Differential Expression of microRNAs in Serum of Patients with Chronic Painful Polyneuropathy and Healthy Age-Matched Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection, Serum Separation and Storage
2.2. miRNA Purification
2.3. miRNA Pre Library Preparation Quality Control
2.4. miRNA Library Preparation
2.5. Adapter Dimer Removal and miRNA Library Pre Sequencing Quantification/Quality Control
2.6. Next Generation Sequencing
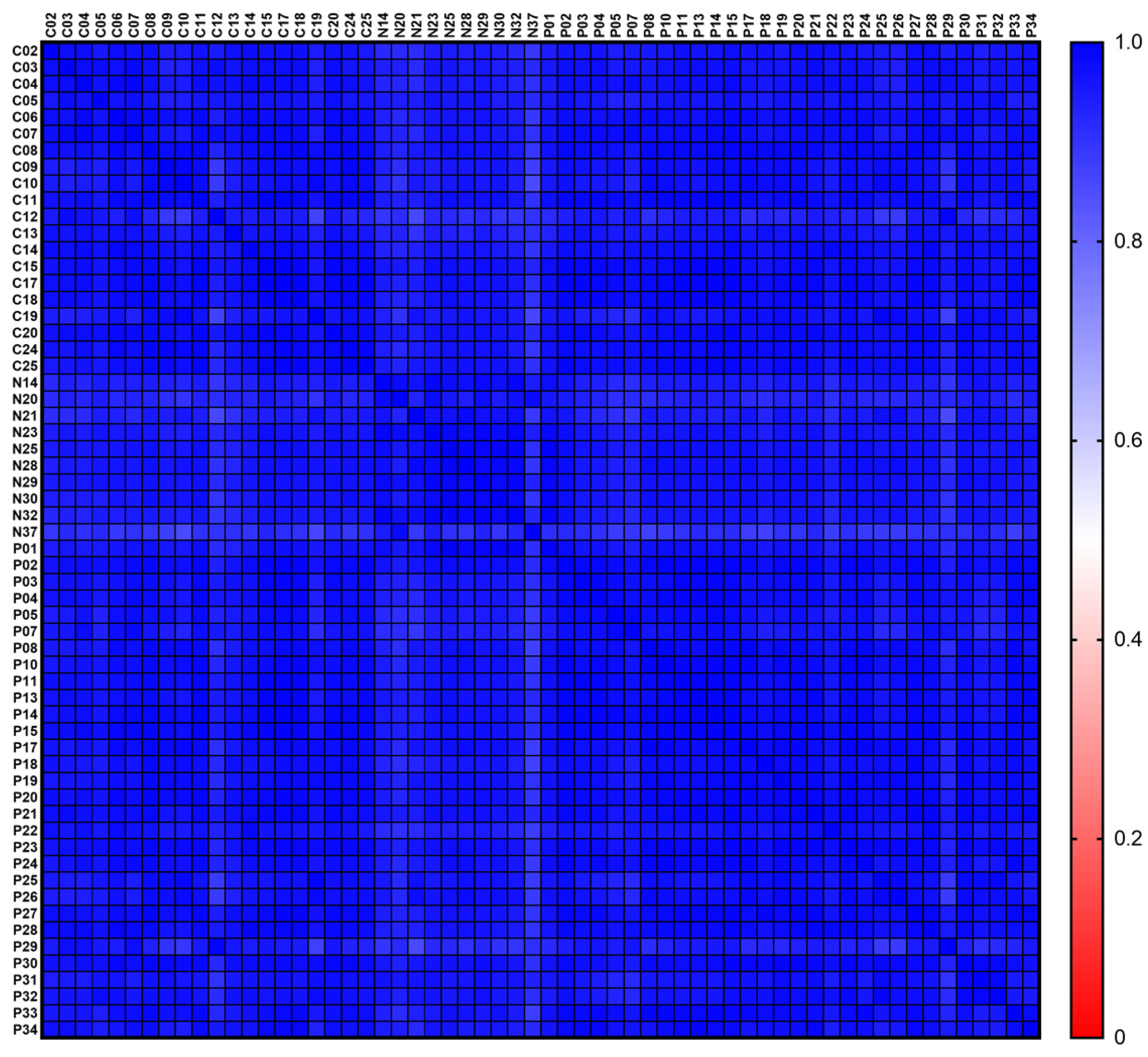
2.7. Sample-to-Sample Correlation
2.8. miRNA Differential Expression Analysis, GO Analysis and miRDB Target Prediction
3. Results
3.1. Patients and Controls
3.2. Next Generation Sequencing
3.3. Sample-to-Sample Correlation
3.4. Differential Expression
3.5. GO Enrichment Analysis and miRNA Target Prediction of Differentially Expressed miRNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, R.; Binder, A.; Wasner, G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef]
- Baron, R.; Wasner, G.; Binder, A. Chronic pain: Genes, plasticity, and phenotypes. Lancet Neurol. 2012, 11, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, K.; Nielsen, J.; Andersen, G.; Ingeman-Nielsen, M.; Arendt-Nielsen, L.; Jensen, T.S. Sensory abnormalities in consecutive, unselected patients with central post-stroke pain. Pain 1995, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Eide, P.K.; Jorum, E.; Stenehjem, A.E. Somatosensory findings in patients with spinal cord injury and central dysaesthesia pain. J. Neurol. Neurosurg. Psychiatry 1996, 60, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Leinders, M.; Üçeyler, N.; Pritchard, R.; Sommer, C.; Sorkin, L. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 2016, 283, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Moen, A.; Jacobsen, D.; Phuyal, S.; Legfeldt, A.; Haugen, F.; Røe, C.; Gjerstad, J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J. Transl. Med. 2017, 15, 89. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wang, Y.; Wang, J.; Feng, S.; Wang, X. The etiological roles of miRNAs, lncRNAs and circRNAs in neuropathic pain: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24592. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Chevillet, J.R.; Lee, I.; Briggs, H.A.; He, Y.; Wang, K. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014, 19, 6080–6105. [Google Scholar] [CrossRef]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. Mol. Mech. Mutagen. 2011, 717, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; Li, Q.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Leinders, M.; Üçeyler, N.; Thomann, A.; Sommer, C. Aberrant microRNA expression in patients with painful peripheral neuropathies. J. Neurol. Sci. 2017, 380, 242–249. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Haroutounian, S.; Kamerman, P.; Baron, R.; Bennett, D.L.; Bouhassira, D.; Cruccu, G.; Freeman, R.; Hansson, P.; Nurmikko, T.; et al. Neuropathic pain: An updated grading system for research and clinical practice. Pain 2016, 157, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y. Gold Standard for Diagnosis of DPN. Front. Endocrinol. 2021, 12, 719356. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- QIAGEN. QIAseq™ miRNA Library QC PCR Handbook: For Quality Control of RNA Isolation for Small RNA Next-Generation Sequencing. Available online: https://www.qiagen.com/us/resources/download.aspx?id=8f2523cc-3af8-4cbf-bdd7-b8f538462755&lang=en (accessed on 30 May 2022).
- de Almeida, L.G.N.; Young, D.; Chow, L.; Nicholas, J.; Lee, A.; Poon, M.-C.; Dufour, A.; Agbani, E.O. Proteomics and Metabolomics Profiling of Platelets and Plasma Mediators of Thrombo-Inflammation in Gestational Hypertension and Preeclampsia. Cells 2022, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hetheridge, C.; Scott, A.N.; Swain, R.; Copeland, J.W.; Higgs, H.; Bicknell, R.; Mellor, H. The novel formin FMNL3 is a cytoskeletal regulator of angiogenesis. J. Cell Sci. 2012, 125, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olinger, E.; Phakdeekitcharoen, P.; Caliskan, Y.; Orr, S.; Mabillard, H.; Pickles, C.; Tse, Y.; Wood, K.; Sayer, J.A.; Genomics England Research Consortium. Biallelic variants in TTC21B as a rare cause of early-onset arterial hypertension and tubuloglomerular kidney disease. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 109–120. [Google Scholar] [CrossRef]
- Su, I.-C.; Su, Y.-K.; Chuang, H.-Y.; Yadav, V.K.; Setiawan, S.A.; Fong, I.-H.; Yeh, C.-T.; Huang, H.-C.; Lin, C.-M. Ubiquitin-Specific Protease 6 n-Terminal-like Protein (USP6NL) and the Epidermal Growth Factor Receptor (EGFR) Signaling Axis Regulates Ubiquitin-Mediated DNA Repair and Temozolomide-Resistance in Glioblastoma. Biomedicines 2022, 10, 1531. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Cheng, P.; Lv, F. Correlation between miRNA target site polymorphisms in the 3′ UTR of AVPR1A and the risk of hypertension in the Chinese Han population. Biosci. Rep. 2019, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Deliu, E.; Arecco, N.; Morandell, J.; Dotter, C.P.; Contreras, X.; Girardot, C.; Käsper, E.-L.; Kozlova, A.; Kishi, K.; Chiaradia, I.; et al. Haploinsufficiency of the intellectual disability gene SETD5 disturbs developmental gene expression and cognition. Nat. Neurosci. 2018, 21, 1717–1727. [Google Scholar] [CrossRef]
- Moore, S.M.; Seidman, J.S.; Ellegood, J.; Gao, R.; Savchenko, A.; Troutman, T.D.; Abe, Y.; Stender, J.; Lee, D.; Wang, S.; et al. Setd5 haploinsufficiency alters neuronal network connectivity and leads to autistic-like behaviors in mice. Transl. Psychiatry 2019, 9, 24. [Google Scholar] [CrossRef]
- Hayano, Y.; Ishino, Y.; Hyun, J.H.; Orozco, C.G.; Steinecke, A.; Potts, E.; Oisi, Y.; Thomas, C.I.; Guerrero-Given, D.; Kim, E.; et al. IgSF11 homophilic adhesion proteins promote layer-specific synaptic assembly of the cortical interneuron subtype. Sci. Adv. 2021, 7, eabf1600. [Google Scholar] [CrossRef]
- Wagh, D.; Terry-Lorenzo, R.; Waites, C.L.; Leal-Ortiz, S.A.; Maas, C.; Reimer, R.J.; Garner, C.C. Piccolo Directs Activity Dependent F-Actin Assembly from Presynaptic Active Zones via Daam1. PLoS ONE 2015, 10, e0120093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Lieu, Z.Z.; Manser, E.; Bershadsky, A.D.; Sheetz, M.P. Formin DAAM1 Organizes Actin Filaments in the Cy-toplasmic Nodal Actin Network. PLoS ONE 2016, 11, e0163915. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Yamakuchi, M.; Ture, S.; Garcia-Hernandez, M.D.L.L.; Ko, K.A.; Modjeski, K.L.; Lomonaco, M.B.; Johnson, A.D.; O’Donnell, C.J.; Takai, Y.; et al. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J. Clin. Investig. 2014, 124, 4503–4516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridge, L.A.; Mitchell, K.; Al-Anbaki, A.; Qureshi, W.M.S.; Stephen, L.A.; Tenin, G.; Lu, Y.; Lupu, I.-E.; Clowes, C.; Robertson, A.; et al. Non-muscle myosin IIB (Myh10) is required for epicardial function and coronary vessel formation during mammalian development. PLoS Genet. 2017, 13, e1007068. [Google Scholar] [CrossRef] [Green Version]
- Dash, B.; Han, C.; Waxman, S.G.; Dib-Hajj, S.D. Nonmuscle myosin II isoforms interact with sodium channel alpha subunits. Mol. Pain 2018, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Hu, S.; Jiao, D.; Li, X.; Qi, S.; Fan, R. Synaptotagmin-4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca2+ influx via TRPM1 channels. Cell Biochem. Funct. 2020, 38, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushita, F.; Kameyama, T.; Kadokawa, Y.; Marunouchi, T. Spatiotemporal expression pattern of Myt/NZF family zinc finger transcription factors during mouse nervous system development. Dev. Dyn. 2014, 243, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shao, Q.; Li, Z.; Gonzalez, G.A.; Lu, F.; Wang, D.; Pu, Y.; Huang, A.; Zhao, C.; He, C.; et al. Myt1L Promotes Differentiation of Oligodendrocyte Precursor Cells and is Necessary for Remyelination after Lysolecithin-Induced Demyelination. Neurosci. Bull. 2018, 34, 247–260. [Google Scholar] [CrossRef]
- Chen, J.; Yen, A.; Florian, C.P.; Dougherty, J.D. MYT1L in the making: Emerging insights on functions of a neurodevelopmental disorder gene. Transl. Psychiatry 2022, 12, 292. [Google Scholar] [CrossRef]
- Liu, W.; Ling, S.; Sun, W.; Liu, T.; Li, Y.; Zhong, G.; Zhao, D.; Zhang, P.; Song, J.; Jin, X.; et al. Circulating microRNAs correlated with the level of coronary artery calcification in symptomatic patients. Sci. Rep. 2015, 5, 16099. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Liu, H.; Wang, C.; Li, H.; Qiao, L. Expression of the microRNA-30 family in pulmonary arterial hypertension and the role of microRNA-30d-5p in the regulation of pulmonary arterial smooth muscle cell toxicity and apoptosis. Exp. Ther. Med. 2022, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dang, J.; Lin, X.; Wang, M.; Liu, Y.; Chen, J.; Chen, Y.; Luo, X.; Hu, Z.; Weng, W.; et al. RA Fibroblast-Like Synoviocytes Derived Extracellular Vesicles Promote Angiogenesis by miRNA-1972 Targeting p53/mTOR Signaling in Vascular Endotheliocyte. Front. Immunol. 2022, 13, 793855. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.W.; Marcon, B.H.; Angulski, A.B.B.; Martins, S.D.T.; Leitolis, A.; Stimamiglio, M.A.; Senegaglia, A.C.; Correa, A.; Alves, L.R. Selective Loading and Variations in the miRNA Profile of Extracellular Vesicles from Endothelial-like Cells Cultivated under Normoxia and Hypoxia. Int. J. Mol. Sci. 2022, 23, 10066. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Feng, B.; Wang, W.; Liu, D.; Zhao, X.; Yu, C.; Wang, X.; Gao, Y. MiR-550a-3p restores damaged vascular smooth muscle cells by inhibiting thrombomodulin in an in vitro atherosclerosis model. Eur. J. Histochem. EJH 2022, 66, 3429. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ren, Y.; Liu, Y.; Cheng, Y.; Liu, Y. Circulating miR-3135b and miR-107 are potential biomarkers for severe hyper-tension. J. Hum. Hypertens. 2021, 35, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Machal, J.; Novak, J.; Hezova, R.; Zlamal, F.; Vasku, A.; Slaby, O.; Bienertova-Vasku, J. Polymorphism in miR-31 and miR-584 binding site in the angiotensinogen gene differentially influences body fat distribution in both sexes. Genes Nutr. 2015, 10, 488. [Google Scholar] [CrossRef]
- Choi, H.; Koh, H.W.L.; Zhou, L.; Cheng, H.; Loh, T.P.; Rizi, E.P.; Toh, S.A.; Ronnett, G.V.; Huang, B.E.; Khoo, C.M. Plasma Protein and MicroRNA Biomarkers of Insulin Resistance: A Network-Based Integrative -Omics Analysis. Front. Physiol. 2019, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- PMC-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pmc/ (accessed on 21 September 2022).
- Cameron, N.E.; Eaton, S.E.M.; Cotter, M.A.; Tesfaye, S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001, 44, 1973–1988. [Google Scholar] [CrossRef] [Green Version]
- Dyck, P.J.; Hansen, S.; Karnes, J.; O’Brien, P.; Yasuda, H.; Windebank, A.; Zimmerman, B. Capillary number and percentage closed in human diabetic sural nerve. Proc. Natl. Acad. Sci. USA 1985, 82, 2513–2517. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.L.; Alfonso, J.; Monyer, H.; Kuner, R. Neurogenesis in the adult brain functionally contributes to the maintenance of chronic neuropathic pain. Sci. Rep. 2021, 11, 18549. [Google Scholar] [CrossRef]
- Hiraga, S.-I.; Itokazu, T.; Nishibe, M.; Yamashita, T. Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm. Regen. 2022, 42, 15. [Google Scholar] [CrossRef] [PubMed]
| Name | Log2 Fold Change | Fold Change | p-Value | FDR p-Value | Bonferroni |
|---|---|---|---|---|---|
| hsa-miR-3135b | −2.65 | −6.30 | 3.53 × 10−14 | 2.26 × 10−11 | 5.94 × 10−11 |
| hsa-miR-584-5p | −1.39 | −2.62 | 8.50 × 10−10 | 2.72 × 10−7 | 1.43 × 10−6 |
| hsa-miR-12136 | −1.92 | −3.80 | 4.46 × 10−7 | 9.51 × 10−5 | 7.50 × 10−4 |
| hsa-miR-550a-3p | 2.09 | 4.27 | 1.45 × 10−5 | 2.07 × 10−3 | 0.02 |
| GO Term | Description | DE Genes | p-Values |
|---|---|---|---|
| 0030947 | regulation of vascular endothelial growth factor receptor signaling pathway | 4 | 1.90 × 10−4 |
| 0030949 | positive regulation of vascular endothelial growth factor receptor signaling pathway | 4 | 1.90 × 10−4 |
| 1904018 | positive regulation of vasculature development | 10 | 4.62 × 10−3 |
| 0045766 | positive regulation of angiogenesis | 9 | 9.00 × 10−3 |
| 0009967 | positive regulation of signal transduction | 9 | 0.01 |
| 0010647 | positive regulation of cell communication | 9 | 0.01 |
| 0023056 | positive regulation of signaling | 9 | 0.01 |
| 0051094 | positive regulation of developmental process | 13 | 0.02 |
| 0010656 | negative regulation of muscle cell apoptotic process | 5 | 0.02 |
| 0090287 | regulation of cellular response to growth factor stimulus | 6 | 0.02 |
| 0010664 | negative regulation of striated muscle cell apoptotic process | 4 | 0.02 |
| 0010667 | negative regulation of cardiac muscle cell apoptotic process | 4 | 0.02 |
| 0090050 | positive regulation of cell migration involved in sprouting angiogenesis | 5 | 0.02 |
| miRNA | Target Rank | Target Score | Gene Symbol | Gene Description |
|---|---|---|---|---|
| hsa-miR-3135b | 1 | 99 | LRRC27 | leucine rich repeat containing 27 |
| 2 | 96 | FMNL3 | formin like 3 | |
| 3 | 96 | TTC21B | tetratricopeptide repeat domain 21B | |
| hsa-miR-584-5p | 1 | 96 | USP6NL | USP6 N-terminal like |
| 2 | 95 | AVPR1A | arginine vasopressin receptor 1A | |
| 3 | 94 | SETD5 | SET domain containing 5 | |
| hsa-miR-12136 | 1 | 100 | IGSF11 | immunoglobulin superfamilymember 11 |
| 2 | 100 | DAAM1 | dishevelled associated activator of morphogenesis 1 | |
| 3 | 100 | STXBP5 | syntaxin binding protein 5 | |
| hsa-miR-550a-3p | 1 | 98 | MYH10 | myosin heavy chain 10 |
| 2 | 98 | SYT4 | synaptotagmin 4 | |
| 3 | 95 | MYT1L | myelin transcription factor 1 like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, A.; Fabig, S.-C.; Kersebaum, D.; Hüllemann, P.; Baron, R.; Roch, T.; Babel, N.; Seitz, H. Differential Expression of microRNAs in Serum of Patients with Chronic Painful Polyneuropathy and Healthy Age-Matched Controls. Biomedicines 2023, 11, 764. https://doi.org/10.3390/biomedicines11030764
Pellegrino A, Fabig S-C, Kersebaum D, Hüllemann P, Baron R, Roch T, Babel N, Seitz H. Differential Expression of microRNAs in Serum of Patients with Chronic Painful Polyneuropathy and Healthy Age-Matched Controls. Biomedicines. 2023; 11(3):764. https://doi.org/10.3390/biomedicines11030764
Chicago/Turabian StylePellegrino, Antonio, Sophie-Charlotte Fabig, Dilara Kersebaum, Philipp Hüllemann, Ralf Baron, Toralf Roch, Nina Babel, and Harald Seitz. 2023. "Differential Expression of microRNAs in Serum of Patients with Chronic Painful Polyneuropathy and Healthy Age-Matched Controls" Biomedicines 11, no. 3: 764. https://doi.org/10.3390/biomedicines11030764





