The Usefulness of Nanotechnology in Improving the Prognosis of Lung Cancer
Abstract
:1. Introduction
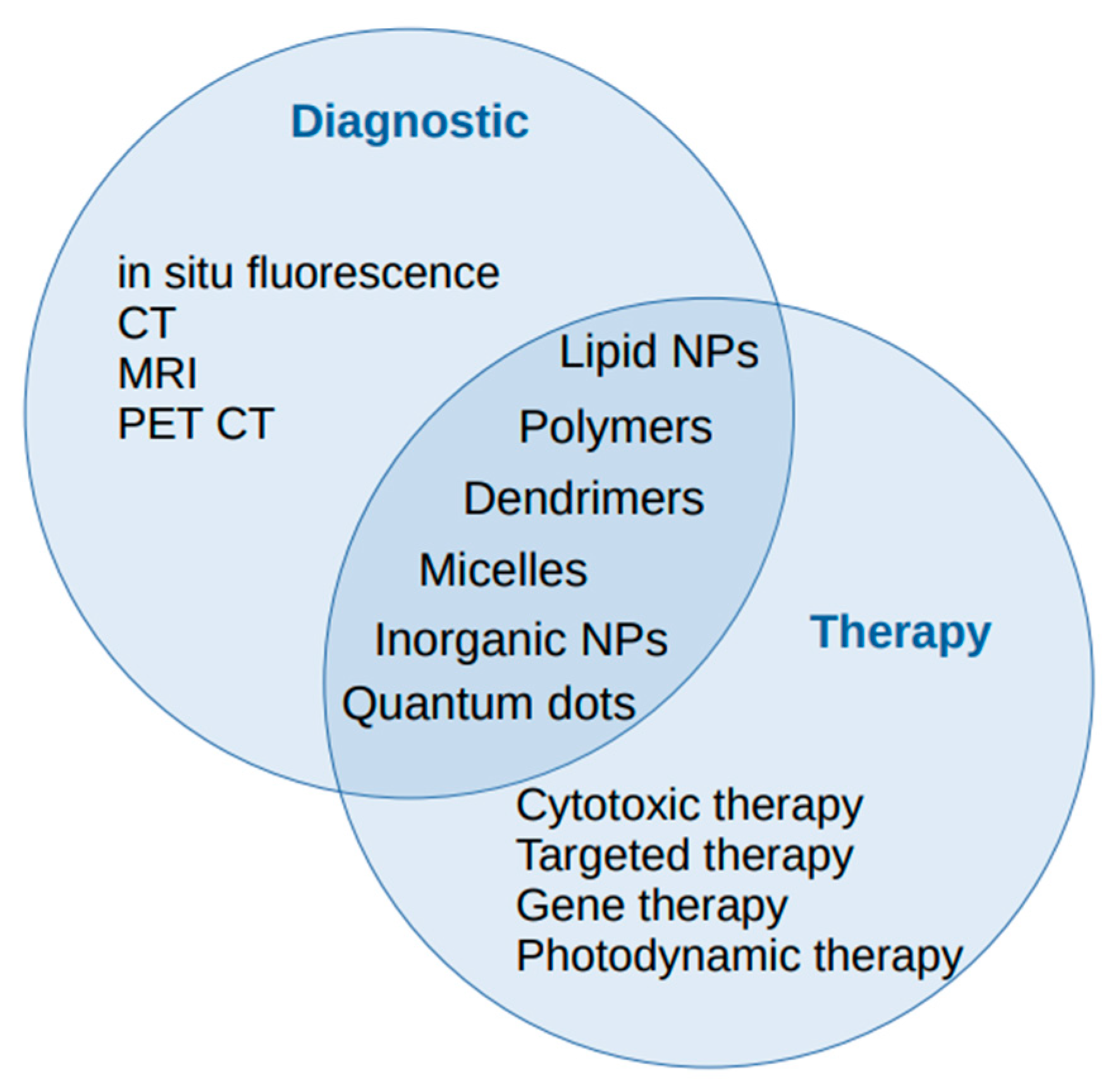
2. Nanoparticles
2.1. Lipid-Based Nanoparticles
2.2. Polymeric Nanoparticles
2.3. Inorganic NPs
2.4. Quantum Dots
3. Nanotechnologies and Lung Cancer Diagnosis
3.1. Imagistics
3.2. Biomarkers
4. Nanotechnologies and Lung Cancer Therapy
5. Limits and Drawbacks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Lin, H.; Datar, R.H. Medical Applications of Nanotechnology. Natl. Med. J. India 2006, 19, 27–32. [Google Scholar] [PubMed]
- GLOBOCAN—Online Database Providing Global Cancer Statistics Link: Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 20 December 2022).
- Ramaswamy, A. Lung Cancer Screening: Review and 2021 Update. Curr. Pulmonol. Rep. 2022, 11, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-Small-Cell Lung Cancer. Nat. Rev. Dis. Prim. 2015, 1, 15009. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Li, K.; Zhan, W.; Jia, M.; Zhao, Y.; Liu, Y.; Jha, R.K.; Zhou, L. Dual Loading of Nanoparticles with Doxorubicin and Icotinib for the Synergistic Suppression of Non-Small Cell Lung Cancer. Int. J. Med. Sci. 2020, 17, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid Optimization of Liposome Characteristics Using a Combined Microfluidics and Design-of-Experiment Approach. Drug Deliv. Transl. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zhang, Y.-Q.; Wang, Z.-Z.; Wu, K.; Lou, J.-N.; Qi, X.-R. Enhanced Brain Distribution and Pharmacodynamics of Rivastigmine by Liposomes Following Intranasal Administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to PEGylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Ju, Y.; Carreño, J.M.; Simon, V.; Dawson, K.; Krammer, F.; Kent, S.J. Impact of Anti-PEG Antibodies Induced by SARS-CoV-2 MRNA Vaccines. Nat. Rev. Immunol. 2022, 20, 1–2. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle Toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, G.; Saini, S.; Rana, A.C. Innovative growth in developing new methods for formulating solid lipid nanoparticles and microparticles. J. Drug Deliv. Ther. 2012, 2, 146–150. [Google Scholar] [CrossRef]
- Manjunath, K.; Reddy, J.S.; Venkateswarlu, V. Solid Lipid Nanoparticles as Drug Delivery Systems. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 127. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Leung, J.; Tam, Y.Y.C.; Cullis, P.R. On the Role of Helper Lipids in Lipid Nanoparticle Formulations of SiRNA. Nanoscale 2019, 11, 21733–21739. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; Dalla-Costa, T.; Guterres, S.S. Freeze-Drying Polymeric Colloidal Suspensions: Nanocapsules, Nanospheres and Nanodispersion. A Comparative Study. Eur. J. Pharm. Biopharm. 2003, 56, 501–505. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric Nanoparticles, Nanospheres and Nanocapsules, for Cutaneous Applications. Drug Target Insights 2007, 2, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [Green Version]
- Aibani, N.; Khan, T.N.; Callan, B. Liposome Mimicking Polymersomes; A Comparative Study of the Merits of Polymersomes in Terms of Formulation and Stability. Int. J. Pharm. X 2020, 2, 100040. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Feijen, J. Polymersomes for Drug Delivery: Design, Formation and Characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Mauro, L.; Naimo, G.D.; Amantea, D.; Cirillo, G.; Tavano, L.; Casaburi, I.; Nicoletta, F.P.; Alvarez-Lorenzo, C.; Iemma, F. Facile Synthesis of PH-Responsive Polymersomes Based on Lipidized PEG for Intracellular Co-Delivery of Curcumin and Methotrexate. Coll. Surf. B Biointerfaces 2018, 167, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Deng, Q.; Li, Y.; Zhou, S. Engineering Docetaxel-Loaded Micelles for Non-Small Cell Lung Cancer: A Comparative Study of Microfluidic and Bulk Nanoparticle Preparation. RSC Adv. 2018, 8, 31950–31966. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Ji, W.; Panus, D.; Palumbo, R.N.; Wang, C. Block Copolymer Micelles with Acid-Labile Ortho Ester Side-Chains: Synthesis, Characterization, and Enhanced Drug Delivery to Human Glioma Cells. J. Control. Release 2011, 151, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic Nanoparticles for Cancer Imaging and Therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luanpitpong, S.; Castranova, V.; Tse, W.; Lu, Y.; Pongrakhananon, V.; Rojanasakul, Y. Carbon Nanotubes Induce Malignant Transformation and Tumorigenesis of Human Lung Epithelial Cells. Nano Lett. 2011, 11, 2796–2803. [Google Scholar] [CrossRef] [Green Version]
- Farmand, M.; Jahanpeyma, F.; Gholaminejad, A.; Azimzadeh, M.; Malaei, F.; Shoaie, N. Carbon Nanostructures: A Comprehensive Review of Potential Applications and Toxic Effects. 3 Biotech 2022, 12, 159. [Google Scholar] [CrossRef]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of Iron Oxide Nanocubes: High-Resolution In Situ Monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an Important Inorganic Nanoparticle Applied in Drug Carrier Systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [Green Version]
- Barkat, A.; Beg, S.; Panda, S.K.; S Alharbi, K.; Rahman, M.; Ahmed, F.J. Functionalized Mesoporous Silica Nanoparticles in Anticancer Therapeutics. Semin. Cancer Biol. 2021, 69, 365–375. [Google Scholar] [CrossRef]
- Mazumder, S.; Dey, R.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Review: Biofunctionalized Quantum Dots in Biology and Medicine. J. Nanomater. 2009, 2009, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent Quantum Dots for Multiplexed Biological Detection and Imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, Y.; Peymani, P.; Afifi, S. Quantum Dot: Magic Nanoparticle for Imaging, Detection and Targeting. Acta Bio-Medica Atenei Parm. 2009, 80, 156–165. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of Quantum Dots in Biology and Medicine. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1048, pp. 323–334. ISBN 978-3-319-72040-1. [Google Scholar]
- Li, Q.; Li, X.; Zhao, C. Strategies to Obtain Encapsulation and Controlled Release of Small Hydrophilic Molecules. Front. Bioeng. Biotechnol. 2020, 8, 437. [Google Scholar] [CrossRef]
- Sharma, G.; Anabousi, S.; Ehrhardt, C.; Ravi Kumar, M.N.V. Liposomes as Targeted Drug Delivery Systems in the Treatment of Breast Cancer. J. Drug Target. 2006, 14, 301–310. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M.; et al. Stimulus-Responsive Liposomes as Smart Nanoplatforms for Drug Delivery Applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.M.; Pearce, T.R.; Adil, M.; Kokkoli, E. Preparation and Characterization of Liposome-Encapsulated Plasmid DNA for Gene Delivery. Langmuir 2013, 29, 9208–9215. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Liposomes in Drug Delivery: Progress and Limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle–Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Arif, U.; Haider, S.; Haider, A.; Khan, N.; Alghyamah, A.A.; Jamila, N.; Khan, M.I.; Almasry, W.A.; Kang, I.-K. Biocompatible Polymers and Their Potential Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 3608–3619. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Hu, J.; Sheng, Y.; Shi, J.; Yu, B.; Yu, Z.; Liao, G. Long Circulating Polymeric Nanoparticles for Gene/Drug Delivery. Curr. Drug Metab. 2018, 19, 723–738. [Google Scholar] [CrossRef]
- Paul, S.; Das, B.; Sharma, H.K. A Review on Bio-Polymers Derived from Animal Sources with Special Reference to Their Potential Applications. J. Drug Deliv. Ther. 2021, 11, 209–223. [Google Scholar] [CrossRef]
- Neuse, E.W. Synthetic Polymers as Drug-Delivery Vehicles in Medicine. Met.-Based Drugs 2008, 2008, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Stránská, E.; Mandys, V.; Waitzová, D. On Toxicity Question of Synthetic Polymers and Their Extracts Tested In Vitro and In Vivo. Polim. Med. 1981, 11, 27–37. [Google Scholar]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of Metallic Nanoparticles. WIREs Nanomed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef]
- Hernando, A.; Crespo, P.; García, M.A. Metallic Magnetic Nanoparticles. Sci. World J. 2005, 5, 972–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Urban, R.M.; Tomlinson, M.J.; Hall, D.J.; Jacobs, J.J. Accumulation in Liver and Spleen of Metal Particles Generated at Nonbearing Surfaces in Hip Arthroplasty. J. Arthroplast. 2004, 19, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Muddapur, U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Nie, S. Next-Generation Quantum Dots. Nat. Biotechnol. 2009, 27, 732–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giroux, M.S.; Zahra, Z.; Salawu, O.A.; Burgess, R.M.; Ho, K.T.; Adeleye, A.S. Assessing the Environmental Effects Related to Quantum Dot Structure, Function, Synthesis and Exposure. Environ. Sci. Nano 2022, 9, 867–910. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C.W. Are Quantum Dots Toxic? Exploring the Discrepancy Between Cell Culture and Animal Studies. Acc. Chem. Res. 2013, 46, 662–671. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for Early Cancer Detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef]
- Higgins, M.J.; Ettinger, D.S. Chemotherapy for Lung Cancer: The State of the Art in 2009. Expert Rev. Anticancer Ther. 2009, 9, 1365–1378. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [Green Version]
- Hollings, N.; Shaw, P. Diagnostic Imaging of Lung Cancer. Eur. Respir. J. 2002, 19, 722–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toumazis, I.; Bastani, M.; Han, S.S.; Plevritis, S.K. Risk-Based Lung Cancer Screening: A Systematic Review. Lung Cancer 2020, 147, 154–186. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Polymeric Contrast Agents for Medical Imaging. Curr. Pharm. Biotechnol. 2000, 1, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Weichert, J.P.; Lee, F.T.; Longino, M.A.; Chosy, S.G.; Counsell, R.E. Lipid-Based Blood-Pool CT Imaging of the Liver. Acad. Radiol. 1998, 5, S16–S19. [Google Scholar] [CrossRef]
- de Vries, A.; Custers, E.; Lub, J.; van den Bosch, S.; Nicolay, K.; Grüll, H. Block-Copolymer-Stabilized Iodinated Emulsions for Use as CT Contrast Agents. Biomaterials 2010, 31, 6537–6544. [Google Scholar] [CrossRef]
- Kweon, S.; Lee, H.-J.; Hyung, W.J.; Suh, J.; Lim, J.S.; Lim, S.-J. Liposomes Coloaded with Iopamidol/Lipiodol as a RES-Targeted Contrast Agent for Computed Tomography Imaging. Pharm. Res. 2010, 27, 1408–1415. [Google Scholar] [CrossRef]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold Nanoparticles as Contrast Agents in X-ray Imaging and Computed Tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [Green Version]
- Gefter, W.B. Magnetic Resonance Imaging in the Evaluation of Lung Cancer. Semin. Roentgenol. 1990, 25, 73–84. [Google Scholar] [CrossRef]
- Sim, A.J.; Kaza, E.; Singer, L.; Rosenberg, S.A. A Review of the Role of MRI in Diagnosis and Treatment of Early Stage Lung Cancer. Clin. Transl. Radiat. Oncol. 2020, 24, 16–22. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent Advances in Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for In Vitro and In Vivo Cancer Nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging Applications for Ferumoxytol as a Contrast Agent in MRI: Emerging Applications of Ferumoxytol. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Kuschner, W.G.; Rydzak, C.E.; Maclean, C.C.; Demas, A.N.; Shigemitsu, H.; Chan, J.K.; Owens, D.K. Test Performance of Positron Emission Tomography and Computed Tomography for Mediastinal Staging in Patients with Non–Small-Cell Lung Cancer: A Meta-Analysis. Ann. Intern. Med. 2003, 139, 879. [Google Scholar] [CrossRef] [PubMed]
- Birim, Ö.; Kappetein, A.P.; Stijnen, T.; Bogers, A.J.J.C. Meta-Analysis of Positron Emission Tomographic and Computed Tomographic Imaging in Detecting Mediastinal Lymph Node Metastases in Nonsmall Cell Lung Cancer. Ann. Thorac. Surg. 2005, 79, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.L.; Henriksen, J.R.; Binderup, T.; Elema, D.R.; Rasmussen, P.H.; Hag, A.M.; Kjær, A.; Andresen, T.L. In Vivo Evaluation of PEGylated 64Cu-Liposomes with Theranostic and Radiotherapeutic Potential Using Micro PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.L.; Binderup, T.; Jølck, R.I.; Rasmussen, P.; Henriksen, J.R.; Pfeifer, A.K.; Kjær, A.; Andresen, T.L. Positron Emission Tomography Evaluation of Somatostatin Receptor Targeted 64Cu-TATE-Liposomes in a Human Neuroendocrine Carcinoma Mouse Model. J. Control. Release 2012, 160, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical Biosensors for the Detection of Lung Cancer Biomarkers: A Review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum Dot Based Nano-Biosensors for Detection of Circulating Cell Free MiRNAs in Lung Carcinogenesis: From Biology to Clinical Translation. Front. Genet. 2018, 9, 616. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Qi, H.; Teng, J.; Su, B.; Chen, H.; Wang, C.; Xia, Q. Identification of Serum MiRNAs by Nano-Quantum Dots Microarray as Diagnostic Biomarkers for Early Detection of Non-Small Cell Lung Cancer. Tumor Biol. 2016, 37, 7777–7784. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Zhang, Y.; Lau, C.; Lu, J. Simultaneous Detection of Two Lung Cancer Biomarkers Using Dual-Color Fluorescence Quantum Dots. Analyst 2011, 136, 1399. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Li, G.; Jing, F.; Mao, H.; Jin, Q.; Zhai, W.; Zhang, H.; Zhao, J.; Jia, C. Multiplexed Detection of Lung Cancer Biomarkers Based on Quantum Dots and Microbeads. Talanta 2016, 156–157, 48–54. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of Rare Circulating Tumour Cells in Cancer Patients by Microchip Technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [Green Version]
- Maheswaran, S.; Sequist, L.V.; Nagrath, S.; Ulkus, L.; Brannigan, B.; Collura, C.V.; Inserra, E.; Diederichs, S.; Iafrate, A.J.; Bell, D.W.; et al. Detection of Mutations in EGFR in Circulating Lung-Cancer Cells. N. Engl. J. Med. 2008, 359, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Wolfbeis, O.S. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtzer, R. Nanoparticles as Computed Tomography Contrast Agents: Current Status and Future Perspectives. Nanomed. 2012, 7, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Mortezazadeh, T. Glucosamine Conjugated Gadolinium (III) Oxide Nanoparticles as a Novel Targeted Contrast Agent for Cancer Diagnosis in MRI. J. Biomed. Phys. Eng. 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Li, H.; Xiong, Z.; Shen, M.; Conti, P.S.; Shi, X.; Chen, K. Polyethyleneimine-Coated Manganese Oxide Nanoparticles for Targeted Tumor PET/MR Imaging. ACS Appl. Mater. Interfaces 2018, 10, 34954–34964. [Google Scholar] [CrossRef] [PubMed]
- Drobota, M.; Vlad, S.; Gradinaru, L.M.; Bargan, A.; Radu, I.; Butnaru, M.; Rîmbu, C.M.; Ciobanu, R.C.; Aflori, M. Composite Materials Based on Gelatin and Iron Oxide Nanoparticles for MRI Accuracy. Materials 2022, 15, 3479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hang, D.; Li, Y.; Zhang, J.; Wu, H.; Wang, H.; Tian, E.; Yan, J. Role of Gd2O3-doped Carbon-11-choline-lenvatinib Nanoparticles Contrast Agent PET/CT in the Diagnosis of Patients with Lung Cancer. Oncol. Lett. 2019, 19, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W. Bacteria-like Mesoporous Silica-Coated Gold Nanorods for Positron Emission Tomography and Photoacoustic Imaging-Guided Chemo-Photothermal Combined Therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef]
- Weiss, R.B.; Donehower, R.C.; Wiernik, P.H.; Ohnuma, T.; Gralla, R.J.; Trump, D.L.; Baker, J.R.; Van Echo, D.A.; Von Hoff, D.D.; Leyland-Jones, B. Hypersensitivity Reactions from Taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, L.D.; Luu, T.H.; McKoy, J.M.; Samaras, A.T.; Fisher, M.J.; Carias, E.E.; Raisch, D.W.; Calhoun, E.A.; Bennett, C.L. Cremophor EL-Containing Paclitaxel-Induced Anaphylaxis: A Call to Action. Community Oncol. 2009, 6, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; Sparreboom, A.; Mross, K. Peripheral Neuropathy: A Persisting Challenge in Paclitaxel-Based Regimes. Eur. J. Cancer 2006, 42, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Montana, M.; Ducros, C.; Verhaeghe, P.; Terme, T.; Vanelle, P.; Rathelot, P. Albumin-Bound Paclitaxel: The Benefit of This New Formulation in the Treatment of Various Cancers. J. Chemother. 2011, 23, 59–66. [Google Scholar] [CrossRef]
- Ramalingam, S.; Belani, C.P. Paclitaxel for Non-Small Cell Lung Cancer. Expert Opin. Pharmacother. 2004, 5, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Brachmann, C.; Liu, X.; Pierce, D.W.; Dey, J.; Kerwin, W.S.; Li, Y.; Zhou, S.; Hou, S.; Carleton, M.; et al. Albumin-Bound Nanoparticle (Nab) Paclitaxel Exhibits Enhanced Paclitaxel Tissue Distribution and Tumor Penetration. Cancer Chemother. Pharmacol. 2015, 76, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil–Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Gradishar, W.J. Albumin-Bound Paclitaxel: A next-Generation Taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Q.; Yi, K.; Chen, N.; Li, X.; Yang, S.; Hou, X.; Zhao, J.; Yuan, X.; Kang, C. Engineering Lipusu with Lysophosphatidylcholine for Improved Tumor Cellular Uptake and Anticancer Efficacy. J. Mater. Chem. B 2022, 10, 1833–1842. [Google Scholar] [CrossRef]
- Shi, M.; Gu, A.; Tu, H.; Huang, C.; Wang, H.; Yu, Z.; Wang, X.; Cao, L.; Shu, Y.; Wang, H.; et al. Comparing Nanoparticle Polymeric Micellar Paclitaxel and Solvent-Based Paclitaxel as First-Line Treatment of Advanced Non-Small-Cell Lung Cancer: An Open-Label, Randomized, Multicenter, Phase III Trial. Ann. Oncol. 2021, 32, 85–96. [Google Scholar] [CrossRef]
- Lu, J.; Gu, A.; Wang, W.; Huang, A.; Han, B.; Zhong, H. Polymeric Micellar Paclitaxel (Pm-Pac) Prolonged Overall Survival for NSCLC Patients without Pleural Metastasis. Int. J. Pharm. 2022, 623, 121961. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, J.; Guo, S.; Zhao, Z.; Wang, F. Formulations, Pharmacodynamic and Clinical Studies of Nanoparticles for Lung Cancer Therapy–An Overview. Curr. Drug Metab. 2018, 19, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Shen, W.; Wang, L.; Wang, C.; Pu, J. Study on the Mechanism of Action of Paclitaxel-Loaded Polylactic-Co-Glycolic Acid Nanoparticles in Non-Small-Cell Lung Carcinoma Cells. Comput. Math. Methods Med. 2022, 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Minchinton, A.I.; Tannock, I.F. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Panuţa, A.; Radu, I.; Gafton, B.; Ioanid, N.; Terinte, C.; Ferariu, D.; Buna-Arvinte, M.; Scripcariu, D.V.; Scripcariu, V. Multiple versus Unifocal Breast Cancer: Clinicopathological and Immunohistochemical Differences. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2019, 60, 103–110. [Google Scholar]
- Primeau, A.J.; Rendon, A.; Hedley, D.; Lilge, L.; Tannock, I.F. The Distribution of the Anticancer Drug Doxorubicin in Relation to Blood Vessels in Solid Tumors. Clin. Cancer Res. 2005, 11, 8782–8788. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, M.S.; Upadhyay, U.; Goodwin, R.; Tyler, B.; Brem, H. Local Delivery of Doxorubicin for the Treatment of Malignant Brain Tumors in Rats. Anticancer Res. 2005, 25, 3825–3831. [Google Scholar]
- Sztandera, K.; Działak, P.; Marcinkowska, M.; Stańczyk, M.; Gorzkiewicz, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Sugar Modification Enhances Cytotoxic Activity of PAMAM-Doxorubicin Conjugate in Glucose-Deprived MCF-7 Cells–Possible Role of GLUT1 Transporter. Pharm. Res. 2019, 36, 140. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced Cardiotoxicity and Comparable Efficacy in a Phase IIItrial of Pegylated Liposomal Doxorubicin HCl(CAELYXTM/Doxil®) versus Conventional Doxorubicin Forfirst-Line Treatment of Metastatic Breast Cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Chittasupho, C.; Lirdprapamongkol, K.; Kewsuwan, P.; Sarisuta, N. Targeted Delivery of Doxorubicin to A549 Lung Cancer Cells by CXCR4 Antagonist Conjugated PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 529–538. [Google Scholar] [CrossRef]
- Inoue, A. Progress in Individualized Treatment for EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Proc. Jpn. Acad. Ser. B 2020, 96, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Javed Shaikh, M.A.; Afzal, O.; Alfawaz Altamimi, A.S.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Al-Abbasi, F.A.; Singh, S.K.; Dua, K.; et al. An Overview of Epithelial Growth Factor Receptor (EGFR) Inhibitors in Cancer Therapy. Chem. Biol. Interact. 2022, 366, 110108. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare Epidermal Growth Factor Receptor (EGFR) Mutations in Non-Small Cell Lung Cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, J.; Zhang, J.; Xie, Y.; Soh, J.; Shigematsu, H.; Zhang, W.; Yamamoto, H.; Peyton, M.; Girard, L.; Lockwood, W.W.; et al. Alterations in Genes of the EGFR Signaling Pathway and Their Relationship to EGFR Tyrosine Kinase Inhibitor Sensitivity in Lung Cancer Cell Lines. PLoS ONE 2009, 4, e4576. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus Cisplatin plus Docetaxel in Patients with Non-Small-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Maruvka, Y.E.; Michor, F.; Pao, W. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor–Resistant Disease. J. Clin. Oncol. 2013, 31, 1070–1080. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-G.; Liu, Y.-N.; Tsai, M.-F.; Chang, Y.-L.; Yu, C.-J.; Yang, P.-C.; Yang, J.C.-H.; Wen, Y.-F.; Shih, J.-Y. The Mechanism of Acquired Resistance to Irreversible EGFR Tyrosine Kinase Inhibitor-Afatinib in Lung Adenocarcinoma Patients. Oncotarget 2016, 7, 12404–12413. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and Management of 3rd-generation EGFR-TKI Resistance in Advanced Non-small Cell Lung Cancer (Review). Int. J. Oncol. 2021, 59, 90. [Google Scholar] [CrossRef]
- Ni, X.L.; Chen, L.X.; Zhang, H.; Yang, B.; Xu, S.; Wu, M.; Liu, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; et al. In Vitro and In Vivo Antitumor Effect of Gefitinib Nanoparticles on Human Lung Cancer. Drug Deliv. 2017, 24, 1501–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the Preparation Process for Human Serum Albumin (HSA) Nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Koli, U.; Dandekar, P.; Jain, R. Investigating Behaviour of Polymers in Nanoparticles of Chitosan Oligosaccharides Coated with Hyaluronic Acid. Polymer 2016, 93, 44–52. [Google Scholar] [CrossRef]
- Pedrosa, S.S.; Pereira, P.; Correia, A.; Gama, F.M. Targetability of Hyaluronic Acid Nanogel to Cancer Cells: In Vitro and In Vivo Studies. Eur. J. Pharm. Sci. 2017, 104, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Li, W. HA/HSA Co-Modified Erlotinib–Albumin Nanoparticles for Lung Cancer Treatment. Drug Des. Devel. Ther. 2018, 12, 2285–2292. [Google Scholar] [CrossRef] [Green Version]
- Elbatanony, R.S.; Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Chauhan, G.; Kunda, N.K.; Gupta, V. Afatinib-Loaded Inhalable PLGA Nanoparticles for Localized Therapy of Non-Small Cell Lung Cancer (NSCLC)—Development and In-Vitro Efficacy. Drug Deliv. Transl. Res. 2021, 11, 927–943. [Google Scholar] [CrossRef]
- Chen, W.; Yu, D.; Sun, S.-Y.; Li, F. Nanoparticles for Co-Delivery of Osimertinib and Selumetinib to Overcome Osimertinib-Acquired Resistance in Non-Small Cell Lung Cancer. Acta Biomater. 2021, 129, 258–268. [Google Scholar] [CrossRef]
- Ali, A.A.A.; Hsu, F.-T.; Hsieh, C.-L.; Shiau, C.-Y.; Chiang, C.-H.; Wei, Z.-H.; Chen, C.-Y.; Huang, H.-S. Erlotinib-Conjugated Iron Oxide Nanoparticles as a Smart Cancer-Targeted Theranostic Probe for MRI. Sci. Rep. 2016, 6, 36650. [Google Scholar] [CrossRef] [Green Version]
- Iwahara, T.; Fujimoto, J.; Wen, D.; Cupples, R.; Bucay, N.; Arakawa, T.; Mori, S.; Ratzkin, B.; Yamamoto, T. Molecular Characterization of ALK, a Receptor Tyrosine Kinase Expressed Specifically in the Nervous System. Oncogene 1997, 14, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Grande, E.; Bolós, M.-V.; Arriola, E. Targeting Oncogenic ALK: A Promising Strategy for Cancer Treatment. Mol. Cancer Ther. 2011, 10, 569–579. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Sun, Y.; Wang, L.-Z.; Yu, Y.-C.; Ding, X. Cytoplasmic C-Ros Oncogene 1 Receptor Tyrosine Kinase Expression May Be Associated with the Development of Human Oral Squamous Cell Carcinoma. Oncol. Lett. 2015, 10, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebele, R.C.; Pilling, A.B.; Aisner, D.L.; Kutateladze, T.G.; Le, A.T.; Weickhardt, A.J.; Kondo, K.L.; Linderman, D.J.; Heasley, L.E.; Franklin, W.A.; et al. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non–Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 1472–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-L.; Xiang, Z.-J.; Yang, J.-H.; Wang, W.-J.; Xiang, R.-L. Effect of Alectinib versus Crizotinib on Progression-Free Survival, Central Nervous System Efficacy and Adverse Events in ALK-Positive Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2020, 9, 1782–1796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Dai, S.-P.; Xu, Y.-Q.; Li, T.; Xie, J.; Li, C.; Zhang, Z.-H. Crizotinib-Loaded Polymeric Nanoparticles in Lung Cancer Chemotherapy. Med. Oncol. 2015, 32, 193. [Google Scholar] [CrossRef]
- Zhong, T.; Liu, X.; Li, H.; Zhang, J. Co-Delivery of Sorafenib and Crizotinib Encapsulated with Polymeric Nanoparticles for the Treatment of in Vivo Lung Cancer Animal Model. Drug Deliv. 2021, 28, 2108–2118. [Google Scholar] [CrossRef]
- Muller, I.B.; de Langen, A.J.; Giovannetti, E.; Peters, G.J. Anaplastic Lymphoma Kinase Inhibition in Metastatic Non-Small Cell Lung Cancer: Clinical Impact of Alectinib. OncoTargets Ther. 2017, 10, 4535–4541. [Google Scholar] [CrossRef] [Green Version]
- Kodama, T.; Tsukaguchi, T.; Yoshida, M.; Kondoh, O.; Sakamoto, H. Selective ALK Inhibitor Alectinib with Potent Antitumor Activity in Models of Crizotinib Resistance. Cancer Lett. 2014, 351, 215–221. [Google Scholar] [CrossRef]
- Larkins, E.; Blumenthal, G.M.; Chen, H.; He, K.; Agarwal, R.; Gieser, G.; Stephens, O.; Zahalka, E.; Ringgold, K.; Helms, W.; et al. FDA Approval: Alectinib for the Treatment of Metastatic, ALK-Positive Non–Small Cell Lung Cancer Following Crizotinib. Clin. Cancer Res. 2016, 22, 5171–5176. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Gu, W.; Li, J.; Chen, C.; Xu, Z.P. Silencing PD-1 and PD-L1 with Nanoparticle-Delivered Small Interfering RNA Increases Cytotoxicity of Tumor-Infiltrating Lymphocytes. Nanomedicine 2019, 14, 955–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold Nanoshells-Mediated Bimodal Photodynamic and Photothermal Cancer Treatment Using Ultra-Low Doses of near Infra-Red Light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold Nanoparticles as Novel Agents for Cancer Therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Jin, H.; Huang, X.; Shi, Y.; Liu, Z.; Ben, S. PPy@Fe3O4 Nanoparticles Inhibit Tumor Growth and Metastasis Through Chemodynamic and Photothermal Therapy in Non-Small Cell Lung Cancer. Front. Chem. 2021, 9, 789934. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, B.; Liu, Y.; Wan, Y.; Liu, Q.; Xu, H.; Liang, R.; Shi, Y.; Tu, P.; Wu, H.; et al. Mitochondria-Targeting Polydopamine-Coated Nanodrugs for Effective Photothermal- and Chemo-Synergistic Therapies against Lung Cancer. Regen. Biomater. 2022, 9, rbac051. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum Dots and Nanoparticles for Photodynamic and Radiation Therapies of Cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [Green Version]
- Uprety, B.; Abrahamse, H. Semiconductor Quantum Dots for Photodynamic Therapy: Recent Advances. Front. Chem. 2022, 10, 946574. [Google Scholar] [CrossRef]
- He, B.; Jin, H.-Y.; Wang, Y.-W.; Fan, C.-M.; Wang, Y.-F.; Zhang, X.-C.; Liu, J.-X.; Li, R.; Liu, J.-W. Carbon Quantum Dots/Bi4O5Br2 Photocatalyst with Enhanced Photodynamic Therapy: Killing of Lung Cancer (A549) Cells In Vitro. Rare Met. 2022, 41, 132–143. [Google Scholar] [CrossRef]
- Chang, J.-E.; Yoon, I.-S.; Sun, P.-L.; Yi, E.; Jheon, S.; Shim, C.-K. Anticancer Efficacy of Photodynamic Therapy with Hematoporphyrin-Modified, Doxorubicin-Loaded Nanoparticles in Liver Cancer. J. Photochem. Photobiol. B 2014, 140, 49–56. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Śmiałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Gold Nanoparticles for Cancer Radiotherapy: A Review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, W.; Kumar, R.; Sridhar, S.; Korideck, H.; Zygmanski, P.; Cormack, R.A.; Berbeco, R.; Makrigiorgos, G.M. Targeted Radiotherapy with Gold Nanoparticles: Current Status and Future Perspectives. Nanomedicine 2014, 9, 1063–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Altundal, Y.; Moreau, M.; Sajo, E.; Kumar, R.; Ngwa, W. Potential for Enhancing External Beam Radiotherapy for Lung Cancer Using High-Z Nanoparticles Administered via Inhalation. Phys. Med. Biol. 2015, 60, 7035–7043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kibria, G.; Hatakeyama, H.; Ohga, N.; Hida, K.; Harashima, H. Dual-Ligand Modification of PEGylated Liposomes Shows Better Cell Selectivity and Efficient Gene Delivery. J. Control. Release 2011, 153, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasungu, L.; Hoekstra, D. Cationic Lipids, Lipoplexes and Intracellular Delivery of Genes. J. Control. Release 2006, 116, 255–264. [Google Scholar] [CrossRef]
- Nimesh, S. Polyethylenimine as a Promising Vector for Targeted SiRNA Delivery. Curr. Clin. Pharmacol. 2012, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Vardhan, H.; Ajmal, G.; Bonde, G.V.; Kapoor, R.; Mittal, A.; Mishra, B. Formulation, Optimization, Hemocompatibility and Pharmacokinetic Evaluation of PLGA Nanoparticles Containing Paclitaxel. Drug Dev. Ind. Pharm. 2019, 45, 365–378. [Google Scholar] [CrossRef]
- Duan, Y.; Shen, C.; Zhang, Y.; Luo, Y. Advanced Diagnostic and Therapeutic Strategies in Nanotechnology for Lung Cancer. Front. Oncol. 2022, 12, 1031000. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Holt, D.; Heitjan, D.; Deshpande, C.; Venegas, O.; Jiang, J.; Judy, R.; DeJesus, E.; Madajewski, B.; Oh, K.; et al. Intraoperative Near-Infrared Imaging Can Identify Pulmonary Nodules. Ann. Thorac. Surg. 2014, 98, 1223–1230. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Bin-Jumah, M.; AL-Abdan, M.; Albasher, G.; Alarifi, S. Effects of Green Silver Nanoparticles on Apoptosis and Oxidative Stress in Normal and Cancerous Human Hepatic Cells In Vitro. Int. J. Nanomed. 2020, 15, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Urbán, P.; Bogni, A.; Ponti, J.; Gioria, S.; Kinsner-Ovaskainen, A. The Agglomeration State of Nanoparticles Can Influence the Mechanism of Their Cellular Internalisation. J. Nanobiotechnol. 2017, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of Size, Surface Charge, and Agglomeration State of Nanoparticle Dispersions for Toxicological Studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Oberdörster, G.; Ferin, J.; Lehnert, B.E. Correlation between Particle Size, In Vivo Particle Persistence, and Lung Injury. Environ. Health Perspect. 1994, 102, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Swetha, K.L.; Roy, A. Tumor Heterogeneity and Nanoparticle-Mediated Tumor Targeting: The Importance of Delivery System Personalization. Drug Deliv. Transl. Res. 2018, 8, 1508–1526. [Google Scholar] [CrossRef]
- Cagliani, R.; Gatto, F.; Bardi, G. Protein Adsorption: A Feasible Method for Nanoparticle Functionalization? Materials 2019, 12, 1991. [Google Scholar] [CrossRef] [Green Version]
- Martínez, R.; Navarro Poupard, M.F.; Álvarez, A.; Soprano, E.; Migliavacca, M.; Carrillo-Carrión, C.; Polo, E.; Pelaz, B.; del Pino, P. Nanoparticle Behavior and Stability in Biological Environments. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 5–18. ISBN 978-0-12-816662-8. [Google Scholar]
| Nanoparticles Type | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| Liposomes |
|
| [47,48,49,50,51,52] | |
| Solid lipid nanoparticles |
|
| [19,53,54,55] | |
| Polymeric nanoparticles | Natural polymers |
|
| [56,57,58,59] |
| Synthetic polymers |
|
| [56,60,61] | |
| Metallic nanoparticles |
|
| [62,63,64,65,66] | |
| Quantum dots |
|
| [67,68,69,70] | |
| Procedure | Nanoparticles | Role | Reference |
|---|---|---|---|
| Fluorescence—in situ examination | Fluorescent and non-fluorescent NPs (e.g., quantum dots, silica-coated with fluorophores) | Fluorescent agents | [34,42,96] |
| Computer tomography | Gold nanoparticles | Targeted contrast agent | [79,97] |
| Magnetic resonance imaging | SPIONs, gadolinium oxide-based NPs, manganese oxide NPs | Improved contrast agents | [83,98,99,100] |
| Positron emission tomography | Gd2O3-doped carbon-11-choline (GdCho), gold/mesoporous silica hybrid nanoparticles, manganese oxide NPs | Improved contrast agents | [99,101,102] |
| Study Type | Description | Primary Outcome | NCT Number | Number of Participants |
|---|---|---|---|---|
| Phase IV | Efficacy and safety of paclitaxel liposome and cisplatin compared with gemcitabine and cisplatin as first-line therapy in advanced squamous non-small-cell lung cancer | Progression-free survival | NCT02996214 | 536 |
| Phase II | ABI-009, human albumin-bound rapamycin, in patients with metastatic, unresectable, low, or intermediate grade neuroendocrine tumors of the lung or gastro-enteropancreatic system who have progressed or been intolerant to everolimus | Disease control rate | NCT03670030 | 5 |
| Phase II | Safety and efficacy of BIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy to patients with non-small-cell lung cancer | Objective response rate | NCT01792479 | 64 |
| Phase II | BIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy for patients with KRAS positive or squamous cell non-small cell lung cancer | Disease control rate | NCT02283320 | 69 |
| Phase II | Carboplatin and paclitaxel albumin-stabilized nanoparticle formulation together with radiation therapy and erlotinib in treating patients with Stage III NSCLC that cannot be removed by surgery | Overall survival at 12 months | NCT00553462 | 78 |
| Phase II | Paclitaxel albumin-stabilized nanoparticle formulation given together with carboplatin in treating patients with stage IIIB, stage IV, or recurrent NSCLC | Overall response rate | NCT00729612 | 63 |
| Phase I-II | Side effects and optimal dose of ABI-007 (paclitaxel albumin-stabilized nanoparticle formulation) efficacy in treating patients with stage IV NSCLC | Target lesion response (safety, tolerability, antitumor activity) | NCT00077246 | 64 |
| Phase II | CRLX101 (camptothecin (CPT) conjugated to a cyclodextrin-based polymer) vs. best supportive care (BSC) in advanced non-small-cell lung cancer (NSCLC) | Overall survival | NCT01380769 | 157 |
| Phase II | Paclitaxel albumin-stabilized nanoparticle formulation (Abraxane) in treating patients with previously treated advanced non-small-cell lung cancer. | Overall response rate | NCT01620190 | 26 |
| Phase I/II | Safety and antitumor activity of ABI-007 (a unique protein formulation of paclitaxel) in weekly administration in naïve patients with advanced non-small cell lung cancer | Establishing the toxicity | NCT00073723 | 75 |
| Phase I | TargomiRs (targeted minicells containing a microRNA mimic) as 2nd or 3rd line treatment for patients with recurrent malignant pleural mesothelioma and non-small-cell lung cancer. | Establishing maximum tolerated dose and dose-limiting toxicities | NCT02369198 | 27 |
| Phase II | Effectiveness of nab-paclitaxel + carboplatin + MPDL3280A (monoclonal antibody directed against the protein ligand programmed cell death-1 ligand 1 (PD-L1) for treatment of non-small-cell lung carcinoma (NSCLC) | Major pathologic response rate | NCT02716038 | 39 |
| Phase I/II | Combination therapy with NC-6004 (nanoparticle-cisplatin) and gemcitabine in patients with advanced solid tumors or non-small-cell lung, biliary, and bladder cancer | Progression-free survival | NCT02240238 | 209 |
| Procedure | Nanoparticles | Role | Reference |
|---|---|---|---|
| Photothermal therapy | Gold nanoparticles, Fe3O4, polydopamine | Fluorescent dye, photosensitizer, theragnostic agent | [154,155,156,157] |
| Photodynamic therapy | Quantum dots, photosensitizer nanoparticles (hypocrellin B) | Photosensitizer | [158,159,160,161] |
| Radiation therapy | Gold and platinum-based NPs | Sensitizer | [162,163,164] |
| Gene therapy | Liposomal nucleic acid delivery system (lipofectamine), solid lipid- and polymer-based gene delivery vectors | Nucleic acid delivery systems | [165,166,167] |
| Chemotherapy | Polymers, dendrimers, liposome-based drug delivery systems (various chemotherapeutic agents) | Carriers, targeted carriers | [112,115,120,121,168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordeianu, G.; Filip, N.; Cernomaz, A.; Veliceasa, B.; Hurjui, L.L.; Pinzariu, A.C.; Pertea, M.; Clim, A.; Marinca, M.V.; Serban, I.L. The Usefulness of Nanotechnology in Improving the Prognosis of Lung Cancer. Biomedicines 2023, 11, 705. https://doi.org/10.3390/biomedicines11030705
Bordeianu G, Filip N, Cernomaz A, Veliceasa B, Hurjui LL, Pinzariu AC, Pertea M, Clim A, Marinca MV, Serban IL. The Usefulness of Nanotechnology in Improving the Prognosis of Lung Cancer. Biomedicines. 2023; 11(3):705. https://doi.org/10.3390/biomedicines11030705
Chicago/Turabian StyleBordeianu, Gabriela, Nina Filip, Andrei Cernomaz, Bogdan Veliceasa, Loredana Liliana Hurjui, Alin Constantin Pinzariu, Mihaela Pertea, Andreea Clim, Mihai Vasile Marinca, and Ionela Lacramioara Serban. 2023. "The Usefulness of Nanotechnology in Improving the Prognosis of Lung Cancer" Biomedicines 11, no. 3: 705. https://doi.org/10.3390/biomedicines11030705
APA StyleBordeianu, G., Filip, N., Cernomaz, A., Veliceasa, B., Hurjui, L. L., Pinzariu, A. C., Pertea, M., Clim, A., Marinca, M. V., & Serban, I. L. (2023). The Usefulness of Nanotechnology in Improving the Prognosis of Lung Cancer. Biomedicines, 11(3), 705. https://doi.org/10.3390/biomedicines11030705










