Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction
Abstract
:1. Introduction
2. Estrogens, Their Synthesis, and Mechanisms of Action
2.1. Estrogens
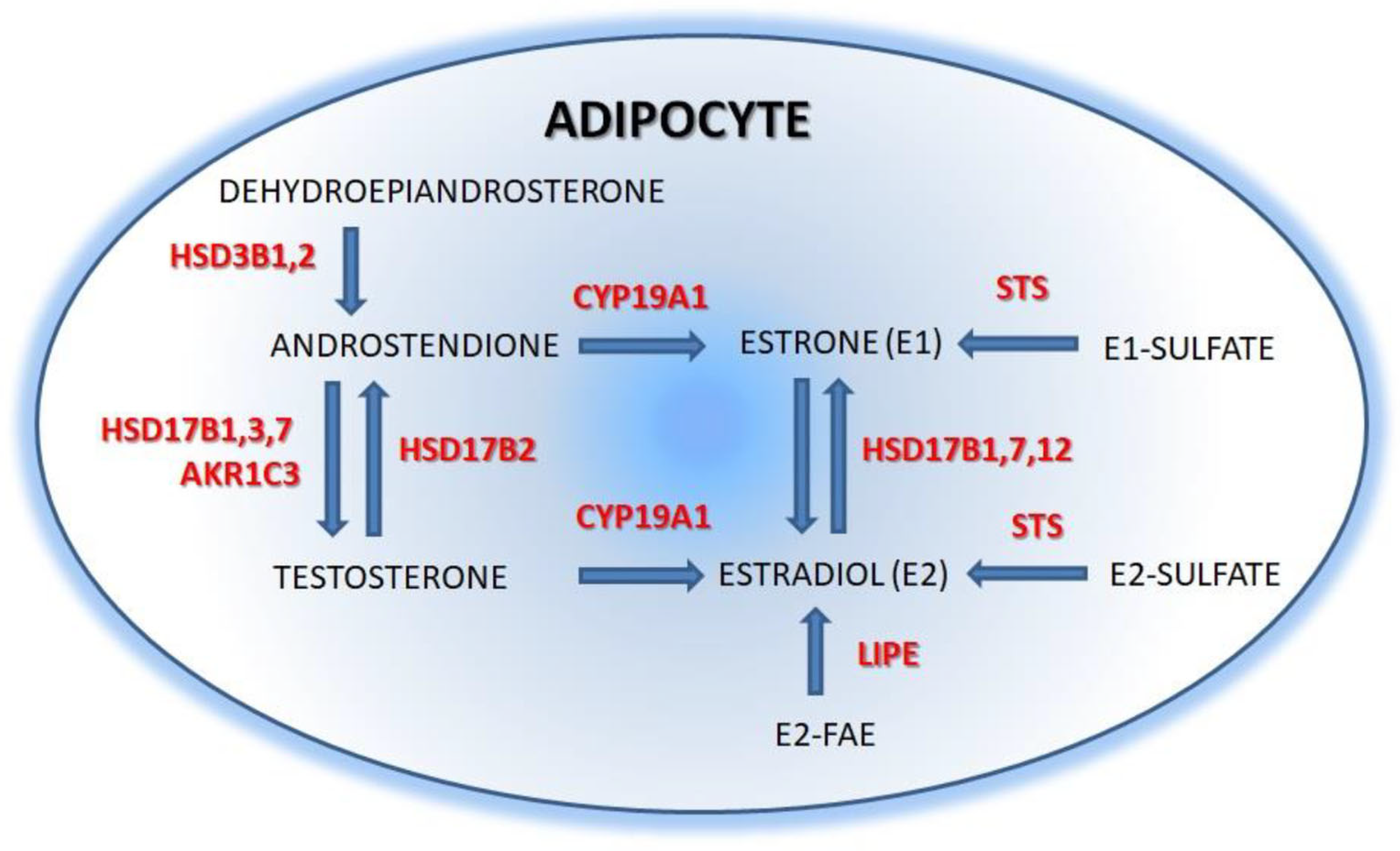
2.2. Estrogens Synthesis
2.3. Estrogen Mechanisms of Action
3. Estrogens in the Regulation of Adipocyte Proliferation and Fate
3.1. Estrogens in the Regulation of Stem Cell Differentiation and Preadipocyte Proliferation
3.2. Estrogens in the Regulation of White and Brown Adipogenesis
4. Estrogens in the Regulation of Adipose Tissue Metabolism
4.1. Estrogens in the Regulation of Lipolysis/Lipogenesis
4.2. Estrogens in the Regulation of Adipose Tissue Insulin Sensitivity
4.3. Estrogens in the Regulation of Adipokines Secretion
4.4. Estrogens in the Regulation of Metabolic Inflammation
5. Therapeutic Potential of Estrogen in the Treatment of Obesity
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef]
- Woo, C.-Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.-U. Mitochondrial Dysfunction in Adipocytes as a Primary Cause of Adipose Tissue Inflammation. Diabetes Metab. J. 2019, 43, 247–256. [Google Scholar] [CrossRef]
- Okunogbe, A.; Nugent, R.; Spencer, G.; Ralston, J.; Wilding, J. Economic impacts of overweight and obesity: Current and future estimates for eight countries. BMJ Glob. Health 2021, 6, e006351. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sam, S. Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. Horm. Mol. Biol. Clin. Investig. 2018, 33. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, E.; Wing, A.; Holtrup, B.; Sebo, Z.; Kaplan, J.L.; Saavedra-Peña, R.; Church, C.D.; Colman, L.; Berry, R.; Rodeheffer, M.S. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016, 24, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by upregu-lating a2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor a. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syn-drome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef] [Green Version]
- Bjune, J.-I.; Strømland, P.P.; Jersin, R.; Mellgren, G.; Dankel, S.N. Metabolic and Epigenetic Regulation by Estrogen in Adipocytes. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Piaggesi, L.; De Simone, L.; Orlandi, R.; Genazzani, A.R. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J. Clin. Endocrinol. Metab. 1997, 82, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; De Simone, L.; Orlandi, R.; Genazzani, A. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas 2001, 39, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hor-mone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- White, U.A.; Tchoukalova, Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 377–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovre, D.; Lindsey, S.H.; Mauvais-Jarvis, F. Effect of menopausal hormone therapy on components of the metabolic syndrome. Ther. Adv. Cardiovasc. Dis. 2016, 11, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Mattison, D.R.; Karyakina, N.; Goodman, M.; LaKind, J.S. Pharmaco- and toxicokinetics of selected exogenous and endog-enous estrogens: A review of the data and identification of knowledge gaps. Crit. Rev. Toxicol. 2014, 44, 696–724. [Google Scholar] [CrossRef] [PubMed]
- Buscato, M.; Davezac, M.; Zahreddine, R.; Adlanmerini, M.; Métivier, R.; Fillet, M.; Cobraiville, G.; Moro, C.; Foidart, J.-M.; Lenfant, F.; et al. Estetrol prevents Western diet–induced obesity and atheroma independently of hepatic estrogen receptor α. Am. J. Physiol. Metab. 2021, 320, E19–E29. [Google Scholar] [CrossRef]
- Kumar, R.S.; Goyal, N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. 2021, 269, 119091. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Potter, B.V. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013, 137, 27–49. [Google Scholar] [CrossRef]
- Xu, B.; Lovre, D.; Mauvais-Jarvis, F. Effect of selective estrogen receptor modulators on metabolic homeostasis. Biochimie 2016, 124, 92–97. [Google Scholar] [CrossRef]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [Green Version]
- Tchernof, A.; Mansour, M.F.; Pelletier, M.; Boulet, M.-M.; Nadeau, M.; Luu-The, V. Updated survey of the steroid-converting enzymes in human adipose tissues. J. Steroid Biochem. Mol. Biol. 2015, 147, 56–69. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Zhao, A.; Tao, T.; Mao, X.; Zhang, P.; Liu, W. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J. Steroid Biochem. Mol. Biol. 2012, 132, 120–126. [Google Scholar] [CrossRef]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Czajka-Oraniec, I.; Simpson, E.R. Aromatase research and its clinical significance. Endokrynol. Pol. 2010, 61, 126–134. [Google Scholar]
- Zhao, H.; Zhou, L.; Shangguan, A.J.; Bulun, S.E. Aromatase expression and regulation in breast and endometrial cancer. J. Mol. Endocrinol. 2016, 57, R19–R33. [Google Scholar] [CrossRef] [Green Version]
- Hetemäki, N.; Savolainen-Peltonen, H.; Tikkanen, M.J.; Wang, F.; Paatela, H.; Hämäläinen, E.; Turpeinen, U.; Haanpää, M.; Vihma, V.; Mikkola, T.S. Estrogen Metabolism in Abdominal Subcutaneous and Visceral Adipose Tissue in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2017, 102, 4588–4595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, D.G.; Shoback, D. Greenspan’s Basic & Clinical Endocrinology, 7th ed.; McGraw-Hill Medical: New York, NY, USA, 2011. [Google Scholar]
- Wang, F.; Vihma, V.; Badeau, M.; Savolainen-Peltonen, H.; Leidenius, M.; Mikkola, T.; Turpeinen, U.; Hämäläinen, E.; Ikonen, E.; Wähälä, K.; et al. Fatty acyl esterification and deesterification of 17β-estradiol in human breast subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 2012, 97, 3349–3356. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Vihma, V.; Soronen, J.; Turpeinen, U.; Hämäläinen, E.; Savolainen-Peltonen, H.; Mikkola, T.S.; Naukkarinen, J.; Pietiläinen, K.H.; Jauhiainen, M.; et al. 17β-Estradiol and estradiol fatty acyl esters and estro-gen-converting enzyme expression in adipose tissue in obese men and women. J. Clin. Endocrinol. Metab. 2013, 98, 4923–4931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef]
- Bélanger, C.; Hould, F.-S.; Lebel, S.; Biron, S.; Brochu, G.; Tchernof, A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids 2006, 71, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Honma, S.; Shibata, Y.; Yamashita, K.; Watanabe, Y.; Maekubo, H.; Okuyama, M.; Takashima, A.; Takeshita, N. An Innovative LC-MS/MS-Based Method for Determining CYP 17 and CYP 19 Activity in the Adipose Tissue of Pre- and Postmenopausal and Ovariectomized Women Using 13C-Labeled Steroid Substrates. J. Clin. Endocrinol. Metab. 2014, 99, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetemäki, N.; Mikkola, T.S.; Tikkanen, M.J.; Wang, F.; Hämäläinen, E.; Turpeinen, U.; Haanpää, M.; Vihma, V.; Savo-lainen-Peltonen, H. Adipose tissue estrogen production and metabolism in premenopausal women. J. Steroid Biochem. Mol. Biol. 2021, 209, 105849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The Dynamic Structure of the Estrogen Receptor. J. Amino Acids 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Nilsson, M.; Dahlman, I.; Rydén, M.; Nordström, E.A.; Gustafsson, J.A.; Arner, P.; Dahlman-Wright, K. Oestrogen receptor alpha gene expression levels are reduced in obese compared to normal weight females. Int. J. Obes. 2007, 31, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Koźniewski, K.; Wąsowski, M.; Jonas, M.I.; Lisik, W.; Jonas, M.; Binda, A.; Jaworski, P.; Tarnowski, W.; Noszczyk, B.; Puzianowska-Kuźnicka, M.; et al. Epigenetic Regulation of Estrogen Receptor Genes’ Expressions in Adipose Tissue in the Course of Obesity. Int. J. Mol. Sci. 2022, 23, 5989. [Google Scholar] [CrossRef]
- Shin, J.H.; Hur, J.Y.; Seo, H.S.; Jeong, Y.A.; Lee, J.K.; Oh, M.J.; Kim, T.; Saw, H.S.; Kim, S.H. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids 2007, 72, 592–599. [Google Scholar] [CrossRef]
- Hammes, S.R.; Levin, E.R. Extranuclear Steroid Receptors: Nature and Actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Filardo, E.J.; Thomas, P. Minireview: G Protein-Coupled Estrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef] [Green Version]
- Yaşar, P.; Ayaz, G.; User, S.D.; Güpür, G.; Muyan, M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod. Med. Biol. 2016, 16, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity Is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Cuenca, J.A.; De La Peña-Sosa, G.; De La Vega-Moreno, K.; Banderas-Lares, D.Z.; Salamanca-García, M.; Mar-tínez-Hernández, J.E.; Vera-Gómez, E.; Hernández-Patricio, A.; Zamora-Alemán, C.R.; Domínguez-Pérez, G.A.; et al. Enlarged adipocytes from subcutaneous vs. visceral adipose tissue differentially contribute to metabolic dysfunction and atherogenic risk of patients with obesity. Sci. Rep. 2021, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; del Romero, A.; Peláez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, R.; Inoue, D.; Shibata, M.; Saika, M.; Kido, S.; Ooka, H.; Tomiyama, H.; Sakamoto, Y.; Matsumoto, T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 2002, 143, 2349–2356. [Google Scholar] [CrossRef]
- Zhang, W.; Schmull, S.; Du, M.; Liu, J.; Lu, Z.; Zhu, H.; Xue, S.; Lian, F. Estrogen Receptor α and β in Mouse: Adipose-Derived Stem Cell Proliferation, Migration, and Brown Adipogenesis In Vitro. Cell. Physiol. Biochem. 2016, 38, 2285–2299. [Google Scholar] [CrossRef]
- Hong, L.; Colpan, A.; Peptan, I.A.; Daw, J.; George, A.; Evans, C.A. 17-Beta estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Eng. 2007, 13, 1197–1203. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Ferreira, L.M.; Milani, A.; Arrigoni, E.; Brini, A.T. 17β-estradiol differently affects osteogenic differ-entiation of mesenchymal stem/stromal cells from adipose tissue and bone marrow. Differentiation 2016, 92, 291–297. [Google Scholar] [CrossRef]
- Hong, L.; Colpan, A.; Peptan, I.A. Modulations of 17-beta estradiol on osteogenic and adipogenic differentiations of human mesenchymal stem cells. Tissue Eng. 2006, 12, 2747–2753. [Google Scholar] [CrossRef]
- Heine, P.A.; Taylor, J.A.; Iwamoto, G.A.; Lubahn, D.B.; Cooke, P.S. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.E.; Thorburn, A.W.; Britt, K.L.; Hewitt, K.N.; Wreford, N.G.; Proietto, J.; Oz, O.K.; Leury, B.J.; Robertson, K.M.; Yao, S.; et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA 2000, 97, 12735–12740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffei, L.; Rochira, V.; Zirilli, L.; Antunez, P.; Aranda, C.; Fabre, B.; Simone, M.L.; Pignatti, E.; Simpson, E.R.; Houssami, S.; et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: Reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin. Endocrinol. 2007, 67, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Maffei, L.; Murata, Y.; Rochira, V.; Tubert, G.; Aranda, C.; Vazquez, M.; Clyne, C.D.; Davis, S.R.; Simpson, E.R.; Carani, C. Dysmetabolic Syndrome in a Man with a Novel Mutation of the Aromatase Gene: Effects of Testosterone, Alendronate, and Estradiol Treatment. J. Clin. Endocrinol. Metab. 2004, 89, 61–70. [Google Scholar] [CrossRef]
- Rochira, V.; Carani, C. Aromatase deficiency in men: A clinical perspective. Nat. Rev. Endocrinol. 2009, 5, 559–568. [Google Scholar] [CrossRef]
- Bulun, S.E. Aromatase and estrogen receptor α deficiency. Fertil. Steril. 2014, 101, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.; Yoon, M. 17β-Estradiol inhibition of PPARγ-induced adipogenesis and adipocyte-specific gene expression. Acta Pharmacol. Sin. 2011, 32, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naaz, A.; Holsberger, D.R.; Iwamoto, G.A.; Nelson, A.; Kiyokawa, H.; Cooke, P.S. Loss of cyclin-dependent kinase inhibitors produces adipocyte hyperplasia and obesity. FASEB J. 2004, 18, 1925–1927. [Google Scholar] [CrossRef]
- Walker, V.R.; Korach, K.S. Estrogen Receptor Knockout Mice as a Model for Endocrine Research. ILAR J. 2004, 45, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, C.; Hellberg, N.; Parini, P.; Vidal, O.; Bohlooly, M.; Rudling, M.; Lindberg, M.K.; Warner, M.; Angelin, B.; Gustafsson, J. Obesity and Disturbed Lipoprotein Profile in Estrogen Receptor-α-Deficient Male Mice. Biochem. Biophys. Res. Commun. 2000, 278, 640–645. [Google Scholar] [CrossRef]
- Anderson, L.A.; McTernan, P.G.; Barnett, A.H.; Kumar, S. The effects of androgens and estrogens on preadipocyte proliferation in human adipose tissue: Influence of gender and site. J. Clin. Endocrinol. Metab. 2001, 86, 5045–5051. [Google Scholar] [CrossRef]
- Tchoukalova, Y.D.; Votruba, S.B.; Tchkonia, T.; Giorgadze, N.; Kirkland, J.L.; Jensen, M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc. Natl. Acad. Sci. USA 2010, 107, 18226–18231. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, C.; Olsson, T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr. Med. Chem. 2007, 14, 2918–2924. [Google Scholar] [CrossRef]
- McInnes, K.J.; Andersson, T.C.; Simonytė, K.; Söderström, I.; Mattsson, C.; Seckl, J.R.; Olsson, T. Association of 11β-hydroxysteroid dehydrogenase type I expression and activity with estrogen receptor β in adipose tissue from post-menopausal women. Menopause 2012, 19, 1347–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonyte, K.; Rask, E.; Näslund, I.; Angelhed, J.-E.; Lönn, L.; Olsson, T.; Mattsson, C. Obesity Is Accompanied by Disturbances in Peripheral Glucocorticoid Metabolism and Changes in FA Recycling. Obesity 2009, 17, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Chazenbalk, G.; Singh, P.; Irge, D.; Shah, A.; Abbott, D.H.; Dumesic, D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013, 78, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, E.; Chazenbalk, G.D.; Aguilera, P.; Madrigal, V.; Grogan, T.; Elashoff, D.; Dumesic, D.A.; Abbott, D.H. Impaired Preadipocyte Differentiation Into Adipocytes in Subcutaneous Abdominal Adipose of PCOS-Like Female Rhesus Monkeys. Endocrinology 2014, 155, 2696–2703. [Google Scholar] [CrossRef] [Green Version]
- Shahnazaryan, U.; Wójcik, M.; Bednarczuk, T.; Kuryłowicz, A. Role of Obesogens in the Pathogenesis of Obesity. Medicina 2019, 55, 515. [Google Scholar] [CrossRef] [Green Version]
- Salehpour, A.; Shidfar, F.; Hedayati, M.; Neshatbini Tehrani, A.; Farshad, A.A.; Mohammadi, S. Bisphenol A enhances adi-pogenic signaling pathways in human mesenchymal stem cells. Genes Environ. 2020, 42, 13. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, Y.; Wei, W.; Zhao, Y.; Jiang, Y.; Wang, N.; Li, X.; Chen, X. Association between Prenatal Exposure to Bisphenol a and Birth Outcomes: A Systematic Review with Meta-Analysis. Medicine 2019, 98, e17672. [Google Scholar] [CrossRef]
- Zhong, Q.; Peng, M.; He, J.; Yang, W.; Huang, F. Association of prenatal exposure to phenols and parabens with birth size: A systematic review and meta-analysis. Sci. Total. Environ. 2020, 703, 134720. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Lapid, K.; Lim, A.; Clegg, D.J.; Zeve, D.; Graff, J.M. Oestrogen signalling in white adipose progenitor cells inhibits differ-entiation into brown adipose and smooth muscle cells. Nat. Commun. 2014, 5, 5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.S.; Frank, A.P.; Fátima, L.A.; Palmer, B.F.; Öz, O.K.; Clegg, D.J. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol. Metab. 2018, 18, 51–59. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Bruun, J.M.; Kristensen, K.; Richelsen, B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem. Biophys. Res. Commun. 2001, 288, 191–197. [Google Scholar] [CrossRef] [PubMed]
- De Morentin, P.B.M.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Cohade, C.; Mourtzikos, K.A.; Wahl, R.L. “USA-Fat”: Prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 1267–1270. [Google Scholar] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfannenberg, C.; Werner, M.K.; Ripkens, S.; Stef, I.; Deckert, A.; Schmadl, M.; Reimold, M.; Häring, H.-U.; Claussen, C.D.; Stefan, N. Impact of Age on the Relationships of Brown Adipose Tissue With Sex and Adiposity in Humans. Diabetes 2010, 59, 1789–1793. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Brychta, R.J.; Linderman, J.D.; Smith, S.; Courville, A.; Dieckmann, W.; Herscovitch, P.; Millo, C.M.; Remaley, A.; Lee, P.; et al. Brown Fat Activation Mediates Cold-Induced Thermogenesis in Adult Humans in Response to a Mild Decrease in Ambient Temperature. J. Clin. Endocrinol. Metab. 2013, 98, E1218–E1223. [Google Scholar] [CrossRef]
- Quarta, C.; Mazza, R.; Pasquali, R.; Pagotto, U. Role of sex hormones in modulation of brown adipose tissue activity. J. Mol. Endocrinol. 2012, 49, R1–R7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Cuenca, S.; Monjo, M.; Frontera, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Sex Steroid Receptor Expression Profile in Brown Adipose Tissue. Effects of Hormonal Status. Cell. Physiol. Biochem. 2007, 20, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Van den Beukel, J.C.; Grefhorst, A.; Hoogduijn, M.J.; Steenbergen, J.; Mastroberardino, P.G.; Dor, F.J.M.F.; Themmen, A.P.N. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity 2015, 23, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Harnichar, A.E.; Zubiría, M.G.; Giordano, A.P.; Miguel, I.; Rey, M.A.; Spinedi, E.; Giovambattista, A. Inhibitory effect of androgens on white adipose tissue thermogenic capacity. Mol. Cell. Endocrinol. 2022, 543. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Non-shivering thermogenesis as a mechanism to facilitate sustainable weight loss. Obes. Rev. 2017, 18, 819–831. [Google Scholar] [CrossRef]
- Bartness, T.J.; Wade, G.N. Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behav. Neurosci. 1984, 98, 674–685. [Google Scholar] [CrossRef]
- Jensen, M.D.; Cryer, P.E.; Johnson, C.M.; Murray, M.J. Effects of epinephrine on regional free fatty acid and energy me-tabolism in men and women. Am. J. Physiol. 1996, 270, E259–E264. [Google Scholar]
- Gavin, K.M.; Cooper, E.E.; Raymer, D.K.; Hickner, R. Estradiol effects on subcutaneous adipose tissue lipolysis in premenopausal women are adipose tissue depot specific and treatment dependent. Am. J. Physiol. Metab. 2013, 304, E1167–E1174. [Google Scholar] [CrossRef] [Green Version]
- Kurylowicz, A.; Jonas, M.; Lisik, W.; Jonas, M.; Wicik, Z.A.; Wierzbicki, Z.; Chmura, A.; Puzianowska-Kuznicka, M. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogenesis-related genes in adipose tissues. J. Transl. Med. 2015, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef] [Green Version]
- Newell-Fugate, A.E. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017, 153, R133–R149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanski, S.A.; Nelson, R.M.; Jensen, M.D. Meal fatty acid uptake in adipose tissue: Gender effects in nonobese humans. Am. J. Physiol. Metab. 2000, 279, E455–E462. [Google Scholar] [CrossRef] [Green Version]
- Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacasa, D.; Agli, B.; Pecquery, R.; Giudicelli, Y. Influence of Ovariectomy and Regional Fat Distribution on the Membranous Transducing System Controlling Lipolysis in Rat Fat Cells. Endocrinology 1991, 128, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Palin, S.L.; McTernan, P.G.; Anderson, L.A.; Sturdee, D.W.; Barnett, A.H.; Kumar, S. 17Beta-estradiol and anti-estrogen ICI:compound 182,780 regulate expression of lipoprotein lipase and hormone-sensitive lipase in isolated subcutaneous ab-dominal adipocytes. Metabolism 2003, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Ramis, J.M.; Salinas, R.; García-Sanz, J.M.; Moreiro, J.; Proenza, A.M.; Lladó, I. Depot- and Gender-related Differences in the Lipolytic Pathway of Adipose Tissue from Severely Obese Patients. Cell. Physiol. Biochem. 2006, 17, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Douchi, T.; Yamamoto, S.; Oki, T.; Kuwahata, R.; Nagata, Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet. Gynecol. 2001, 97, 338–342. [Google Scholar] [PubMed]
- Keller, C.; Larkey, L.; Distefano, J.K.; Boehm-Smith, E.; Records, K.; Robillard, A.; Veres, S.; Al-Zadjali, M.; O’Brian, A.M. Perimenopausal obesity. J. Womens Health 2010, 19, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, L.; Zang, H.; Hirschberg, A.L.; Gustafsson, J.-A.; Arner, P.; Dahlman-Wright, K. Key lipogenic gene expression can be decreased by estrogen in human adipose tissue. Fertil. Steril. 2008, 90, 44–48. [Google Scholar] [CrossRef]
- Tchernof, A.; Desmeules, A.; Richard, C.; Laberge, P.; Daris, M.; Mailloux, J.; Rheaume, C.; Dupont, P. Ovarian Hormone Status and Abdominal Visceral Adipose Tissue Metabolism. J. Clin. Endocrinol. Metab. 2004, 89, 3425–3430. [Google Scholar] [CrossRef] [Green Version]
- Santosa, S.; Jensen, M.D. Adipocyte Fatty Acid Storage Factors Enhance Subcutaneous Fat Storage in Postmenopausal Women. Diabetes 2013, 62, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Zang, H.; Rydén, M.; Wåhlen, K.; Dahlman-Wright, K.; Arner, P.; Hirschberg, A.L. Effects of testosterone and estrogen treatment on lipolysis signaling pathways in subcutaneous adipose tissue of postmenopausal women. Fertil. Steril. 2007, 88, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Faulds, G.; Rydén, M.; Ek, I.; Wahrenberg, H.; Arner, P. Mechanisms behind Lipolytic Catecholamine Resistance of Subcutaneous Fat Cells in the Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.L.; MacPherson, R.; Castellani, L.; Cervone, D.; Anderson, E.; Wright, D.C.; Dyck, D.J. Estradiol does not directly regulate adipose lipolysis. Adipocyte 2017, 6, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, A.P.; Palmer, B.F.; Clegg, D.J. Do estrogens enhance activation of brown and beiging of adipose tissues? Physiol. Behav. 2018, 187, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Naaz, A.; Zakroczymski, M.; Heine, P.; Taylor, J.; Saunders, P.; Lubahn, D.; Cooke, P.S. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): A potential role for estrogen receptor beta (ERbeta). Horm. Metab. Res. 2002, 34, 758–763. [Google Scholar] [CrossRef]
- Varlamov, O.; Chu, M.P.; McGee, W.K.; Cameron, J.L.; O’Rourke, R.; Meyer, K.A.; Bishop, C.; Stouffer, R.L.; Roberts, C. Ovarian Cycle-Specific Regulation of Adipose Tissue Lipid Storage by Testosterone in Female Nonhuman Primates. Endocrinology 2013, 154, 4126–4135. [Google Scholar] [CrossRef] [Green Version]
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Oya, J.; Nakagami, T.; Yamamoto, Y.; Fukushima, S.; Takeda, M.; Endo, Y.; Uchigata, Y. Effects of Age on Insulin Resistance and Secretion in Subjects without Diabetes. Intern. Med. 2014, 53, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Pu, D.; Tan, R.; Yu, Q.; Wu, J. Metabolic syndrome in menopause and associated factors: A meta-analysis. Climacteric 2017, 20, 583–591. [Google Scholar] [CrossRef]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Pereira, R.I.; Casey, B.A.; Swibas, T.A.; Erickson, C.B.; Wolfe, P.; Van Pelt, R.E. Faculty Opinions recommendation of Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J. Clin. Endocrinol. Metab. 2015, 100, 4456–4462. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-E.; Choi, J.; Park, J.; Lee, J.-K.; Shin, A.; Park, S.M.; Kang, D.; Choi, J.-Y. Associations of postmenopausal hormone therapy with metabolic syndrome among diabetic and non-diabetic women. Maturitas 2018, 121, 76–82. [Google Scholar] [CrossRef]
- Macotela, Y.; Boucher, J.; Tran, T.T.; Kahn, C.R. Sex and depot differences in adipocyte insulin sensitivity and glucose me-tabolism. Diabetes 2009, 58, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Zhou, B.; Li, Y.; Wu, Z.; Meng, H.; Wang, G. Sex Differences in the Effect of Testosterone on Adipose Tissue Insulin Resistance From Overweight to Obese Adults. J. Clin. Endocrinol. Metab. 2021, 106, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Palou, M.; Pomar, C.A.; Rodríguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2021, 23, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.W.; Shin, J.H.; Seo, H.S.; Lee, J.K.; Oh, M.J.; Kim, T.; Saw, H.S.; Kim, S.H.; Hur, J.Y. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity 2008, 16, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Casabiell, X.; Piñeiro, V.; Peino, R.; Lage, M.; Camiña, J.; Gallego, R.; Vallejo, L.G.; Dieguez, C.; Casanueva, F.F. Gender Differences in Both Spontaneous and Stimulated Leptin Secretion by Human Omental Adipose Tissue in Vitro: Dexamethasone and Estradiol Stimulate Leptin Release in Women, But Not in Men1. J. Clin. Endocrinol. Metab. 1998, 83, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Gui, Y.; Silha, J.V.; Murphy, L.J. Sexual Dimorphism and Regulation of Resistin, Adiponectin, and Leptin Expression in the Mouse. Obes. Res. 2004, 12, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Van Sinderen, M.L.; Steinberg, G.; Jorgensen, S.B.; Honeyman, J.; Chow, J.D.; Herridge, K.A.; Winship, A.; Dimitriadis, E.; Jones, M.; Simpson, E.R.; et al. Effects of Estrogens on Adipokines and Glucose Homeostasis in Female Aromatase Knockout Mice. PLoS ONE 2015, 10, e0136143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, H.; Shimomura, Y.; Nakanishi, Y.; Futawatari, T.; Ohtani, K.; Sato, N.; Mori, M. Estrogen increases in vivo leptin production in rats and human subjects. J. Endocrinol. 1997, 154, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Yoo, S.W.; Cho, G.J.; Kim, T.; Hur, J.Y.; Park, Y.K.; Lee, K.W.; Kim, S.H. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause 2007, 14, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Castracane, V.; Kraemer, R.R.; Franken, M.A.; Kraemer, G.R.; Gimpel, T. Serum leptin concentration in women: Effect of age, obesity, and estrogen administration. Fertil. Steril. 1998, 70, 472–477. [Google Scholar] [CrossRef]
- Sharma, A.; Mah, M.; Ritchie, R.H.; De Blasio, M.J. The adiponectin signalling pathway—A therapeutic target for the cardiac complications of type 2 diabetes? Pharmacol. Ther. 2022, 232, 108008. [Google Scholar] [CrossRef]
- Böttner, A.; Kratzsch, J.; Müller, G.; Kapellen, T.M.; Blüher, S.; Keller, E.; Blüher, M.; Kiess, W. Gender Differences of Adiponectin Levels Develop during the Progression of Puberty and Are Related to Serum Androgen Levels. J. Clin. Endocrinol. Metab. 2004, 89, 4053–4061. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens decrease plasma adiponectin, an insu-lin-sensitizing adipocyte-derived protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef] [Green Version]
- Babaei, P.; Mehdizadeh, R.; Ansar, M.M.; Damirchi, A. Effects of ovariectomy and estrogen replacement therapy on visceral adipose tissue and serum adiponectin levels in rats. Menopause Int. 2010, 16, 100–104. [Google Scholar] [CrossRef]
- Pektaş, M.; Kurt, A.H.; Ün, I.; Tiftik, R.N.; Büyükafşar, K. Effects of 17β-estradiol and progesterone on the production of adipokines in differentiating 3T3-L1 adipocytes: Role of Rho-kinase. Cytokine 2015, 72, 130–134. [Google Scholar] [CrossRef]
- Horenburg, S.; Fischer-Posovszky, P.; Debatin, K.M.; Wabitsch, M. Influence of sex hormones on adiponectin ex-pression in human adipocytes. Horm. Metab. Res. 2008, 40, 779–786. [Google Scholar] [CrossRef]
- Combs, T.P.; Berg, A.H.; Rajala, M.W.; Klebanov, S.; Iyengar, P.; Jimenez-Chillaron, J.C.; Patti, M.E.; Klein, S.L.; Weinstein, R.S.; Scherer, P.E. Sexual Differentiation, Pregnancy, Calorie Restriction, and Aging Affect the Adipocyte-Specific Secretory Protein Adiponectin. Diabetes 2003, 52, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Chan, K.W.; Hoo, R.L.C.; Wang, Y.; Tan, K.C.B.; Zhang, J.; Chen, B.; Lam, M.C.; Tse, C.; Cooper, G.; et al. Testosterone Selectively Reduces the High Molecular Weight Form of Adiponectin by Inhibiting Its Secretion from Adipocytes. J. Biol. Chem. 2005, 280, 18073–18080. [Google Scholar] [CrossRef] [Green Version]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Domienik-Karlowicz, J.; Puzianowska-Kuznicka, M. Ad-iponectin/resistin interplay in serum and in adipose tissue of obese and normal-weight individuals. Diabetol. Metab. Syndr. 2017, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Babaei, P.; Damirchi, A.; Pourrahim Ghouroughchi, A. The Effect of Estrogen on Visceral Fat, Serum Omentin-1 and Insulin Resistance in Ovariectomized Rats. J. Ardabil. Univ. Med. Sci. 2016, 16, 189–199. [Google Scholar]
- Borowski, A.; Siemińska, L. Serum Omentin Levels in Patients with Prostate Cancer and Associations with Sex Steroids and Metabolic Syndrome. J. Clin. Med. 2020, 9, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.; Krsek, M.; Skrha, J.; Sucharda, P.; Nyomba, B.; Murphy, L. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Lee, M.J.; Chang, H.H.; Hung, P.F.; Kao, Y.H. 17 beta-estradiol stimulates resistin gene expression in 3T3-L1 adipocytes via the estrogen receptor, extracellularly regulated kinase, and CCAAT/enhancer binding protein-alpha pathways. Endocrinology 2006, 147, 4496–4504. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-W.; Seow, K.-M.; Ho, L.-T.; Chien, Y.; Chung, D.-Y.; Chang, C.-L.; Lai, Y.-H.; Hwang, J.-L.; Juan, C.-C. Resistin mRNA levels are downregulated by estrogen in vivo and in vitro. FEBS Lett. 2004, 579, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Seidel, E.R. Estrogens induce visfatin expression in 3T3-L1 cells. Peptides 2010, 31, 271–274. [Google Scholar] [CrossRef]
- Zangooei, M.; Nourbakhsh, M.; Ghahremani, M.H.; Meshkani, R.; Khedri, A.; Shadboorestan, A.; Afra, H.S.; Shahmohamadnejad, S.; Mirmiranpour, H.; Khaghani, S. Investigating the effect of visfatin on ERalpha phosphorylation (Ser118 and Ser167) and ERE-dependent transcriptional activity. EXCLI J. 2018, 17, 516–525. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, M.; Griffin, C.; Singer, K. The Role of Sex and Sex Hormones in Regulating Obesity-Induced Inflammation. Sex Gend. Factors Affect. Metab. Homeost. Diabetes Obes. 2017, 1043, 65–86. [Google Scholar] [CrossRef]
- Ribas, V.; Drew, B.G.; Le, J.A.; Soleymani, T.; Daraei, P.; Sitz, D.; Mohammad, L.; Henstridge, D.C.; Febbraio, M.A.; Hewitt, S.C.; et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic ho-meostasis and accelerates atherosclerotic lesion development. Proc. Natl. Acad. Sci. USA 2011, 108, 16457–16462. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.E.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.D.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef]
- Toniolo, A.; Tedesco, S.; Cappellari, R.; Vegeto, E.; Maggi, A.; Avogaro, A.; Bolego, C.; Cignarella, A.; Fadini, G.P. Alternative Activation of Human Macrophages Is Rescued by Estrogen Treatment In Vitro and Impaired by Menopausal Status. J. Clin. Endocrinol. Metab. 2015, 100. [Google Scholar] [CrossRef] [Green Version]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.J.; Pedersen, A.T.; Lindegaard, B.; Nielsen, S.D. Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS ONE 2020, 15, e0235174. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2018, 3, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkerton, J.V. Hormone Therapy for Postmenopausal Women. N. Engl. J. Med. 2020, 382, 446–455. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenfeld, C.L. Relationship of obesity and high urinary enterolignan concentrations in 6806 children and adults: Analysis of National Health and Nutrition Examination Survey data. Eur. J. Clin. Nutr. 2013, 67, 887–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Liu, Q.; Zhang, Q.; Gu, A.; Jiang, Z.-Y. Urinary enterolactone is associated with obesity and metabolic alteration in men in the US National Health and Nutrition Examination Survey 2001–10. Br. J. Nutr. 2015, 113, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glisic, M.; Kastrati, N.; Musa, J.; Milic, J.; Asllanaj, E.; Fernandez, E.P.; Nano, J.; Rosales, C.O.; Amiri, M.; Kraja, B.; et al. Phytoestrogen supplementation and body composition in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2018, 115, 74–83. [Google Scholar] [CrossRef]
- Carrera-Quintanar, L.; López Roa, R.I.; Quintero-Fabián, S.; Sánchez-Sánchez, M.A.; Vizmanos, B.; Ortuño-Sahagún, D. Phy-tochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018, 2018, 9734845. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.S.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef]
- López, M.; Lelliott, C.J.; Tovar, S.; Kimber, W.; Gallego, R.; Virtue, S.; Blount, M.; Vázquez, M.J.; Finer, N.; Powles, T.J.; et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 2006, 55, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Gandini, S.; Guerrieri-Gonzaga, A.; Iodice, S.; Ruscica, M.; Bonanni, B.; Gulisano, M.; Magni, P.; Formelli, F.; Decensi, A. Effect of Fenretinide and Low-Dose Tamoxifen on Insulin Sensitivity in Premenopausal Women at High Risk for Breast Cancer. Cancer Res. 2008, 68, 9512–9518. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-J.; Lim, Y.S.; Kim, M.S.; Lee, B.; Kim, B.-Y.; Kim, Z.; Lee, J.E.; Lee, M.H.; Kim, S.G.; Kim, Y.S. Risk of fatty liver after long-term use of tamoxifen in patients with breast cancer. PLoS ONE 2020, 15, e0236506. [Google Scholar] [CrossRef]
- Yoneyama, K.; Nakagawa, M. Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia. Breast J. 2019, 25, 788–789. [Google Scholar] [CrossRef] [PubMed]
- Francucci, C.M.; Pantaleo, D.; Iori, N.; Camilletti, A.; Massi, F.; Boscaro, M. Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J. Endocrinol. Investig. 2005, 28, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.E.; Samson, M.M.; Emmelot-Vonk, M.H.; Verhaar, H.J.J. Raloxifene and body composition and muscle strength in postmenopausal women: A randomized, double-blind, placebo-controlled trial. Eur. J. Endocrinol. 2010, 162, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Pelt, R.; Gozansky, W.; Wolfe, P.; Kittelson, J.; Jankowski, C.; Schwartz, R.; Kohrt, W. Estrogen or raloxifene during postmenopausal weight loss: Adiposity and cardiometabolic outcomes. Obesity 2013, 22, 1024–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Li, N.; Gaman, M.-A.; Wang, N. Raloxifene has favorable effects on the lipid profile in women explaining its beneficial effect on cardiovascular risk: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 166, 105512. [Google Scholar] [CrossRef]
- Pickar, J.H.; Boucher, M.; Morgenstern, D. Tissue selective estrogen complex (TSEC): A review. Menopause 2018, 25, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Messig, M.; Yu, C.R.; Assaf, A.R.; Komm, B.S.; Mirkin, S.; Boucher, M. The effect of conjugated estro-gens/bazedoxifene therapy on body weight of postmenopausal women: Pooled analysis of five randomized, place-bo-controlled trials. Menopause 2016, 23, 376–382. [Google Scholar] [CrossRef]
- Lobo, R.A.; Pinkerton, J.V.; Gass, M.L.; Dorin, M.H.; Ronkin, S.; Pickar, J.H.; Constantine, G. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil. Steril. 2009, 92, 1025–1038. [Google Scholar] [CrossRef]
- Skouby, S.O.; Pan, K.; Thompson, J.R.; Komm, B.S.; Mirkin, S. Effects of conjugated estrogens/bazedoxifene on lipid and coagulation variables: A randomized placebo- and active-controlled trial. Menopause 2015, 22, 640–649. [Google Scholar] [CrossRef]
- Solomon, Z.J.; Mirabal, J.R.; Mazur, D.J.; Kohn, T.P.; Lipshultz, L.I.; Pastuszak, A.W. Selective Androgen Receptor Modu-lators: Current Knowledge and Clinical Applications. Sex. Med. Rev. 2019, 7, 84–94. [Google Scholar] [CrossRef]
- Machek, S.B.; Cardaci, T.D.; Wilburn, D.T.; Willoughby, D.S. Considerations, possible contraindications, and potential mechanisms for deleterious effect in recreational and athletic use of selective androgen receptor modulators (SARMs) in lieu of anabolic androgenic steroids: A narrative review. Steroids 2020, 164, 108753. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, S.; Barillà, F.; Tajmir, R.; Meloni, M.; Della Morte, D.; Bellia, A.; Di Daniele, N.; Lauro, D.; Andreadi, A. The New Role of SGLT2 Inhibitors in the Management of Heart Failure: Current Evidence and Future Perspective. Pharmaceutics 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
| Process/Function | Compound | Experimental Settings | Effect | References |
|---|---|---|---|---|
| Stem cell differentiation | 17 β-E2 | mouse BMSC | ↑ differentiation towards osteoblasts | [47] |
| propylpyrazoletriol (ERα agonist) | murine ASC | ↑↑↑ differentiation towards adipocytes | [48] | |
| diarylpropionitrile (ERβ agonist) | murine ASC | ↑ differentiation towards adipocytes | [48] | |
| 17 β-E2 | human ASC | ↑↑↑ differentiation towards adipocytes | [49,50] | |
| Preadipocyte proliferation | 17 β-E2 | subcutaneous and visceral preadipocytes | ↑↑↑ proliferation in women ↑ proliferation in men | [62] |
| E1 | subcutaneous and visceral preadipocytes | ↑ proliferation in women and men | [62] | |
| Adipocyte browning/beiging | propylpyrazoletriol (ERα agonist) | 3T3-L1 murine preadipocytes | ↑ expression of beiging markers | [75] |
| propylpyrazoletriol (ERα agonist) | murine primary preadipocytes | ↑ expression of beiging markers | [75] | |
| 17 β-E2 | ovariectomized rats | ↑ expression of browning markers in adipose tissue | [76] | |
| 17 β-E2 | ovariectomized rats | ↑ expression of browning markers in adipose tissue | [77] | |
| Lipolysis/lipogenesis | 17 β-E2 | 3T3-L1 murine preadipocytes | ↓ expression of lipoprotein lipase gene | [94] |
| 17 β-E2 | mature murine white adipocytes | ↓ expression of PPARγ gene (↓ lipogenesis) | [58] | |
| 17 β-E2 | human primary subcutaneous adipocytes (pre-and postmenopausal women) | ↓ activity of lipoprotein lipase and hormone-sensitive lipase | [8] | |
| 17 β-E2 high concentrations | human primary subcutaneous adipocytes (pre-and postmenopausal women) | ↓ expression of lipoprotein lipase gene | [96] | |
| 17 β-E2 low concentrations | human primary subcutaneous adipocytes | ↑ expression of lipoprotein lipase gene | [96] | |
| 17 β-E2 | human subcutaneous adipose tissue samples (postmenopausal women) | ↓ expression of stearoyl-CoA desaturase, acetyl CoA carboxylase alpha, fatty acid desaturase, PPARγ genes | [100] | |
| 17 β-E2 | human subcutaneous adipose tissue samples (postmenopausal women) | =expression of hormone-sensitive lipase gene | [103] | |
| Insulin sensitivity | HRT | meta-analysis of studies in postmenopausal women | ↓ insulin resistance ↓ diabetes onset | [13,115] |
| 17 β-E2 | ovariectomized C57BL/6 mice | ↑ insulin sensitivity | [116] | |
| Adipokine secretion | ||||
| Leptin | propylpyrazoletriol (ERα agonist) | 3T3-L1 murine preadipocytes | ↑ expression of leptin gene | [120] |
| diarylpropionitrile (ERβ agonist) | 3T3-L1 murine preadipocytes | ↓ expression of leptin gene | [120] | |
| 17 β-E2 | primary human omental adipocytes (postmenopausal women) | ↑ expression of leptin gene | [121] | |
| 17 β-E2 | primary human omental adipocytes (men) | ↓ expression of leptin gene | [121] | |
| 17 β-E2 | ovariectomized mice | ↑ leptin gene expression in adipose tissue | [122] | |
| HRT | postmenopausal women | =serum leptin levels | [126] | |
| ethinyl estradiol | premenopausal women | =serum leptin levels | [126] | |
| Adiponectin | 17 β-E2 | 3T3-L1 murine preadipocytes | ↓ adiponectin secretion | [131] |
| 17 β-E2 | human SBGS adipocyte cell line | =adiponectin secretion | [132] | |
| 17 β-E2 | ovariectomized rats | =serum adiponectin levels | [130] | |
| 17 β-E2 | ovariectomized mice | ↑ adiponectin gene expression in adipose tissue | [122] | |
| Omentin | 17 β-E2 | ovariectomized rats | ↑ omentin serum level | [136] |
| Resitin | 17 β-E2 | 3T3-L1 murine preadipocytes | ↑ resistin secretion | [131,140] |
| 17 β-E2 | ovariectomized mice | ↓ resistin gene expression in adipose tissue | [122,141] | |
| Visfatin | 17 β-E2 | 3T3-L1 murine preadipocytes | ↑ visfatin gene expression | [142] |
| E3 | 3T3-L1 murine preadipocytes | ↑↑↑ visfatin gene expression | [142] | |
| Metabolic inflammation | 17 β-E2 | female aromatase knockout mice | ↑ IL-6 and TNFα serum level | [123] |
| HRT | meta-analysis of studies in postmenopausal women | ↓ serum levels of proinflammatory cytokines | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. https://doi.org/10.3390/biomedicines11030690
Kuryłowicz A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines. 2023; 11(3):690. https://doi.org/10.3390/biomedicines11030690
Chicago/Turabian StyleKuryłowicz, Alina. 2023. "Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction" Biomedicines 11, no. 3: 690. https://doi.org/10.3390/biomedicines11030690
APA StyleKuryłowicz, A. (2023). Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines, 11(3), 690. https://doi.org/10.3390/biomedicines11030690







