CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cell Culture and Preparation of MSU Crystals
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Western Blot Analysis
2.6. Transfection of siRNA
2.7. Statistical Analysis
3. Results
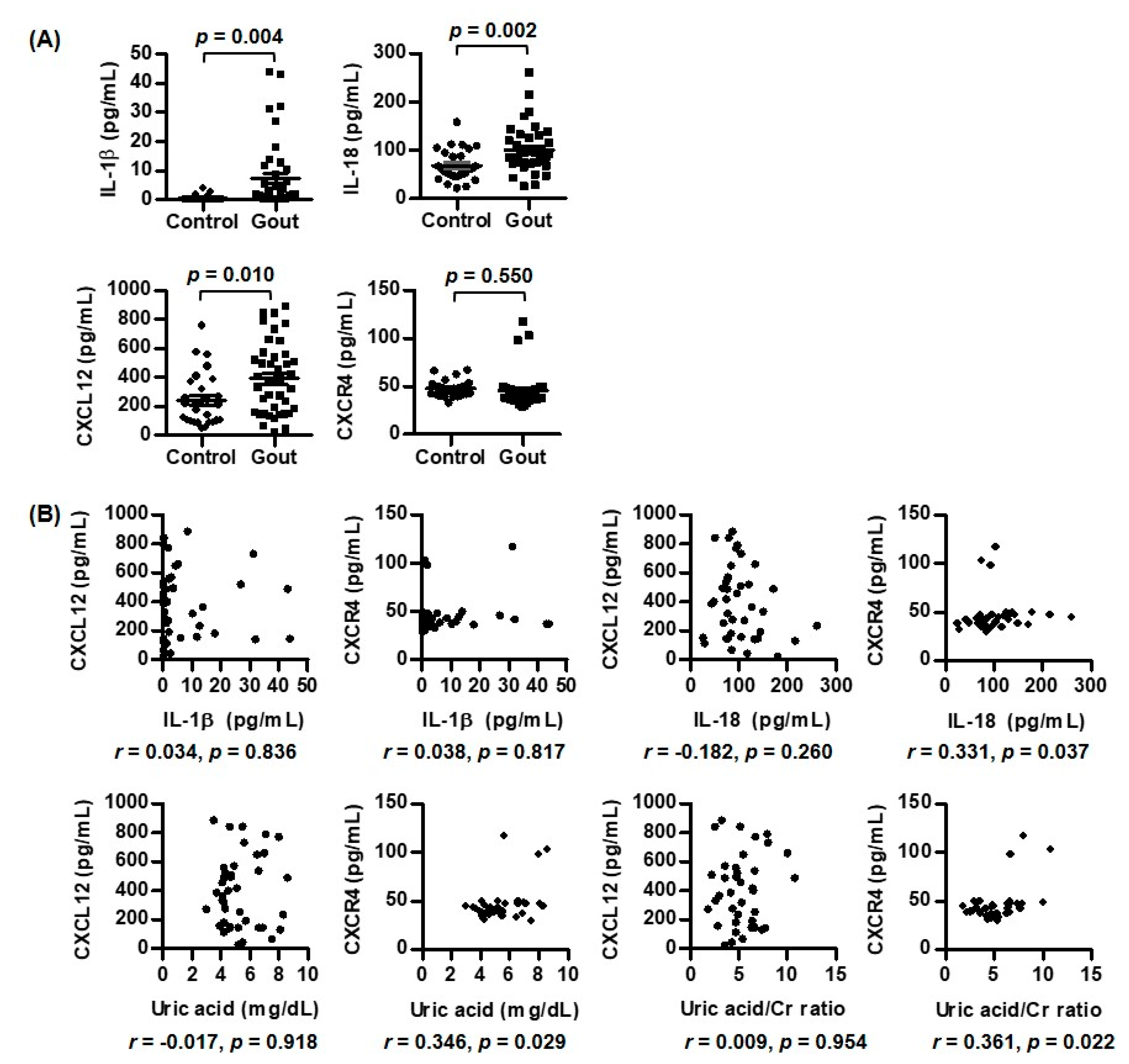
3.1. Comparison of Expression of CXCR4, CXCL12, IL-1β, and IL-18 between Patients with Gout and Controls
| Variables | Gout (n = 40) | Controls (n = 27) |
|---|---|---|
| Sex, male (n, %) | 40 (100.0) | 27 (100.0) |
| Age (years) | 63.0 (54.3, 70.0) | 65.0 (51.8, 70.0) |
| Blood urea nitrogen (mg/dL) | 19.4 (12.8, 27.1) | |
| Creatinine (mg/dL) | 1.0 (0.8, 1.4) | |
| eGFR (mL/min/1.73 m2) | 77.7 (52.4, 100.1) | |
| Erythrocyte sedimentation rate (mm/h) | 11.5 (7.0, 21.8) | |
| C-reactive protein (mg/L) | 0.9 (0.6, 1.9) | |
| Uric acid (mg/dL) | 4.8 (4.2, 6.6) | |
| Uric acid/creatinine | 5.12 (3.65, 6.59) | |
| Medication (n, %) | ||
| Febuxostat | 30 (75.0) | |
| Allopurinol | 3 (7.5) | |
| Benzbromarone | 5 (12.5) | |
| Colchicine | 16 (40.0) | |
| Steroids | 6 (15.0) | |
| Non-steroidal anti-inflammatory drugs | 8 (20.0) | |
| Diuretics | 2 (5.0) |
3.2. Correlations of IL-1β, IL-18, CXCL12, and CXCR4 with Laboratory Variables in Gout Patients
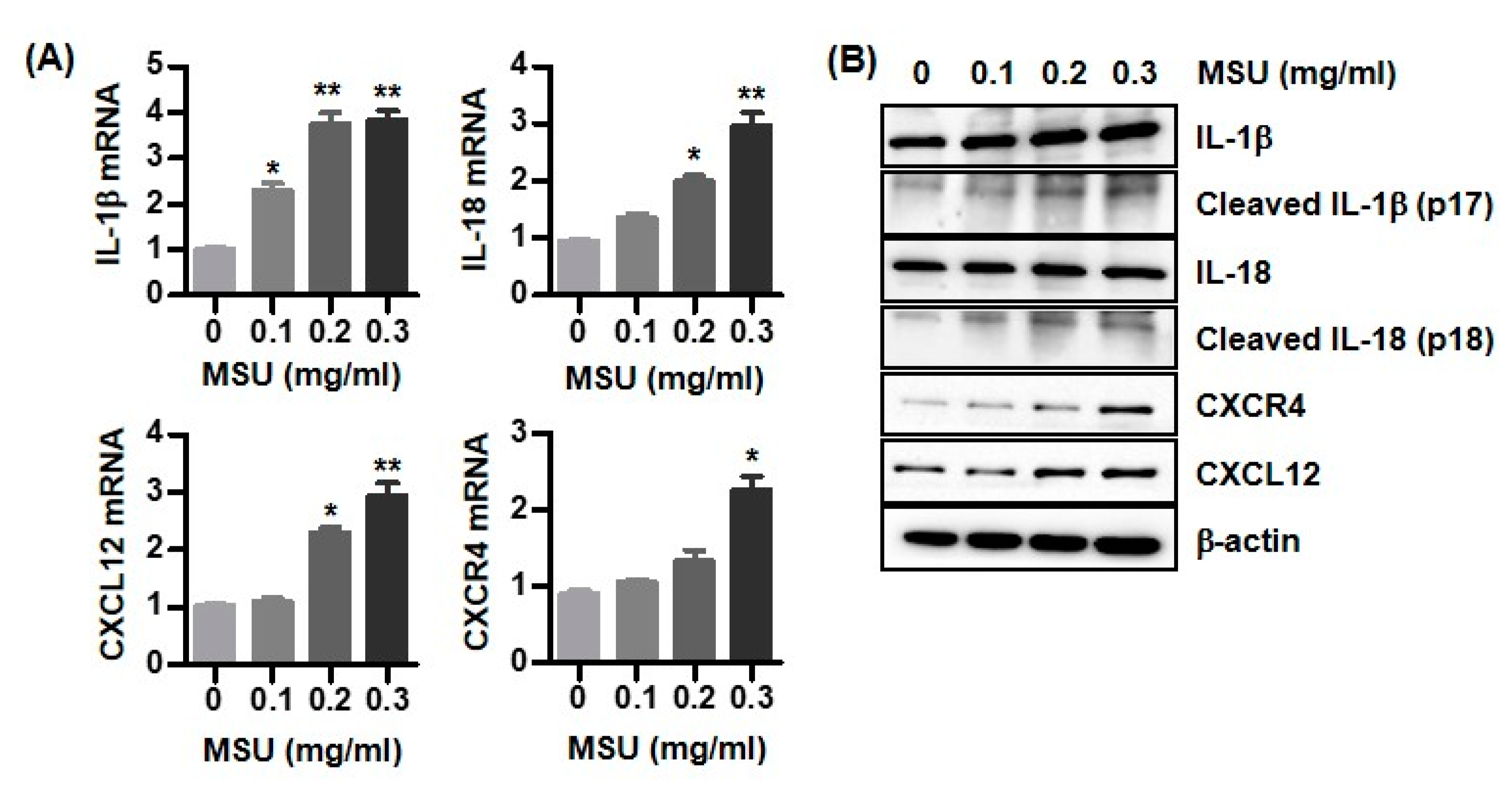
3.3. Expression of IL-1β, IL-18, CXCL12, and CXCR4 in MSU Crystal-Stimulated U937 Cells
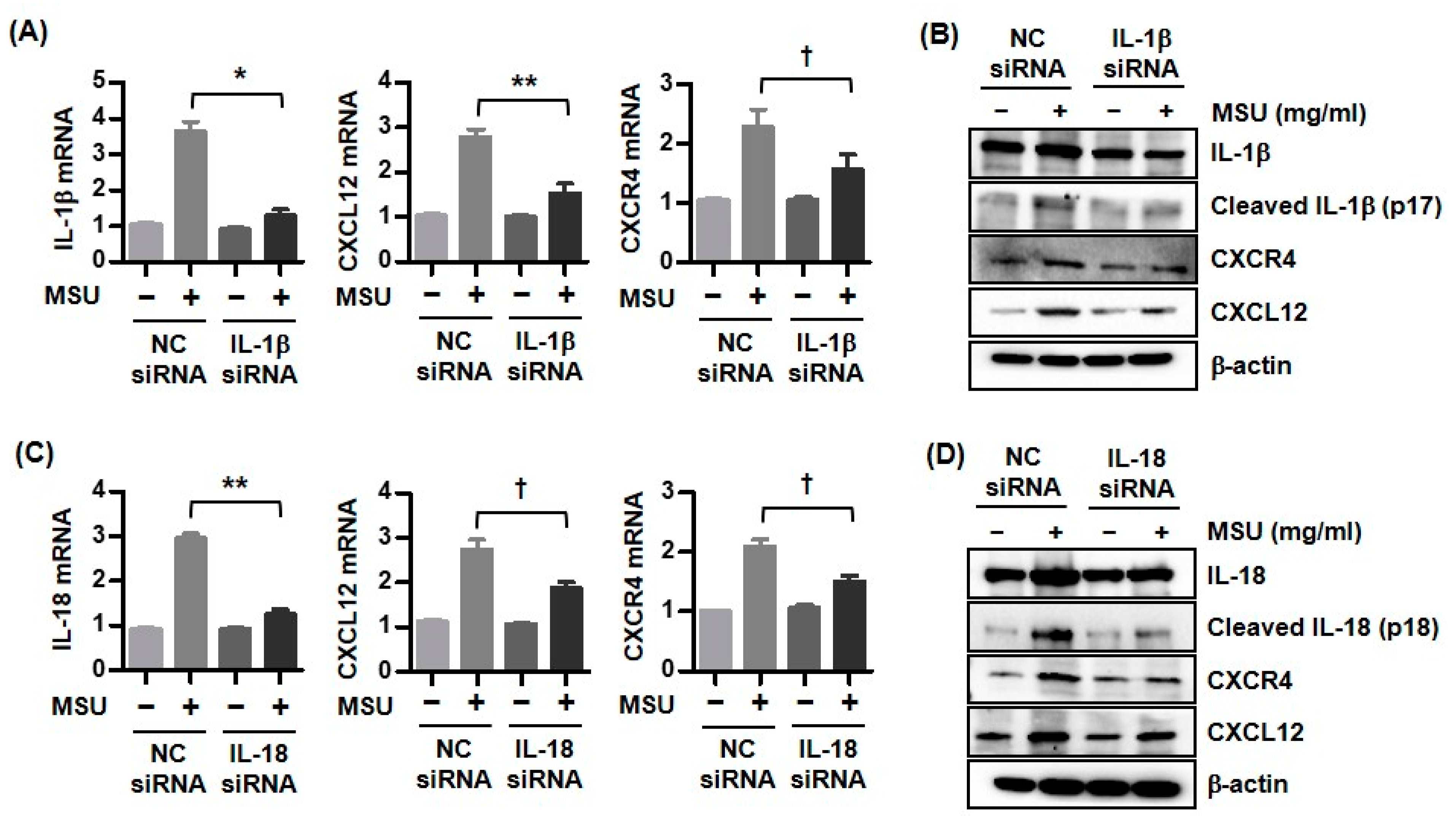
3.4. Expression of CXCR4 and CXCL12 by Downregulation of Either IL-1β or IL-18 in MSU Crystal-Stimulated U937 Cells
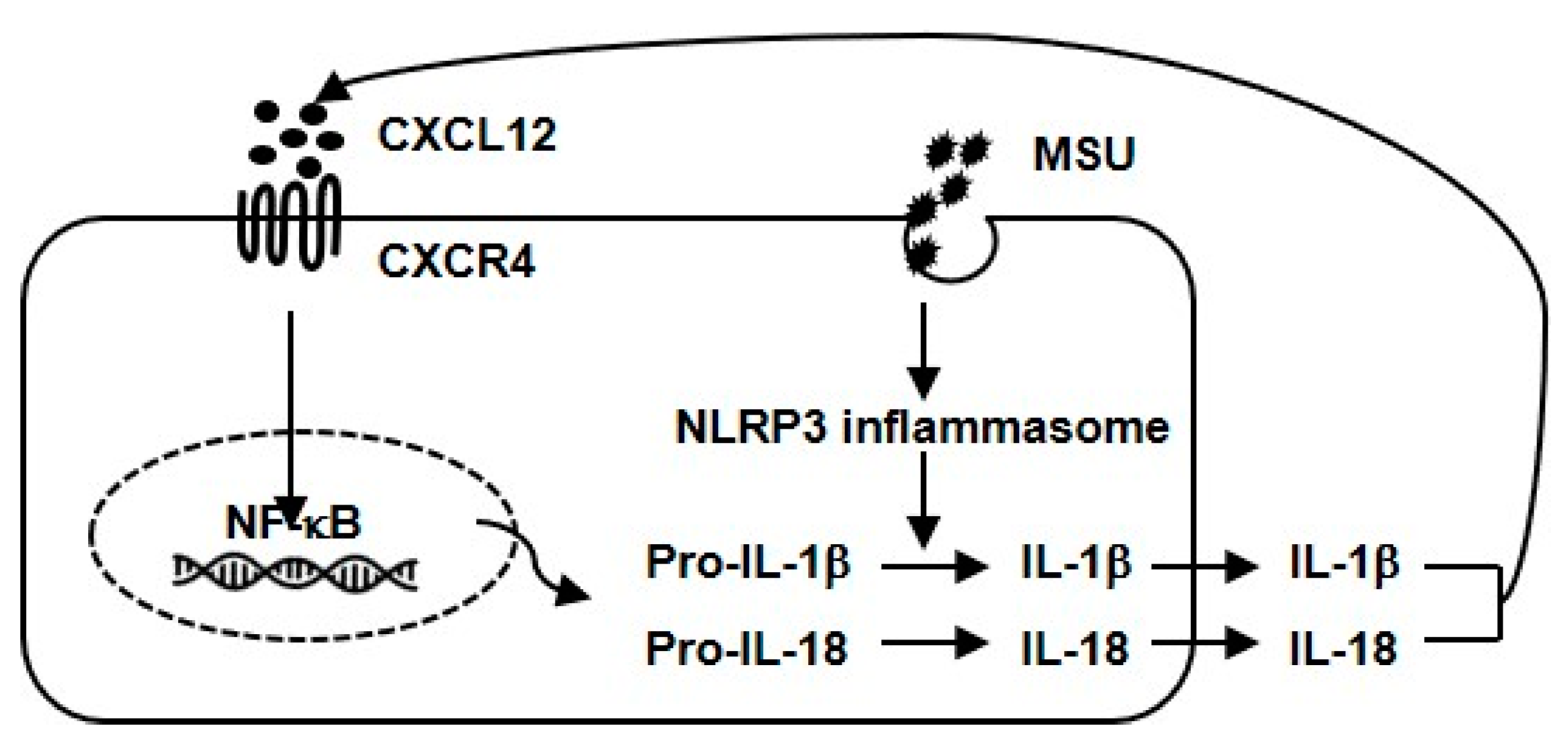
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. The mechanism of the NLRP3 inflammasome activation and pathogenic implication in the pathogenesis of gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Haringman, J.J.; Ludikhuize, J.; Tak, P.P. Chemokines in joint disease: The key to inflammation. Ann. Rheum. Dis. 2004, 63, 1186–1194. [Google Scholar] [CrossRef]
- Kienhorst, L.B.; van Lochem, E.; Kievit, W.; Dalbeth, N.; Merriman, M.E.; Phipps-Green, A.; Loof, A.; van Heerde, W.; Vermeulen, S.; Stamp, L.K.; et al. Gout Is a Chronic Inflammatory Disease in Which High Levels of Interleukin-8 (CXCL8), Myeloid-Related Protein 8/Myeloid-Related Protein 14 Complex, and an Altered Proteome Are Associated With Diabetes Mellitus and Cardiovascular Disease. Arthritis Rheumatol. 2015, 67, 3303–3313. [Google Scholar] [CrossRef] [Green Version]
- Ryckman, C.; McColl, S.R.; Vandal, K.; de Médicis, R.; Lussier, A.; Poubelle, P.E.; Tessier, P.A. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003, 48, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Kanellis, J.; Watanabe, S.; Li, J.H.; Kang, D.H.; Li, P.; Nakagawa, T.; Wamsley, A.; Sheikh-Hamad, D.; Lan, H.Y.; Feng, L.; et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003, 41, 1287–1293. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; Wu, F.; Pan, X.; Yu, J.; Li, Y.; Lu, T.; Li, X.; Lin, Z. Soluble C-X-C chemokine ligand 16 levels are increased in gout patients. Clin. Biochem. 2012, 45, 1368–1373. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11, 2109. [Google Scholar] [CrossRef] [PubMed]
- García-Cuesta, E.M.; Santiago, C.A.; Vallejo-Díaz, J.; Juarranz, Y.; Rodríguez-Frade, J.M.; Mellado, M. The Role of the CXCL12/CXCR4/ACKR3 Axis in Autoimmune Diseases. Front. Endocrinol. (Lausanne). 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, R.; Struyf, S.; Proost, P. The unique structural and functional features of CXCL12. Cell. Mol. Immunol. 2018, 15, 299–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Klerck, B.; Geboes, L.; Hatse, S.; Kelchtermans, H.; Meyvis, Y.; Vermeire, K.; Bridger, G.; Billiau, A.; Schols, D.; Matthys, P. Pro-inflammatory properties of stromal cell-derived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res. Ther. 2005, 7, R1208–R1220. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Zhu, N.; Mao, J.; Huang, L.; Yang, Y.; Zhou, Z.; Wang, L.; Wu, B. Expression levels of CXCR4 and CXCL12 in patients with rheumatoid arthritis and its correlation with disease activity. Exp. Ther. Med. 2020, 20, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, J.; Zhang, J.; Lv, Z.; Guo, F.; Huang, H.; Zhu, W.; Chen, A. CXCL12/CXCR4 Axis Regulates Aggrecanase Activation and Cartilage Degradation in a Post-Traumatic Osteoarthritis Rat Model. Int. J. Mol. Sci. 2016, 17, 1522. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.J.; Xu, T.; Wu, H.T.; Yao, Z.L.; Hou, Y.L.; Xie, Y.H.; Su, J.W.; Cheng, C.Y.; Yang, K.F.; Zhang, X.R.; et al. SDF-1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone 2019, 125, 140–150. [Google Scholar] [CrossRef]
- Hanaoka, H.; Okazaki, Y.; Hashiguchi, A.; Yasuoka, H.; Takeuchi, T.; Kuwana, M. Overexpression of CXCR4 on circulating B cells in patients with active systemic lupus erythematosus. Clin. Exp. Rheumatol. 2015, 33, 863–870. [Google Scholar]
- Aeberli, D.; Kamgang, R.; Balani, D.; Hofstetter, W.; Villiger, P.M.; Seitz, M. Regulation of peripheral classical and non-classical monocytes on infliximab treatment in patients with rheumatoid arthritis and ankylosing spondylitis. RMD Open 2016, 2, e000079. [Google Scholar] [CrossRef] [Green Version]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout Classification Criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015, 67, 2557–2568. [Google Scholar] [CrossRef] [Green Version]
- Choe, J.Y.; Jung, H.Y.; Park, K.Y.; Kim, S.K. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1β expression. Rheumatology 2014, 53, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, S.R.; Conaghan, P.G.; McDermott, M.F. The role of the NLRP3 inflammasome in gout. J. Inflamm. Res. 2011, 4, 39–49. [Google Scholar] [PubMed] [Green Version]
- Sun, L.; Ye, R.D. Role of G protein-coupled receptors in inflammation. Acta. Pharmacol. Sin. 2012, 33, 342–350. [Google Scholar] [CrossRef] [Green Version]
- New, D.C.; Wu, K.; Kwok, A.W.; Wong, Y.H. G protein-coupled receptor-induced Akt activity in cellular proliferation and apoptosis. FEBS J. 2007, 274, 6025–6036. [Google Scholar] [CrossRef] [PubMed]
- Busillo, J.M.; Benovic, J.L. Regulation of CXCR4 signaling. Biochim. Biophys. Acta 2007, 1768, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Xu, Y.; Wei, Y.; He, X. Uric acid induces the expression of TNF-α via the ROS-MAPK-NF-κB signaling pathway in rat vascular smooth muscle cells. Mol. Med. Rep. 2017, 16, 6928–6933. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Choe, J.Y.; Park, K.Y. TXNIP-mediated nuclear factor-κB signaling pathway and intracellular shifting of TXNIP in uric acid-induced NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2019, 511, 725–731. [Google Scholar] [CrossRef]
- Murakami, T.; Ruengsinpinya, L.; Nakamura, E.; Takahata, Y.; Hata, K.; Okae, H.; Taniguchi, S.; Takahashi, M.; Nishimura, R. Cutting Edge: G Protein Subunit β 1 Negatively Regulates NLRP3 Inflammasome Activation. J. Immunol. 2019, 202, 1942–1947. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; An, F.; Mei, J.; Yang, C.; Zhan, Q.; Zhang, Q. IL-1β Pretreatment Improves the Efficacy of Mesenchymal Stem Cells on Acute Liver Failure by Enhancing CXCR4 Expression. Stem. Cells. Int. 2020, 2020, 1498315. [Google Scholar] [CrossRef]
- Tian, X.; Xie, G.; Xiao, H.; Ding, F.; Bao, W.; Zhang, M. CXCR4 knockdown prevents inflammatory cytokine expression in macrophages by suppressing activation of MAPK and NF-κB signaling pathways. Cell. Biosci. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maloberti, A.; Biolcati, M.; Ruzzenenti, G.; Giani, V.; Leidi, F.; Monticelli, M.; Algeri, M.; Scarpellini, S.; Nava, S.; Soriano, F.; et al. The Role of Uric Acid in Acute and Chronic Coronary Syndromes. J. Clin. Med. 2021, 10, 4750. [Google Scholar] [CrossRef] [PubMed]
- Döring, Y.; Pawig, L.; Weber, C.; Noels, H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front. Physiol. 2014, 5, 212. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-K.; Choe, J.-Y.; Park, K.-Y. CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis. Biomedicines 2023, 11, 649. https://doi.org/10.3390/biomedicines11030649
Kim S-K, Choe J-Y, Park K-Y. CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis. Biomedicines. 2023; 11(3):649. https://doi.org/10.3390/biomedicines11030649
Chicago/Turabian StyleKim, Seong-Kyu, Jung-Yoon Choe, and Ki-Yeun Park. 2023. "CXCL12 and CXCR4 as Novel Biomarkers in Uric Acid-Induced Inflammation and Patients with Gouty Arthritis" Biomedicines 11, no. 3: 649. https://doi.org/10.3390/biomedicines11030649





