnusamy, S.; Grimes, B.; Morrison, M.L.; Fowke, J.H.; et al. Racial Differences in Androgen Receptor (AR) and AR Splice Variants (AR-SVs) Expression in Treatment-Naïve Androgen-Dependent Prostate Cancer. Biomedicines 2023, 11, 648. https://doi.org/10.3390/biomedicines11030648
Khan F, Anelo OM, Sadiq Q, Effah W, Price G, Johnson DL, Ponnusamy S, Grimes B, Morrison ML, Fowke JH, et al. Racial Differences in Androgen Receptor (AR) and AR Splice Variants (AR-SVs) Expression in Treatment-Naïve Androgen-Dependent Prostate Cancer. Biomedicines. 2023; 11(3):648. https://doi.org/10.3390/biomedicines11030648
Chicago/Turabian StyleKhan, Farhan, Obianuju Mercy Anelo, Qandeel Sadiq, Wendy Effah, Gary Price, Daniel L. Johnson, Suriyan Ponnusamy, Brandy Grimes, Michelle L. Morrison, Jay H. Fowke, and et al. 2023. "Racial Differences in Androgen Receptor (AR) and AR Splice Variants (AR-SVs) Expression in Treatment-Naïve Androgen-Dependent Prostate Cancer" Biomedicines 11, no. 3: 648. https://doi.org/10.3390/biomedicines11030648





